Temporal Trends and Geographic Variability in the Prescription of Antiretroviral Treatments in People Living with HIV in Spain, 2004–2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
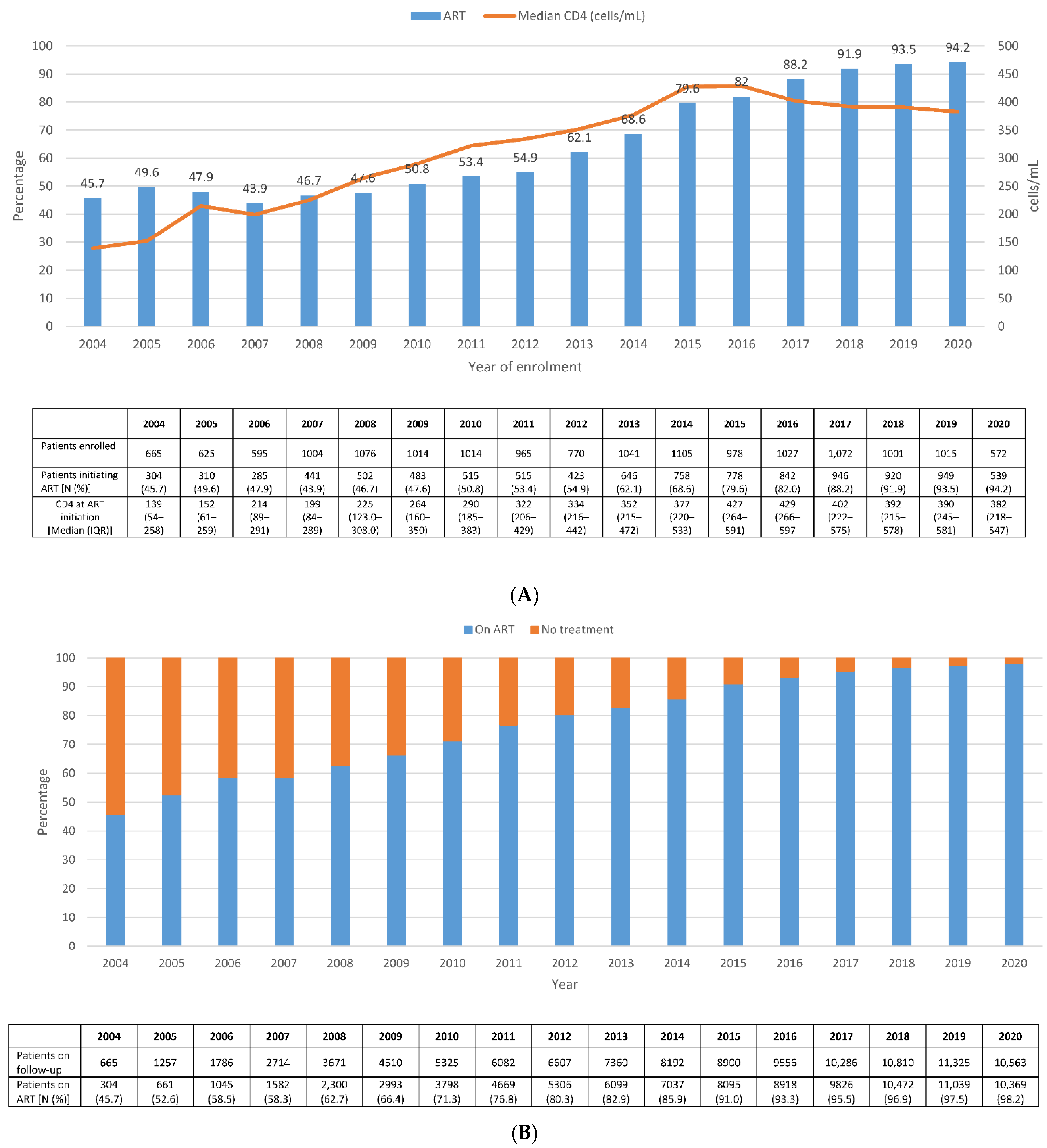
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. UNAIDS 2021 Epidemiological Estimates. Available online: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (accessed on 10 January 2022).
- Gupta, R. The current state of an HIV cure: Innovative approaches and limitations. EBioMedicine 2019, 42, 1–2. [Google Scholar]
- Eaton, E.F.; Tamhane, A.; Davy-Mendez, T.; Mathews, W.C.; Moore, R.D.; Saag, M.S.; Mugavero, M.J. Trends in antiretroviral therapy prescription, durability and modification: New drugs, more changes, but less failure. AIDS 2018, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.I.; Castillo-Mancilla, J.R.; Tashima, K.T.; Landovitz, R.L. Advances in Long-Acting Agents for the Treatment of HIV Infection. Drugs 2020, 80, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.F.; Simões, S.; Carvalheiro, M.; Azevedo Pereira, J.M.; Costa, Q.; Ascenso, A. Novel antiretroviral therapeutic strategies for HIV. Molecules 2021, 26, 5305. [Google Scholar] [CrossRef] [PubMed]
- Alejos, B.; Suárez-García, I.; Bisbal, O.; Iribarren, J.A.; Asensi, V.; Górgolas, M.; Muga, R.; Moreno, S.; Jarrín, I.; CoRIS Cohort. Choice of the initial antiretroviral treatment for HIV-positive individuals in the era of integrase inhibitors. PLoS ONE 2019, 14, e0221598. [Google Scholar] [CrossRef]
- Viciana, P.; Ocampo, A.; Hevia, H.; Palazuelos, M.; Ledesma, F. Tratamiento Antirretroviral de Inicio en Pacientes Infectados por el Virus de la Inmunodeficiencia Humana en España: Decisiones con Relación a Características Inmunovirológicas Específicas (Estudio PERFIL-es). Enferm. Infecc. Microbiol. Clin. 2014, 32, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, I.; González, J.; Berenguer, J.; García, F.; Portilla, J.; Muga, R.; Moreno, S.; Jarrín, I. Reasons for noncompliance with the national guidelines for initial antiretroviral therapy of HIV-infected patients in Spain, 2010–2015. Enferm. Infecc. Microbiol. Clin. 2019, 37, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Servicio de Coordinación Legislativa y Relaciones Institucionales. ORDEN 429/2006, de 3 de Marzo, de la Consejería de Sanidad y Consumo, por la que se Acuerda la Uniformidad de Determinados Medicamentos y se Declara de Gestión Centralizada su Contratación. 2006; pp. 1–2.
- Sociedad Española de Farmacia Hospitalaria SEFH. Guía de Compra Pública de Medicamentos Para las Farmacias Hospitalarias. 2018. Available online: http://www.madrid.org/wleg_pub/secure/normativas/contenidoNormativa.jsf?opcion=VerHtml&nmnorma=3811#no-back-button (accessed on 10 January 2022).
- Sobrino-Vegas, P.; Gutierrez, F.; Berenguer, J.; Labarga, P.; Garcia, F.; Alejos-Ferreras, B.; Muñoz, M.A.; Moreno, S.; del Amo, J.; CoRIS. The Cohort of the Spanish HIV Research Network (CoRIS) and its associated biobank; organizational issues, main findings and losses to follow-up. Enferm. Infecc. Microbiol. Clin. 2011, 29, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.B.; Buchacz, K.; Gebo, K.A.; Hessol, N.A.; Horberg, M.A.; Jacobson, L.P.; Kirk, G.D.; Kitahata, M.M.; Korthuis, P.T.; Moore, R.D.; et al. Trends and disparities in antiretroviral therapy initiation and Virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin. Infect. Dis. 2013, 56, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Panel de Expertos de GeSIDA y Plan Nacional Sobre el SIDA. Documento de Consenso de GeSIDA/Plan Nacional Sobre el SIDA Respecto al Tratamiento Antirretroviral en Adultos Infectados por el Virus de la Inmunodeficiencia Humana (Actualización 2020). Available online: https://gesida-seimc.org/wp-content/uploads/2020/07/TAR_GUIA_GESIDA_2020_COMPLETA_Julio.pdf (accessed on 10 January 2022).
- European AIDS Clinical Society. EACS Guideline. EACS. 2021. Available online: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf (accessed on 10 January 2022).
- Panel de Expertos de GeSIDA y Plan Nacional Sobre el SIDA. Documento de Consenso de GeSIDA/Plan Nacional Sobre el SIDA Respecto al Tratamiento Antirretroviral en Adultos Infectados por el Virus de la Inmunodeficiencia Humana (Actualización 2018). Available online: https://gesida-seimc.org/wp-content/uploads/2018/01/gesida_TAR_adultos_v3-1.pdf (accessed on 10 January 2022).
- Panel de Expertos de GeSIDA y Plan Nacional Sobre el SIDA. Documento de Consenso para Mejorar la Adherencia a la Farmacoterapia en Pacientes con Infección por el Virus de la Inmunodeficiencia en Tratamiento Antirretroviral (Actualización Febrero de 2020). Available online: https://gesida-seimc.org/wp-content/uploads/2020/04/GUIA_GESIDA_febrero_2020_Adherencia.pdf (accessed on 10 January 2022).
- de expertos de GeSIDA, Panel; Plan Nacional Sobre el Sida. GESIDA/National AIDS Plan: Consensus document on antiretroviral therapy in adults infected by the human immunodeficiency virus (Updated January 2015). Enferm. Infecc. Microbiol. Clin. 2015, 33, 543.e1. (In Spanish) [Google Scholar] [CrossRef]
- AIDS Study Group. GeSIDA of the Spanish Society of Infectious Diseases and Clinical Microbiology and the National AIDS Plan. Executive Summary of the GeSIDA/National AIDS Plan Consensus Document on Antiretroviral Therapy in Adults Infected by the Human Immunodeficiency Virus (Updated January 2018). Enferm. Infecc. Microbiol. Clin. 2019, 37, 195–202. Available online: https://www.elsevier.es/en-revista-enfermedades-infecciosas-microbiologia-clinica-english-428-articulo-executive-summary-gesida-national-aids-plan-S2529993X19300243?referer=buscador (accessed on 10 January 2022).
- Zamora, F.J.; Dowers, E.; Yasin, F.; Ogbuagu, O. Dolutegravir and lamivudine combination for the treatment of HIV-1 infection. HIV/AIDS-Res. Palliat. Care 2019, 11, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baril, J.G.; Angel, J.B.; John Gill, M.; Gathe, J.; Cahn, P.; Van Wyk, J.; Walmsley, S. Dual therapy treatment strategies for the management of patients infected with HIV: A systematic review of current evidence in ARV-naive or ARV-experienced, virologically suppressed patients. PLoS ONE 2016, 11, e0148231. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, I.; García-Sempere, A.; Peiró, S.; Rodríguez-Bernal, C.; Sanfélix-Genovés, J.; Sanfélix-Gimeno, G. Trends and Geographical Variability in Osteoporosis Treatment After Hip Fracture: A Multilevel Analysis of 30,965 Patients in the Region of Valencia, Spain. J. Bone Miner. Res. 2020, 35, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Rosell, L.C.; Malats, N.; Los, D. Variabilidad en el Diagnóstico, Tratamiento y Pronóstico del Cáncer Vesical en España. Análisis Según el área Geográfica y la Categoría de Hospital. pp. 231–235. Available online: https://www.atlasvpm.org/wp-content/uploads/2019/06/Variabilidad-en-el-diagn%C3%B3stico-tratamiento-y-pron%C3%B3stico-del-c%C3%A1ncer-vesical-en-espa%C3%B1a.pdf (accessed on 10 January 2022).
- Suárez-García, I.; Sobrino-Vegas, P.; Tejada, A.; Viciana, P.; Ribas, M.; Iribarren, J.; Díaz Menéndez, M.; Rivero, M.; Arazo, P.; del Amo, J. Compliance with national guidelines for HIV treatment and its association with mortality and treatment outcome: A study in a Spanish cohort. HIV Med. 2014, 15, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Garcıa, I.; Gonzalez, J.; Berenguer, J.; Garcia, F.; Portilla, J.; Muga, R. Cost limitations and restricted access to antiretroviral drugs as potential barriers for compliance to HIV treatment guidelines in the CoRIS cohort. In Proceedings of the X Conference of the Spanish AIDS Study Group (GeSIDA), Madrid, Spain; 2018. [Google Scholar]
- Unidad de Vigilancia de VIH y Hepatitis. Vigilancia Epidemiológica del VIH y SIDA en España 2020. Datos Provisionales. Sistema de Información Sobre Nuevos Diagnósticos de VIH. Registro Nacional de Casos de SIDA. Actualización 30 de Junio de 2021. Available online: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/Informe_VIH_SIDA_WEB.pdf (accessed on 10 January 2022).
- Vourli, G.; Pharris, A.; Cazein, F.; Costagliola, D.; Dabis, F.; Del Amo, J.; Delpech, V.; Díaz, A.; Girardi, E.; Gourlay, A.; et al. Are European HIV cohort data within EuroCoord representative of the diagnosed HIV population? AIDS 2019, 33, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caram, M.E.V.; Estes, J.P.; Griggs, J.J.; Lin, P.; Mukherjee, B. Temporal and geographic variation in the systemic treatment of advanced prostate cancer. BMC Cancer 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Algueró, M.; Hernando, V.; Riero, M.; Blanco Ramos, J.R.; de Zarraga Fernández, M.A.; Galindo, P.; Pérez-González, A.; Díaz, A.; Suárez-García, I.; Jarrín, I.; et al. Temporal Trends and Geographic Variability in the Prescription of Antiretroviral Treatments in People Living with HIV in Spain, 2004–2020. J. Clin. Med. 2022, 11, 1896. https://doi.org/10.3390/jcm11071896
Ruiz-Algueró M, Hernando V, Riero M, Blanco Ramos JR, de Zarraga Fernández MA, Galindo P, Pérez-González A, Díaz A, Suárez-García I, Jarrín I, et al. Temporal Trends and Geographic Variability in the Prescription of Antiretroviral Treatments in People Living with HIV in Spain, 2004–2020. Journal of Clinical Medicine. 2022; 11(7):1896. https://doi.org/10.3390/jcm11071896
Chicago/Turabian StyleRuiz-Algueró, Marta, Victoria Hernando, María Riero, José Ramón Blanco Ramos, Miguel Alberto de Zarraga Fernández, Pepa Galindo, Alexandre Pérez-González, Asunción Díaz, Inés Suárez-García, Inma Jarrín, and et al. 2022. "Temporal Trends and Geographic Variability in the Prescription of Antiretroviral Treatments in People Living with HIV in Spain, 2004–2020" Journal of Clinical Medicine 11, no. 7: 1896. https://doi.org/10.3390/jcm11071896
APA StyleRuiz-Algueró, M., Hernando, V., Riero, M., Blanco Ramos, J. R., de Zarraga Fernández, M. A., Galindo, P., Pérez-González, A., Díaz, A., Suárez-García, I., Jarrín, I., & CoRIS cohort. (2022). Temporal Trends and Geographic Variability in the Prescription of Antiretroviral Treatments in People Living with HIV in Spain, 2004–2020. Journal of Clinical Medicine, 11(7), 1896. https://doi.org/10.3390/jcm11071896





