The Impact of the Age, Dyspnoea, and Airflow Obstruction (ADO) Index on the Medical Burden of Chronic Obstructive Pulmonary Disease (COPD)
Abstract
:1. Introduction
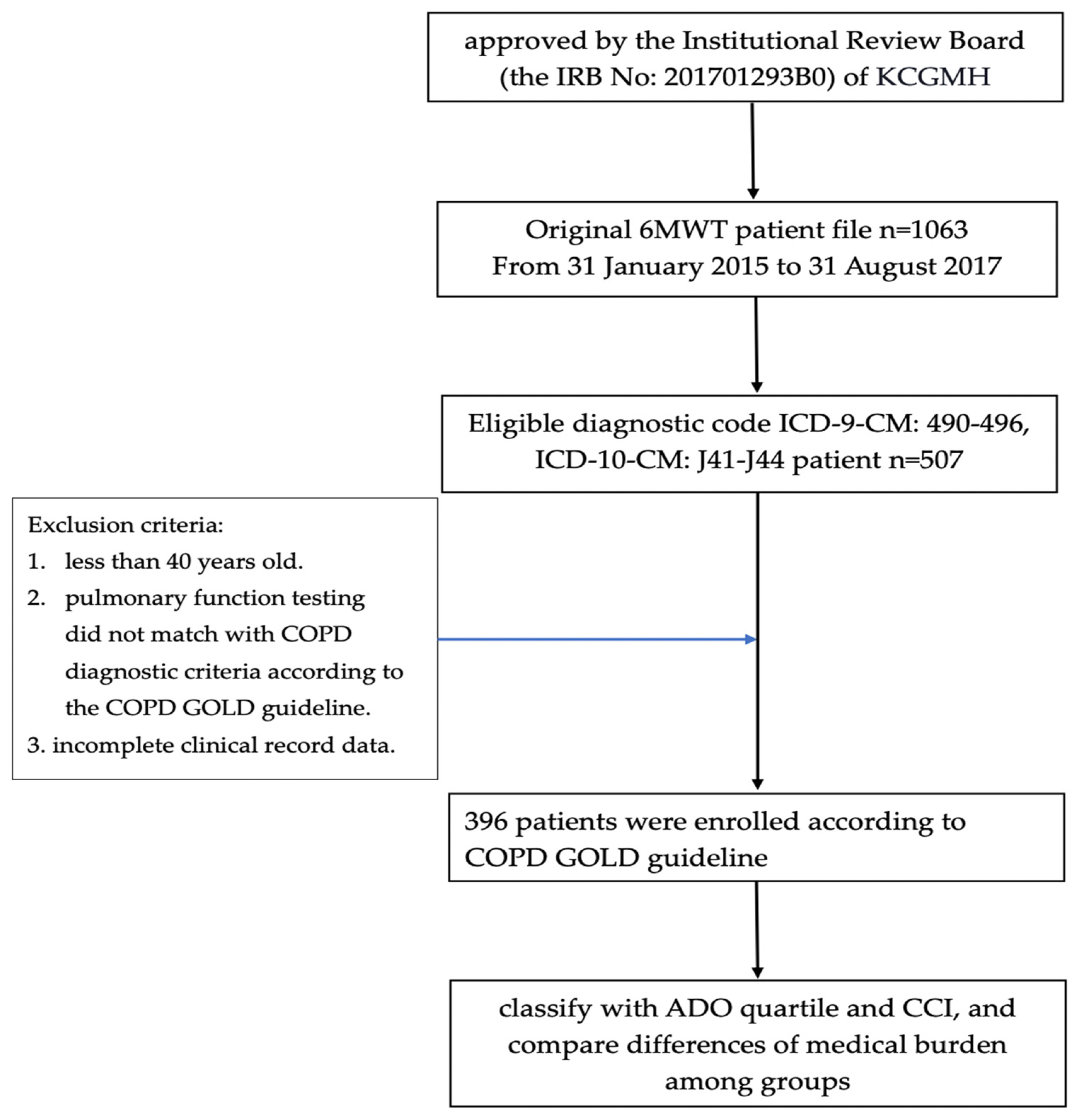
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Clinical Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Top 10 Causes of Death. 9 December 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 30 October 2021).
- Germini, F.; Veronese, G.; Marcucci, M.; Coen, D.; Ardemagni, D.; Montano, N.; Fabbri, A.; Adinolfi, L.E.; Alvisi, A.; Azin, G.; et al. COPD exacerbations in the emergency department: Epidemiology and related costs. A retrospective cohort multicentre study from the Italian Society of Emergency Medicine (SIMEU). Eur. J. Intern. Med. 2018, 51, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.R.; Díez-Manglano, J.; García, F.L.; Peromingo, J.A.D.; Almagro, P.; Aguilar, J.M.V. Management of the COPD Patient with Comorbidities: An Experts Recommendation Document. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1015–1037. [Google Scholar] [CrossRef] [PubMed]
- Negewo, N.A.; Gibson, P.; McDonald, V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015, 20, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- van Manen, J.; Bindels, P.; Ijzermans, C.; van der Zee, J.; Bottema, B.; Schadé, E. Prevalence of comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J. Clin. Epidemiol. 2001, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Shwartz, M.; Iezzoni, L.I.; Moskowitz, M.A.; Ash, A.S.; Sawitz, E. The Importance of Comorbidities in Explaining Differences in Patient Costs. Med. Care 1996, 34, 767–782. [Google Scholar] [CrossRef]
- Charlson, M.E.; Charlson, R.E.; Peterson, J.C.; Marinopoulos, S.S.; Briggs, W.M.; Hollenberg, J.P. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J. Clin. Epidemiol. 2008, 61, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Tzanakis, N.; Hillas, G.; Perlikos, F.; Tsiligianni, I. Managing comorbidities in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 95–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lortet-Tieulent, J.; Soerjomataram, I.; López-Campos, J.L.; Ancochea, J.; Coebergh, J.W.; Soriano, J.B. International trends in COPD mortality, 1995–2017. Eur. Respir. J. 2019, 54, 1901791. [Google Scholar] [CrossRef]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannino, D.M.; Higuchi, K.; Yu, T.-C.; Zhou, H.; Li, Y.; Tian, H.; Suh, K. Economic Burden of COPD in the Presence of Comorbidities. Chest 2015, 148, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puhan, M.; Hansel, N.N.; Sobradillo, P.; Enright, P.; Lange, P.; Hickson, D.; Menezes, A.M.; Ter Riet, G.; Held, U.; Domingo-Salvany, A.; et al. Large-scale international validation of the ADO index in subjects with COPD: An individual subject data analysis of 10 cohorts. BMJ Open 2012, 2, e002152. [Google Scholar] [CrossRef]
- Athlin, Å.; Giezeman, M.; Hasselgren, M.; Montgomery, S.; Lisspers, K.; Ställberg, B.; Janson, C.; Sundh, J. Prediction of Mortality Using Different COPD Risk Assessments–A 12-Year Follow-Up. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 665. [Google Scholar] [CrossRef] [PubMed]
- Keene, S.J.; Jordan, R.E.; Franssen, F.M.; de Vries, F.; Martin, J.; Sitch, A.; Turner, A.M.; Dickens, A.P.; Fitzmaurice, D.; Adab, P. External Validation Of The Updated ADO Score In COPD Patients From The Birmingham COPD Cohort. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2395–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-L.; Lin, M.-H.; Chen, P.-S.; Tsai, Y.-C.; Shen, L.-S.; Kuo, H.-C.; Liu, S.-F. Using the BODE Index and Comorbidities to Predict Health Utilization Resources in Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Plata, V.P.; Cabral, H.J. The Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.-C.; Earnest, A.; Lu, S.-J. A Multidimensional Grading System (BODE Index) as Predictor of Hospitalization for COPD. Chest 2005, 128, 3810–3816. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, Z.; Zhang, J.; Feng, H.; Liang, C.; Liu, D. Charlson comorbidity index as a predictor of short-term outcomes after pulmonary resection. J. Thorac. Dis. 2020, 12, 6670–6679. [Google Scholar] [CrossRef]
- Asai, N.; Ohashi, W.; Sakanashi, D.; Suematsu, H.; Kato, H.; Hagihara, M.; Watanabe, H.; Shiota, A.; Koizumi, Y.; Yamagishi, Y.; et al. Combination of Sequential Organ Failure Assessment (SOFA) score and Charlson Comorbidity Index (CCI) could predict the severity and prognosis of candidemia more accurately than the Acute Physiology, Age, Chronic Health Evaluation II (APACHE II) score. BMC Infect. Dis. 2021, 21, 77. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.G.; Paz-Zulueta, M.; Herrero-Montes, M.; Parás-Bravo, P.; Pérez, M.M. Cost analysis of chronic obstructive pulmonary disease (COPD): A systematic review. Heal. Econ. Rev. 2021, 11, 31. [Google Scholar] [CrossRef]
- Divo, M.J.; Celli, B.R.; Poblador-Plou, B.; Calderón-Larrañaga, A.; De-Torres, J.P.; Gimeno-Feliu, L.A.; Bertó, J.; Zulueta, J.J.; Casanova, C.; Pinto-Plata, V.M.; et al. Chronic Obstructive Pulmonary Disease (COPD) as a disease of early aging: Evidence from the EpiChron Cohort. PLoS ONE 2018, 13, e0193143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ställberg, B.; Janson, C.; Larsson, K.; Johansson, G.; Kostikas, K.; Gruenberger, J.-B.; Gutzwiller, F.S.; Jorgensen, L.; Uhde, M.; Lisspers, K. Real-world retrospective cohort study ARCTIC shows burden of comorbidities in Swedish COPD versus non-COPD patients. NPJ Prim. Care Respir. Med. 2018, 28, 33. [Google Scholar] [CrossRef] [PubMed]
- Verberkt, C.A.; van den Beuken-van Everdingen, M.H.; Dirksen, C.D.; Schols, J.M.; Vanfleteren, L.E.; Franssen, F.M.; Groenen, M.T.; Wouters, E.F.; Janssen, D.J. Healthcare and Societal Costs in Patients with COPD and Breathlessness after Completion of a Comprehensive Rehabilitation Program. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 170–180. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Chen, Y.; Xiao, T.; Fu, C.; Xu, B. Costs of chronic obstructive pulmonary disease in urban areas of China: A cross-sectional study in four cities. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2625–2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakavas, S.; Kotsiou, O.S.; Perlikos, F.; Mermiri, M.; Mavrovounis, G.; Gourgoulianis, K.; Pantazopoulos, I. Pulmonary function testing in COPD: Looking beyond the curtain of FEV1. NPJ Prim. Care Respir. Med. 2021, 31, 23. [Google Scholar] [CrossRef]
- Schünemann, H. From BODE to ADO to outcomes in multimorbid COPD patients. Lancet 2009, 374, 667–668. [Google Scholar] [CrossRef]
- García-Polo, C.; Alcázar-Navarrete, B.; Ruiz-Iturriaga, L.A.; Herrejón, A.; Ros-Lucas, J.A.; García-Sidro, P.; Tirado-Conde, G.; López-Campos, J.L.; Martínez-Rivera, C.; Costán-Galicia, J.; et al. Factors associated with high healthcare resource utilisation among COPD patients. Respir. Med. 2012, 106, 1734–1742. [Google Scholar] [CrossRef] [Green Version]
- Bu, X.N.; Yang, T.; Thompson, M.; Hutchinson, A.F.; Irving, L.B. Changes in the BODE index, exacerbation duration and hospitalisation in a cohort of COPD patients. Singap. Med. J. 2011, 52, 894. [Google Scholar]
- Morales, D.R.; Flynn, R.; Zhang, J.; Trucco, E.; Quint, J.K.; Zutis, K. External validation of ADO, DOSE, COTE and CODEX at predicting death in primary care patients with COPD using standard and machine learning approaches. Respir. Med. 2018, 138, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.-Y.; Chen, Y.-J.; Sheu, S.-M.; Tsai, C.-F.; Chen, W. Misuse of inhalers among COPD patients in a community hospital in Taiwan. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1309–1316. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.-W.; Ruan, S.-Y.; Huang, C.-T.; Tsai, Y.-J.; Lai, F.; Yu, C.-J. Validity of ICD9-CM codes to diagnose chronic obstructive pulmonary disease from National Health Insurance claim data in Taiwan. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3055–3063. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-Y.; Majeed, A.; Kuo, K.N. An overview of the healthcare system in Taiwan. Lond. J. Prim. Care 2010, 3, 115–119. [Google Scholar] [CrossRef] [Green Version]
| Points | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| FEV1 (% predicted) | ≥65% | ≥36–64% | ≤35% | |||
| Dyspnoea (MRC scale) | 0–1 | 2 | 3 | 4 | ||
| Age (years) | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | ≥90 |
| Factors | Mean ± Standard Deviation (SD) or n (%) |
|---|---|
| Male (%) | 382 (96.5) |
| Smoking history (pack-years) | 31.7 ± 18.5 |
| Age (years) | 73.1 ± 9.5 |
| Body-mass index (BMI) | 23.5 ± 4.1 |
| FVC (% of predicted value) | 79.7±16.7 |
| FEV1/FVC (%) | 52.7 ± 10.6 |
| FEV1 (% of predicted value) | 55.2 ± 18.2 |
| GOLD stage (%) | |
| Mild | 46 (11.6) |
| Moderate | 187 (47.2) |
| Severe | 140 (35.4) |
| Very severe | 23 (5.8) |
| DLCO (%) | 68.5 ± 21.0 |
| 6-MWD (m) | 351.9 ± 111.6 |
| mMRC | 1.72 ± 0.9 |
| mMRC dyspnea scale | |
| Scale 0/1/2/3/4 | 25/133/173/56/9 |
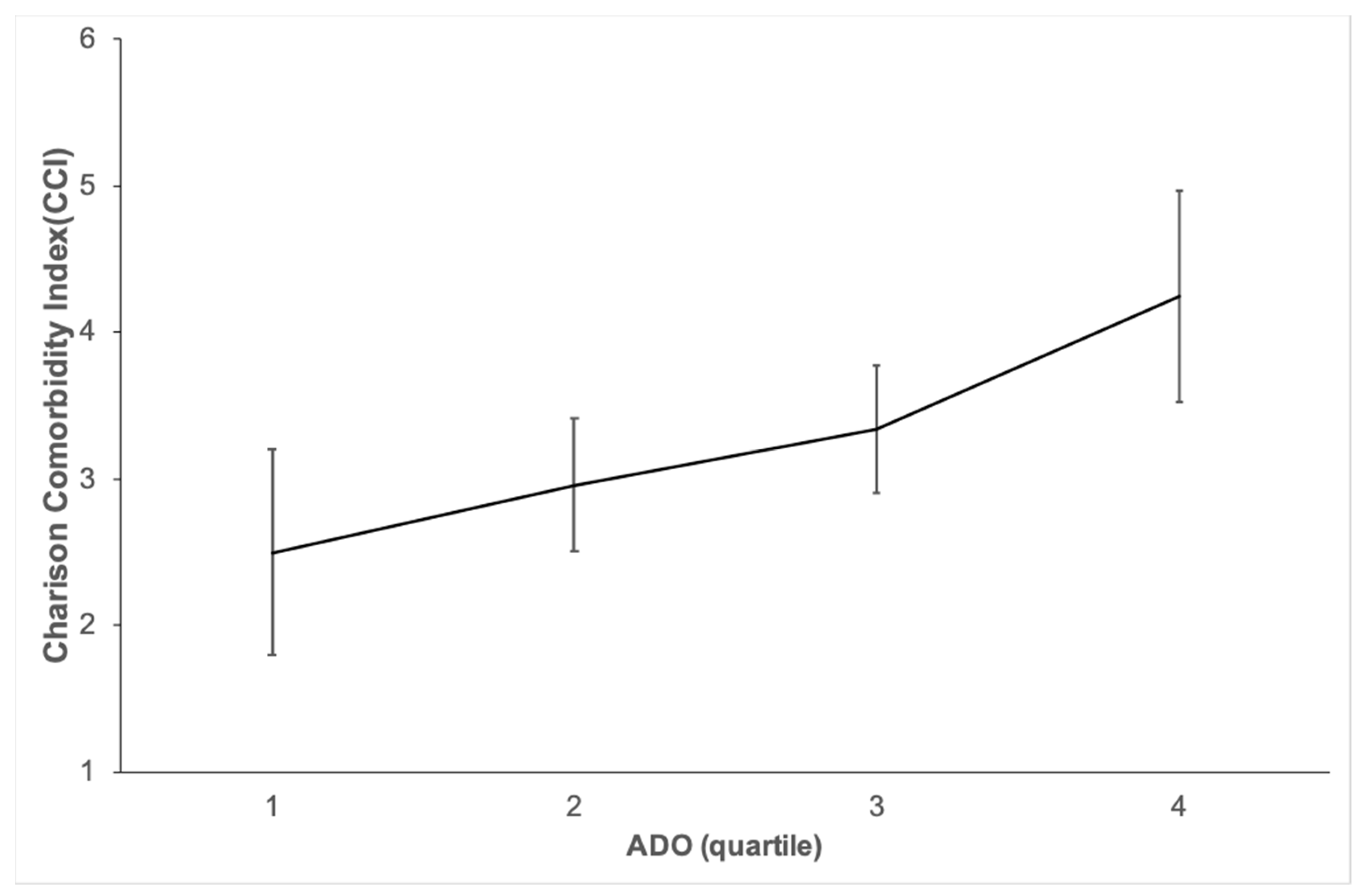
| CCI | 3.3 ± 2.8 |
| BODE INDEX | 3.0 ± 2.1 |
| ADO INDEX | 4.9 ± 1.8 |
| ADO quartile: Q1, Q2, Q3, Q4 | (%) * |
| quartile one | 40 (10.1) |
| quartile two | 124(31.3) |
| quartile three | 152 (38.4) |
| quartile four | 80 (20.2) |
| Classification | ADO Quartile | Frequency or Costs (Mean (95% CI)) | p-Value |
|---|---|---|---|
| number of outpatient visits | 1 | 14.68 (11.92–17.43) | 0.108 |
| 2 | 17.3 (15.26–19.33) | ||
| 3 | 22.68 (17.64–27.73) | ||
| 4 | 19.08 (16.55–21.6) | ||
| outpatient medical expenses | 1 | 40,843.48 (30,640.1–51,046.9) | 0.344 |
| 2 | 65,834.9 (44,039.02–87,630.877) | ||
| 3 | 69,271.15 (54,892.98–83,649.32) | ||
| 4 | 62,963.4 (54,702.35–71,224.45) | ||
| number of hospitalizations | 1 | 0.35 (0.11–0.59) | <0.001 |
| 2 | 0.56 (0.38–0.73) | ||
| 3 | 1.11 (0.82–1.4) | ||
| 4 | 1.69 (1.11–2.26) | ||
| Length of hospital stay | 1 | 2.75 (0.53–4.97) | <0.001 |
| 2 | 4.56 (2.74–6.39) | ||
| 3 | 12.16 (8.37–15.94) | ||
| 4 | 20.25 (12.6–27.9) | ||
| hospitalization expenses | 1 | 11,865.83 (−859.49–24,591.14) | 0.03 |
| 2 | 25,597.92 (9280.94–41,914.9) | ||
| 3 | 35,292.59 (23,548.79–47,036.39) | ||
| 4 | 41,407.2 (24,540–58,274.4) | ||
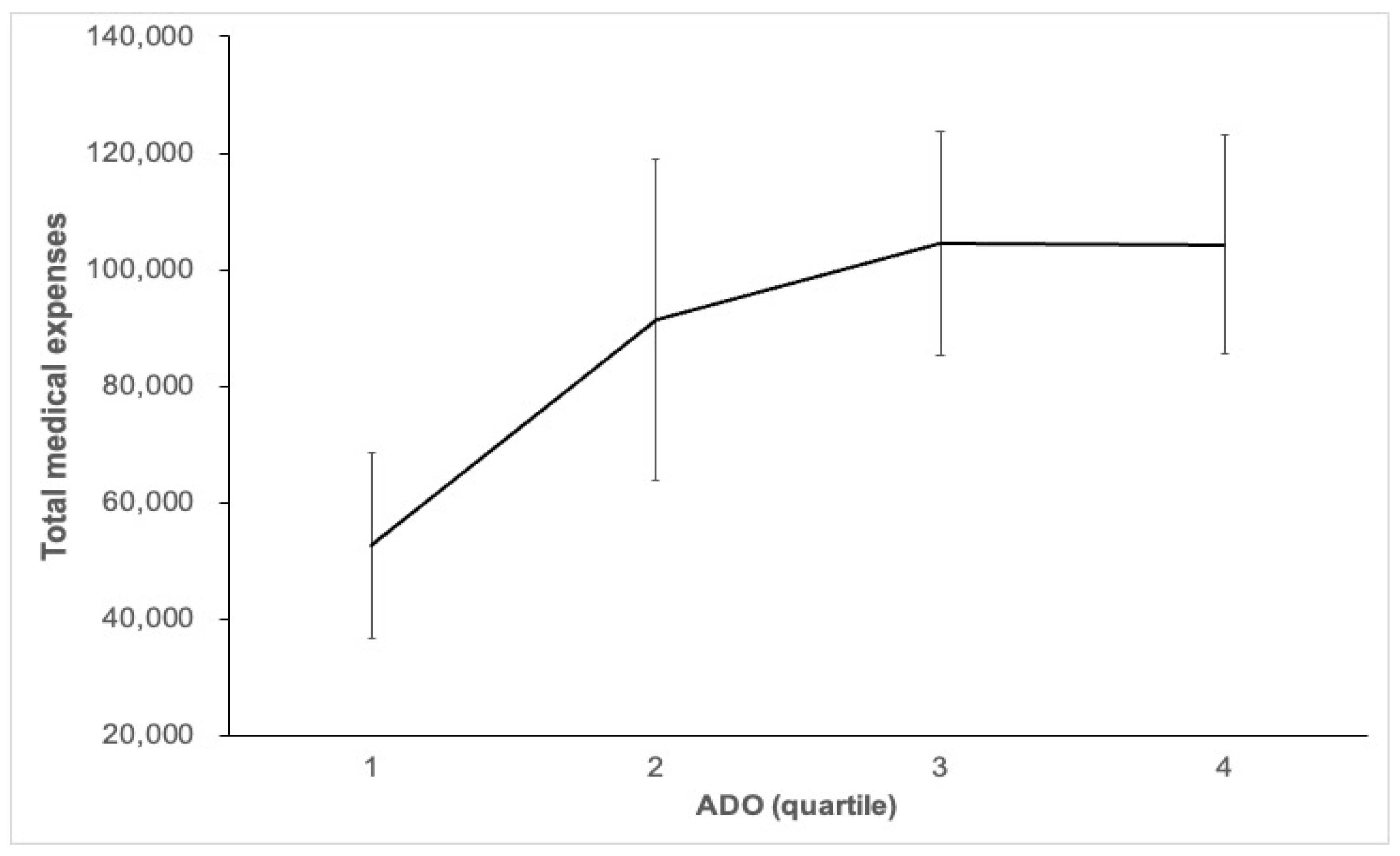
| total medical expenses | 1 | 52,709.3 (36,714.96–68,703.64) | 0.037 |
| 2 | 91,432.81(63,742.03–119,123.6) | ||
| 3 | 104,563.74(85,295.18–123,832.31) | ||
| 4 | 104,370.63 (85,685.52–123,055.73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-L.; Lin, M.-H.; Tsai, Y.-C.; Tseng, C.-W.; Chang, C.-L.; Shen, L.-S.; Kuo, H.-C.; Liu, S.-F. The Impact of the Age, Dyspnoea, and Airflow Obstruction (ADO) Index on the Medical Burden of Chronic Obstructive Pulmonary Disease (COPD). J. Clin. Med. 2022, 11, 1893. https://doi.org/10.3390/jcm11071893
Li C-L, Lin M-H, Tsai Y-C, Tseng C-W, Chang C-L, Shen L-S, Kuo H-C, Liu S-F. The Impact of the Age, Dyspnoea, and Airflow Obstruction (ADO) Index on the Medical Burden of Chronic Obstructive Pulmonary Disease (COPD). Journal of Clinical Medicine. 2022; 11(7):1893. https://doi.org/10.3390/jcm11071893
Chicago/Turabian StyleLi, Chin-Ling, Mei-Hsin Lin, Yuh-Chyn Tsai, Ching-Wan Tseng, Chia-Ling Chang, Lien-Shi Shen, Ho-Chang Kuo, and Shih-Feng Liu. 2022. "The Impact of the Age, Dyspnoea, and Airflow Obstruction (ADO) Index on the Medical Burden of Chronic Obstructive Pulmonary Disease (COPD)" Journal of Clinical Medicine 11, no. 7: 1893. https://doi.org/10.3390/jcm11071893
APA StyleLi, C.-L., Lin, M.-H., Tsai, Y.-C., Tseng, C.-W., Chang, C.-L., Shen, L.-S., Kuo, H.-C., & Liu, S.-F. (2022). The Impact of the Age, Dyspnoea, and Airflow Obstruction (ADO) Index on the Medical Burden of Chronic Obstructive Pulmonary Disease (COPD). Journal of Clinical Medicine, 11(7), 1893. https://doi.org/10.3390/jcm11071893







