The Effect of Hyaluronic Acid and Chondroitin Sulphate-Based Medical Device Combined with Acid Suppression in the Treatment of Atypical Symptoms in Gastroesophageal Reflux Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
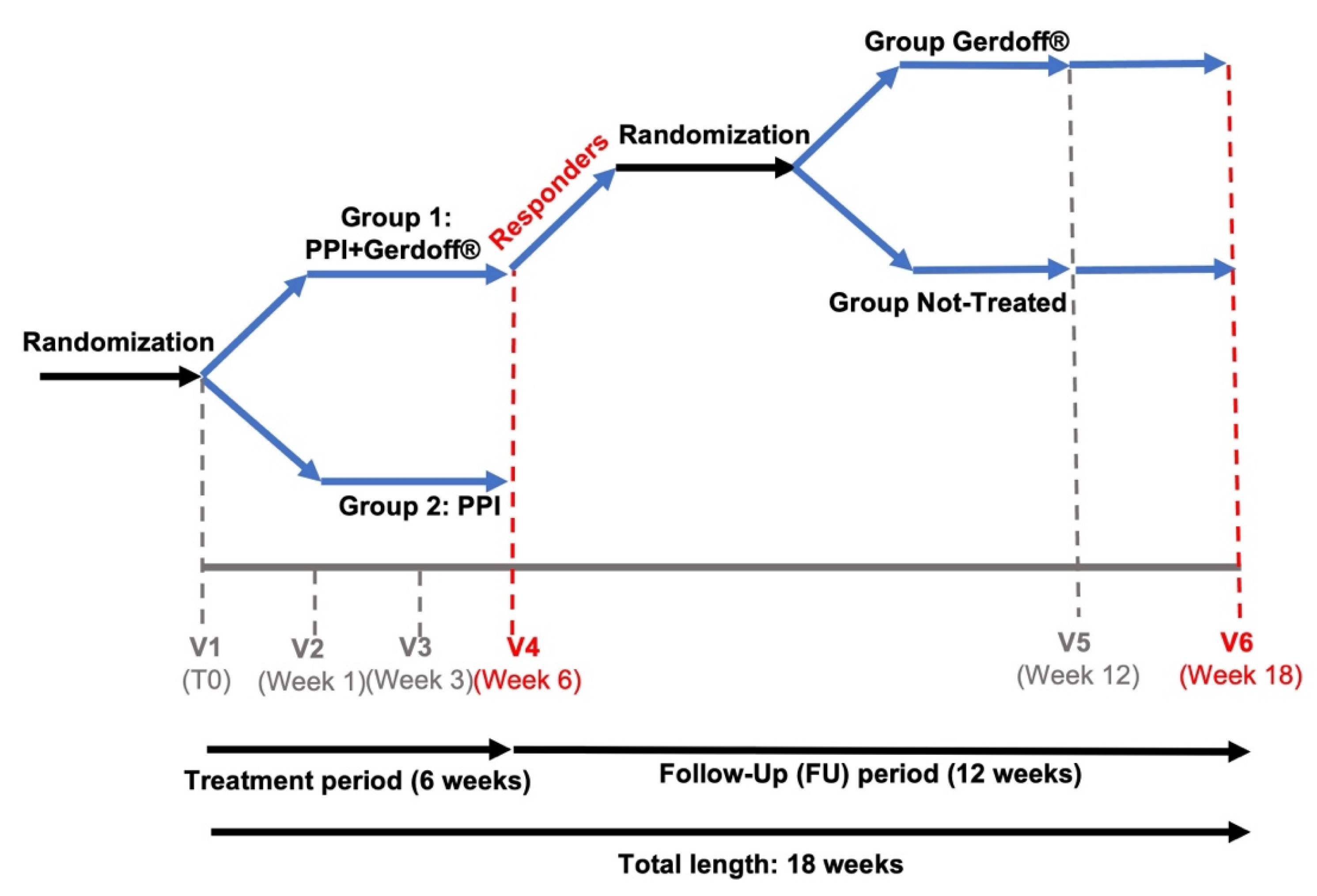
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Total RSI Score during Treatment Period
3.3. Change in Total RSI Score from Baseline to the Other Timepoints
3.4. Change in the Individual RSI Item Score from Baseline to Any Time Point
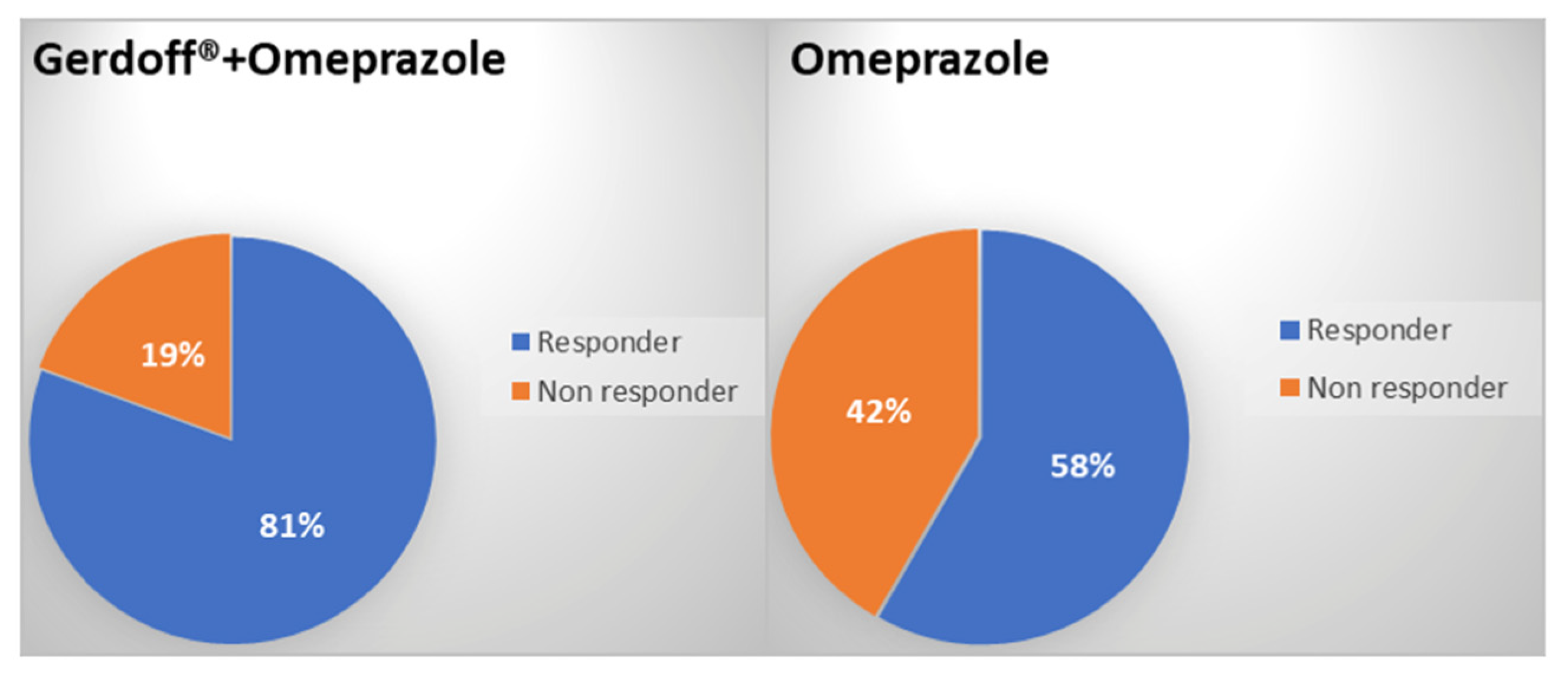
3.5. Number and Percentage of Responder/Non-Responder Patients at Week 6
3.6. Change in the Frequency of Extraesophageal Symptoms Assessed Using the Likert Scale from Baseline to End of Treatment (Week 6) and End of Follow-Up (Week 18)
3.7. Use of Rescue Medication during the Follow-Up Period: Administered Therapy, Frequency of Administration, Timing of Administration, and Administered Dose
3.8. Patient-Reported Satisfaction with Treatment
3.9. Adverse Events (AEs)
4. Discussion
4.1. Comparisons with Other Studies
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vakil, N.; Van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal Definition and Classification of Gastroesophageal Reflux Disease: A Global Evidence-Based Consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef]
- Wong, R.K.; Hanson, D.G.; Waring, P.J.; Shaw, G. ENT manifestations of gastroesophageal reflux. Am. J. Gastroenterol. 2000, 95, S15–S22. [Google Scholar] [CrossRef]
- Dore, M.P.; Pedroni, A.; Pes, G.M.; Maragkoudakis, E.; Tadeu, V.; Pirina, P.; Realdi, G.; Delitala, G.; Malaty, H.M. Effect of antisecretory therapy on atypical symptoms in gastroesophageal reflux disease. Dig. Dis. Sci. 2007, 52, 463–468. [Google Scholar] [CrossRef]
- Pacheco, A.; Cobeta, I.; Wagner, C. Refractory Chronic Cough: New Perspectives in Diagnosis and Treatment. Arch. Bronconeumol. 2013, 49, 151–157. [Google Scholar] [CrossRef]
- Altman, K.W.; Stephens, R.M.; Lyttle, C.S.; Weiss, K.B. Changing Impact of Gastroesophageal Reflux in Medical and Otolaryngology Practice. Laryngoscope 2005, 115, 1145–1153. [Google Scholar] [CrossRef]
- Fraser, A.G. Review article: Gastro-oesophageal reflux and laryngeal symptoms. Aliment. Pharmacol. Ther. 1994, 8, 265–272. [Google Scholar] [CrossRef]
- Yadlapati, R.; Pandolfino, J.E.; Lidder, A.K.; Shabeeb, N.; Jaiyeola, D.-M.; Adkins, C.; Agrawal, N.; Cooper, A.; Price, C.P.E.; Ciolino, J.D.; et al. Oropharyngeal pH Testing Does Not Predict Response to Proton Pump Inhibitor Therapy in Patients with Laryngeal Symptoms. Am. J. Gastroenterol. 2016, 111, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Zerbib, F.; Bredenoord, A.J.; Fass, R.; Kahrilas, P.J.; Roman, S.; Savarino, E.; Sifrim, D.; Vaezi, M.; Yadlapati, R.; Gyawali, C. ESNM/ANMS consensus paper: Diagnosis and management of refractory gastro-esophageal reflux disease. Neurogastroenterol. Motil. 2021, 33, e14075. [Google Scholar] [CrossRef]
- Yadlapati, R.; Gyawali, C.P.; Pandolfino, J.E.; Chang, K.; Kahrilas, P.J.; Katz, P.O.; Katzka, D.; Komaduri, S.; Lipham, J.; Menard-Katcher, P.; et al. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin. Gastroenterol. Hepatol. 2022. S1542-3565(22)00079-9. [Google Scholar] [CrossRef]
- Pellegatta, G.; Spadaccini, M.; Lamonaca, L.; Craviotto, V.; D’Amico, F.; Ceriotti, L.; Meloni, M.; Repici, A. Evaluation of Human Esophageal Epithelium Permeability in Presence of Different Formulations Containing Hyaluronic Acid and Chondroitin Sulphate. Med. Devices Évid. Res. 2020, 13, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Schindler, A.; Mozzanica, F.; Ginocchio, D.; Peri, A.; Bottero, A.; Ottaviani, F. Reliability and Clinical Validity of the Italian Reflux Symptom Index. J. Voice 2010, 24, 354–358. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guidelines for Good Clinical Practice. 1996. Available online: https://www.ich.org/page/efficacy-guidelines (accessed on 10 March 2022).
- World Medical Association (WMA). Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 10 March 2022).
- Wei, C. A meta-analysis for the role of proton pump inhibitor therapy in patients with laryngopharyngeal reflux. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- O’hara, J.; Stocken, D.D.; Watson, G.C.; Fouweather, T.; McGlashan, J.; MacKenzie, K.; Carding, P.; Karagama, Y.; Wood, R.; Wilson, J.A. Use of proton pump inhibitors to treat persistent throat symptoms: Multicentre, double blind, randomised, placebo controlled trial. BMJ 2021, 372, m4903. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Stocken, D.D.; Watson, G.C.; Fouweather, T.; McGlashan, J.; MacKenzie, K.; Carding, P.; Karagama, Y.; Harries, M.; Ball, S.; et al. Lansoprazole for persistent throat symptoms in secondary care: The TOPPITS RCT. Health Technol. Assess. 2021, 25, 1–118. [Google Scholar] [CrossRef]
- Qadeer, M.A.; Phillips, C.O.; Lopez, A.R.; Steward, D.L.; Noordzij, J.P.; Wo, J.M.; Suurna, M.; Havas, T.; Howden, C.W.; Vaezi, M.F. Proton Pump Inhibitor Therapy for Suspected GERD-Related Chronic Laryngitis: A Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2006, 101, 2646–2654. [Google Scholar] [CrossRef]
- Gatta, L.; Vaira, D.; Sorrenti, G.; Zucchini, S.; Sama, C.; Vakil, N. Meta-analysis: The efficacy of proton pump inhibitors for laryngeal symptoms attributed to gastro-oesophageal reflux disease. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews; Centre for Reviews and Dissemination: York, UK, 2007. [Google Scholar]
- Spantideas, N.; Drosou, E.; Bougea, A.; AlAbdulwahed, R. Proton Pump Inhibitors for the Treatment of Laryngopharyngeal Reflux. A Systematic Review. J. Voice 2020, 34, 918–929. [Google Scholar] [CrossRef]
- Chang, A.B.; Connor, F.L.; Petsky, H.L.; Eastburn, M.M.; Lewindon, P.J.; Hall, C.; Wilson, S.J.; Katelaris, P.H. An objective study of acid reflux and cough in children using an ambulatory pHmetry-cough logger. Arch. Dis. Child. 2011, 96, 468–472. [Google Scholar] [CrossRef]
- Hopkins, C.; Yousaf, U.; Pedersen, M. Acid reflux treatment for hoarseness. Cochrane Database Syst. Rev. 2006, CD005054. [Google Scholar] [CrossRef]
- Lechien, J.R.; Saussez, S.; Schindler, A.; Karkos, P.D.; Hamdan, A.L.; Harmegnies, B.; De Marrez, L.G.; Finck, C.; Journe, F.; Paesmans, M.; et al. Clinical outcomes of laryngopharyngeal reflux treatment: A systematic review and meta-analysis. Laryngoscope 2019, 129, 1174–1187. [Google Scholar] [CrossRef]
- Yadlapati, R.; Kaizer, A.M.; Sikavi, D.R.; Greytak, M.; Cai, J.X.; Carroll, T.L.; Gupta, S.; Wani, S.; Menard-Katcher, P.; Wu, T.-C.; et al. Distinct Clinical Physiologic Phenotypes of Patients with Laryngeal Symptoms Referred for Reflux Evaluation. Clin. Gastroenterol. Hepatol. 2022, 20, 776–786. [Google Scholar] [CrossRef]
- Sereg-Bahar, M.; Jerin, A.; Jansa, R.; Stabuc, B.; Hocevar-Boltezar, I. Pepsin and bile acids in saliva in patients with laryngopharyngeal reflux—A prospective comparative study. Clin. Otolaryngol. 2015, 40, 234–239. [Google Scholar] [CrossRef]
- Lechien, J.R.; Saussez, S.; Karkos, P.D. Laryngopharyngeal reflux disease: Clinical presentation, diagnosis and therapeutic challenges in 2018. In Current Opinion in Otolaryngology and Head and Neck Surgery; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2018; Volume 26, pp. 392–402. [Google Scholar] [CrossRef]
- Wilkie, M.D.; Fraser, H.M.; Raja, H. Gaviscon® Advance alone versus co-prescription of Gaviscon® Advance and proton pump inhibitors in the treatment of laryngopharyngeal reflux. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2515–2521. [Google Scholar] [CrossRef]
- Palmieri, B.; Corbascio, D.; Capone, S.; Lodi, D. Preliminary clinical experience with a new natural compound in the treatment of oesophagitis and gastritis: Symptomatic effect. Trends Med. 2009, 9, 219–225. [Google Scholar]
- Palmieri, B.; Merighi, A.; Corbascio, D.; Rottigni, V.; Fistetto, G.; Esposito, A. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3272–3278. [Google Scholar]
- Savarino, V.; Pace, F.; Scarpignato, C.; the Esoxx Study Group. Randomised clinical trial: Mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease—Efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment. Pharmacol. Ther. 2017, 45, 631–642. [Google Scholar] [CrossRef]
- Iannitti, T.; Morales-Medina, J.C.; Merighi, A.; Boarino, V.; Laurino, C.; Vadalà, M.; Palmieri, B. A hyaluronic acid- and chondroitin sulfate-based medical device improves gastritis pain, discomfort, and endoscopic features. Drug Deliv. Transl. Res. 2018, 8, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Boarino, V.; Raguzzi, I.; Marocchi, M.; Merighi, A. Symptomatic response to GERDOFF® in patients with gastro-esophageal reflux disease and poor response to alginates: An exploratory, post-market, open-label study. Turk. J. Gastroenterol. 2020, 31, 466–473. [Google Scholar] [CrossRef]
- Powell, J.; Cocks, H.C. Mucosal changes in laryngopharyngeal reflux-prevalence, sensitivity, specificity and assessment. Laryngoscope 2012, 123, 985–991. [Google Scholar] [CrossRef]
| Gerdoff® + Omeprazole N = 35 | Omeprazole N = 36 | Total N = 71 | |
|---|---|---|---|
| Age (years) | n = 35 | n = 36 | n = 71 |
| Mean (SD) | 49.2 (15.22) | 46.6 (14.36) | 47.9 (14.74) |
| Gender, N (%) | n = 35 | n = 36 | n = 71 |
| Male | 13 (37.1%) | 4 (11.1%) | 17 (23.9%) |
| Female | 22 (62.89) | 32 (88.9%) | 54 (76.1%) |
| Race, N (%) | n = 35 | n = 36 | n = 71 |
| Caucasian | 35 (100.0%) | 36 (100.0%) | 71 (100.0%) |
| Height (cm) | n = 29 | n = 31 | n = 60 |
| Mean (SD) | 167.5 (8.63) | 165.0 (7.77) | 166.2 (8.23) |
| Weight (kg) | n = 29 | n = 31 | n = 60 |
| Mean (SD) | 67.9 (15.65) | 65.0 (13.90) | 66.4 (14.72) |
| BMI (kg/m2) | n = 29 | n = 31 | n = 60 |
| Mean (SD) | 24.1 (4.80) | 23.9 (4.96) | 24.0 (4.84) |
| SBP (mmHg) | n = 31 | n = 31 | n = 62 |
| Mean (SD) | 119.6 (13.35) | 123.3 (15.43) | 121.5 (14.43) |
| DBP (mmHg) | n = 31 | n = 31 | n = 62 |
| Mean (SD) | 73.9 (8.68) | 75.5 (11.28) | 74.7 (10.06) |
| Heart rate (bpm) | n = 31 | n = 31 | n = 62 |
| Mean (SD) | 76.1 (13.81) | 77.6 (10.02) | 76.9 (11.99) |
| Respiratory rate (breaths/min) | n = 30 | n = 30 | n = 60 |
| Mean (SD) | 15.6 (1.92) | 15.2 (2.31) | 15.4 (2.12) |
| Treatment Group | Study Visit | n | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Gerdoff® + omeprazole N = 35 | Baseline/Screening (V1) | 35 | 24.2 | 5.18 | 22 | 20 | 41 |
| 1 week ± 1 day after baseline (V2) | 32 | 15.3 | 7.86 | 15.5 | 2 | 32 | |
| 3 weeks ± 2 days after baseline (V3) | 28 | 12.9 | 7.77 | 10.5 | 0 | 32 | |
| 6 weeks ± 2 days after baseline (V4) | 31 | 7.9 | 6.04 | 7 | 0 | 21 | |
| Omeprazole N = 36 | Baseline/Screening (V1) | 36 | 26 | 4.96 | 24 | 20 | 37 |
| 1 week ± 1 day after baseline (V2) | 35 | 19.6 | 7.2 | 19 | 3 | 37 | |
| 3 weeks ± 2 days after baseline (V3) | 35 | 15.3 | 8.08 | 16 | 2 | 32 | |
| 6 weeks ± 2 days after baseline (V4) | 36 | 12.3 | 8.98 | 10.5 | 0 | 39 |
| Treatment Group | n | Mean | SD | SE of Mean | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Total | 67 | −14.8 | 9.28 | 1.13 | −16.0 | −38 | 13 |
| Gerdoff® + omeprazole | 31 | −16.2 | 7.45 | 1.34 | −17.0 | −38 | 0 |
| Omeprazole | 36 | −13.7 | 10.58 | 1.76 | −13.5 | −34 | 13 |
| Difference Gerdoff® + omeprazole -omeprazole | −2.5 | 9.27 | 2.27 | 95% CI of the difference: −7.03 to 2.04 | |||
| Unpaired t-test: p = 0.2760 (p = 0.2679 with Cochran correction) | |||||||
| Homogeneity of variance: p = 0.0536 | |||||||
| Treatment | Visit | n | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Gerdoff® | After 6 weeks (V4) | 9 | 5.7 | 3 | 5 | 2 | 10 |
| No treatment | 8 | 5.5 | 4.07 | 6.5 | 0 | 10 | |
| Gerdoff® | After 18 weeks (V6) | 9 | 4.2 | 3.53 | 4 | 0 | 11 |
| No treatment | 8 | 5.9 | 8.01 | 3 | 0 | 24 |
| Variable | Treatment | n | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Change at 1 week ± 1 day (V2) | Total | 67 | −7.6 | 7.21 | −8.0 | −32 | 6 |
| Gerdoff® + omeprazole | 32 | −8.9 | 6.83 | −10.0 | −20 | 4 | |
| Omeprazole | 35 | −6.4 | 7.42 | −5.0 | −32 | 6 | |
| Change at 3 weeks ± 2 days (V3) | Total | 63 | −11.1 | 7.86 | −10.0 | −30 | 9 |
| Gerdoff® + omeprazole | 28 | −11.8 | 8 | −13.0 | −23 | 9 | |
| Omeprazole | 35 | −10.7 | 7.82 | −9.0 | −30 | 1 |
| Item | Treatment | Visit | n | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Hoarseness or voice problem | Gerdoff® + omeprazole | Visit 1 (T0) | 35 | 3.2 | 1.35 | 3 | 0 | 5 |
| Visit 2 (T1) | 32 | 1.8 | 1.63 | 1 | 0 | 5 | ||
| Visit 3 (T3) | 28 | 1.5 | 1.45 | 1 | 0 | 5 | ||
| Visit 4 (T6) | 31 | 1.1 | 1.33 | 1 | 0 | 4 | ||
| Omeprazole | Visit 1 (T0) | 36 | 2.8 | 1.54 | 3 | 0 | 5 | |
| Visit 2 (T1) | 35 | 2.4 | 1.63 | 2 | 0 | 5 | ||
| Visit 3 (T3) | 35 | 1.8 | 1.57 | 2 | 0 | 5 | ||
| Visit 4 (T6) | 36 | 1.6 | 1.54 | 1 | 0 | 5 | ||
| Clearing the throat | Gerdoff® + omeprazole | Visit 1 (T0) | 35 | 3.4 | 1.22 | 4 | 1 | 5 |
| Visit 2 (T1) | 32 | 2.5 | 1.5 | 2.5 | 0 | 5 | ||
| Visit 3 (T3) | 28 | 2 | 1.4 | 2 | 0 | 5 | ||
| Visit 4 (T6) | 31 | 1.4 | 1.39 | 1 | 0 | 5 | ||
| Omeprazole | Visit 1 (T0) | 36 | 3.6 | 1 | 4 | 1 | 5 | |
| Visit 2 (T1) | 35 | 3.1 | 1.14 | 3 | 1 | 5 | ||
| Visit 3 (T3) | 35 | 2.2 | 1.32 | 2 | 0 | 5 | ||
| Visit 4 (T6) | 36 | 1.8 | 1.37 | 1 | 0 | 5 | ||
| Excess throat mucus or post-nasal drip | Gerdoff® + omeprazole | Visit 1 (T0) | 35 | 2.7 | 1.76 | 3 | 0 | 5 |
| Visit 2 (T1) | 32 | 2.3 | 1.58 | 3 | 0 | 5 | ||
| Visit 3 (T3) | 28 | 2.1 | 1.53 | 2 | 0 | 5 | ||
| Visit 4 (T6) | 31 | 1.4 | 1.31 | 1 | 0 | 5 | ||
| Omeprazole | Visit 1 (T0) | 36 | 2.8 | 1.77 | 3 | 0 | 5 | |
| Visit 2 (T1) | 35 | 2.6 | 1.65 | 3 | 0 | 5 | ||
| Visit 3 (T3) | 35 | 2.3 | 1.64 | 2 | 0 | 5 | ||
| Visit 4 (T6) | 36 | 1.9 | 1.61 | 2 | 0 | 5 |
| Treatment Group | Study Visit | n | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Gerdoff® + omeprazole N = 35 | Baseline/Screening (V1) | 35 | 19 | 4.301 | 19 | 12 | 32 |
| 1 week ± 1 day after baseline (V2) | 32 | 13.9 | 5.975 | 15 | 3 | 29 | |
| 3 weeks ± 2 days after baseline (V3) | 28 | 11.1 | 5.993 | 13 | 1 | 29 | |
| 6 weeks ± 2 days after baseline (V4) | 31 | 7.4 | 5.667 | 8 | 0 | 31 | |
| 12 weeks ± 3 days after baseline (V5) | 17 | 7.4 | 5.744 | 7 | 0 | 17 | |
| 18 weeks ± 3 days after baseline (V6) | 17 | 4.2 | 5.238 | 2 | 0 | 17 | |
| Omeprazole N = 36 | Baseline/Screening (V1) | 36 | 21 | 4.557 | 18 | 12 | 32 |
| 1 week ± 1 day after baseline (V2) | 35 | 16.4 | 5.977 | 13.5 | 3 | 29 | |
| 3 weeks ± 2 days after baseline (V3) | 35 | 14.4 | 6.735 | 12 | 1 | 25 | |
| 6 weeks ± 2 days after baseline (V4) | 36 | 11.6 | 8.083 | 6 | 0 | 18 |
| Gerdoff® + Omeprazole N = 35 | Omeprazole N = 36 | |
|---|---|---|
| No. of AEs | 28 | 29 |
| No. (%) of patients with AEs | 14 (40.0%) | 12 (33.3%) |
| No. of SAEs | 0 | 1 |
| No. (%) of patients with SAEs | 0 (0.0%) | 1 (2.8%) |
| Intensity of AEs: No. (%) of AEs | ||
| Mild | 23 (82.1%) | 23 (79.3%) |
| Moderate | 5 (17.9%) | 6 (20.7%) |
| Action taken: No. (%) of AEs | ||
| None | 22 (78.6%) | 18 (50.0%) |
| Drug therapy | 6 (17.1%) | 8 (22.2%) |
| Non-drug therapy | 0 (0.0%) | 1 (2.8%) |
| Temporary interruption or dose adjustment | 0 (0.0%) | 1 (2.8%) |
| Hospitalization | 0 (0.0%) | 1 (2.8%) |
| Outcome: No. (%) of AEs | ||
| Resolved | 25 (89.3%) | 23 (79.3%) |
| Unresolved | 0 (0.0%) | 2 (5.6%) |
| Unknown | 3 (10.7%) | 4 (11.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegatta, G.; Mangiavillano, B.; Semeraro, R.; Auriemma, F.; Carlani, E.; Fugazza, A.; Vespa, E.; Repici, A. The Effect of Hyaluronic Acid and Chondroitin Sulphate-Based Medical Device Combined with Acid Suppression in the Treatment of Atypical Symptoms in Gastroesophageal Reflux Disease. J. Clin. Med. 2022, 11, 1890. https://doi.org/10.3390/jcm11071890
Pellegatta G, Mangiavillano B, Semeraro R, Auriemma F, Carlani E, Fugazza A, Vespa E, Repici A. The Effect of Hyaluronic Acid and Chondroitin Sulphate-Based Medical Device Combined with Acid Suppression in the Treatment of Atypical Symptoms in Gastroesophageal Reflux Disease. Journal of Clinical Medicine. 2022; 11(7):1890. https://doi.org/10.3390/jcm11071890
Chicago/Turabian StylePellegatta, Gaia, Benedetto Mangiavillano, Rossella Semeraro, Francesco Auriemma, Elisa Carlani, Alessandro Fugazza, Edoardo Vespa, and Alessandro Repici. 2022. "The Effect of Hyaluronic Acid and Chondroitin Sulphate-Based Medical Device Combined with Acid Suppression in the Treatment of Atypical Symptoms in Gastroesophageal Reflux Disease" Journal of Clinical Medicine 11, no. 7: 1890. https://doi.org/10.3390/jcm11071890
APA StylePellegatta, G., Mangiavillano, B., Semeraro, R., Auriemma, F., Carlani, E., Fugazza, A., Vespa, E., & Repici, A. (2022). The Effect of Hyaluronic Acid and Chondroitin Sulphate-Based Medical Device Combined with Acid Suppression in the Treatment of Atypical Symptoms in Gastroesophageal Reflux Disease. Journal of Clinical Medicine, 11(7), 1890. https://doi.org/10.3390/jcm11071890






