Treatment of Patients with Malignant Peritoneal Mesothelioma
Abstract
:1. Introduction
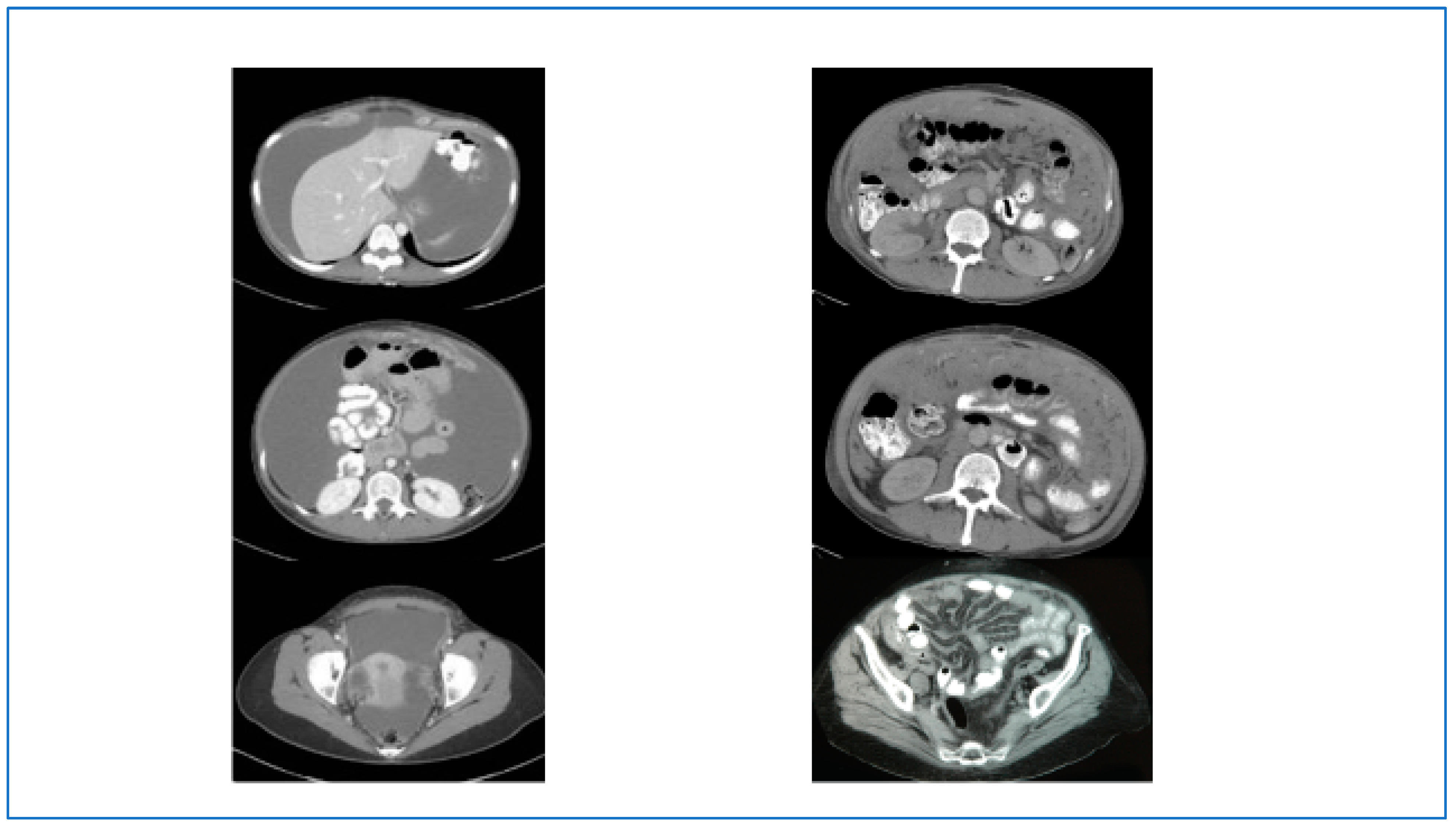
2. Initial Diagnosis and Evaluation
3. Surgical Treatment of MPM
4. Systemic Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R., Jr.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J. Malignant tumor arising from endothelium of peritoneum, and producing mucoid ascitic fluid. J. Path. Bact. 1908, 12, 267–278. [Google Scholar] [CrossRef]
- Winslow, D.J.; Taylor, H.B. Malignant peritoneal mesotheliomas.A clinicopathological analysis of 12 fatal cases. Cancer 1960, 13, 127–136. [Google Scholar] [CrossRef]
- Selikoff, I.J.; Churg, J.; Hammond, E.C. Asbestos Exposure and Neoplasia. JAMA J. Am. Med Assoc. 1964, 188, 22–26. [Google Scholar] [CrossRef]
- Moertel, C. Peritoneal mesothelioma. Gastroenterology 1972, 63, 346–350. [Google Scholar] [CrossRef]
- Antman, K.H.; Pomfret, E.A.; Aisner, J.; MacIntyre, J.; Osteen, R.T.; Greenberger, J.S. Peritoneal mesothelioma: Natural history and response to chemotherapy. J. Clin. Oncol. 1983, 1, 386–391. [Google Scholar] [CrossRef]
- Bijelic, L.; Darcy, K.; Do, J.S.; Tian, C.; Cannon, T. Predictors and Outcomes of Surgery in Peritoneal Mesothelioma: An Analysis of 2000 Patients from the National Cancer Database. Ann. Surg. Oncol. 2020, 27, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Miura, J.T.; Johnston, F.M.; Gamblin, T.C.; Turaga, K.K. Current Trends in the Management of Malignant Peritoneal Mesothelioma. Ann. Surg. Oncol. 2014, 21, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Selikoff, I.J.; Hammond, E.C.; Seidman, H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980, 46, 2736–2740. [Google Scholar] [CrossRef]
- Leblay, N.; Leprêtre, F.; Le Stang, N.; Gautier-Stein, A.; Villeneuve, L.; Isaac, S.; Maillet, D.; Galateau-Sallé, F.; Villenet, C.; Sebda, S.; et al. BAP1 Is Altered by Copy Number Loss, Mutation, and/or Loss of Protein Expression in More Than 70% of Malignant Peritoneal Mesotheliomas. J. Thorac. Oncol. 2017, 12, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2012, 43, 1022–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugarbaker, P.H.; Welch, L.S.; Mohamed, F.; Glehen, O. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg. Oncol. Clin. N. Am. 2003, 12, 605–621. [Google Scholar] [CrossRef]
- Alexander, H.R.; Hanna, N.; Pingpank, J.F. Clinical results of cytoreduction and HIPEC for malignant peritoneal mesothelioma. Cancer Treat Res. 2007, 134, 343–355. [Google Scholar] [CrossRef]
- Yan, T.D.; Haveric, N.; Carmignani, C.P.; Chang, D.; Sugarbaker, P.H. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer 2005, 103, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Cabras, A.D.; Bertulli, R.; Hutanu, I.; Deraco, M. Diffuse malignant peritoneal mesothelioma: Long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur. J. Cancer 2013, 49, 3140–3148. [Google Scholar] [CrossRef]
- Schaub, N.P.; Alimchandani, M.; Quezado, M.; Kalina, P.; Eberhardt, J.S.; Hughes, M.S.; Beresnev, T.; Hassan, R.; Bartlett, D.L.; Libutti, S.K.; et al. A Novel Nomogram for Peritoneal Mesothelioma Predicts Survival. Ann. Surg. Oncol. 2012, 20, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Alexander, R.; Antman, K.; Boffetta, P.; Churg, A.; Coit, D.; Hausner, P.; Kennedy, R.; Kindler, H.; Metintas, M.; et al. Current treatment options and biology of peritoneal mesothelioma: Meeting summary of the first NIH peritoneal mesothelioma conference. Ann. Oncol. 2006, 17, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma: 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, T.D.; Deraco, M.; Baratti, D.; Kusamura, S.; Elias, D.; Glehen, O.; Gilly, F.N.; Levine, E.A.; Shen, P.; Mohamed, F.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: Multi-Institutional Experience. J. Clin. Oncol. 2009, 27, 6237–6242. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.R.; Bartlett, D.L.; Pingpank, J.F.; Libutti, S.K.; Royal, R.; Hughes, M.S.; Holtzman, M.; Hanna, N.; Turner, K.; Beresneva, T.; et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013, 153, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helm, J.H.; Miura, J.T.; Glenn, J.A.; Marcus, R.K.; Larrieux, G.; Jayakrishnan, T.T.; Donahue, A.E.; Gamblin, T.C.; Turaga, K.K.; Johnston, F.M. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2014, 22, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Kepenekian, V.; Elias, D.; Passot, G.; Mery, E.; Goere, D.; Delroeux, D.; Quenet, F.; Ferron, G.; Pezet, D.; Guilloit, J.M.; et al. Diffuse malignant peritoneal mesothelioma: Evaluation of systemic chemotherapy with comprehensive treatment through the RENAPE Database: Multi-Institutional Retrospective Study. Eur. J. Cancer 2016, 65, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Khashab, T.; Terhune, J.; Eckert, R.L.; Hanna, N.; Burke, A.; Alexander, H.R. Preoperative Thrombocytosis Predicts Shortened Survival in Patients with Malignant Peritoneal Mesothelioma Undergoing Operative Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2017, 24, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Naffouje, S.A.; Tulla, K.; Salti, G.I. The impact of chemotherapy and its timing on survival in malignant peritoneal mesothelioma treated with complete debulking. Med. Oncol. 2018, 35, 69. [Google Scholar] [CrossRef] [PubMed]
- Gusani, N.J.; Cho, S.W.; Colovos, C.; Seo, S.; Franko, J.; Richard, S.D.; Edwards, R.P.; Brown, C.K.; Holtzman, M.P.; Zeh, H.J.; et al. Aggressive Surgical Management of Peritoneal Carcinomatosis With Low Mortality in a High-Volume Tertiary Cancer Center. Ann. Surg. Oncol. 2007, 15, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Staats, P.; Lee, M.; Alexander, H.R.; Burke, A.P. Diffuse mesothelioma of the peritoneum: Correlation between histological and clinical parameters and survival in 73 patients. Pathology 2014, 46, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Cabras, A.D.; Deraco, M. Cytoreductive Surgery with Selective Versus Complete Parietal Peritonectomy Followed by Hyperthermic Intraperitoneal Chemotherapy in Patients with Diffuse Malignant Peritoneal Mesothelioma: A Controlled Study. Ann. Surg. Oncol. 2012, 19, 1416–1424. [Google Scholar] [CrossRef]
- Roife, D.; Powers, B.D.; Zaidi, M.Y.; Staley, C.A.; Cloyd, J.M.; Ahmed, A.; Grotz, T.; Leiting, J.; Fournier, K.; Lee, A.J.; et al. CRS/HIPEC with Major Organ Resection in Peritoneal Mesothelioma Does not Impact Major Complications or Overall Survival: A Retrospective Cohort Study of the US HIPEC Collaborative. Ann. Surg. Oncol. 2020, 27, 4996–5004. [Google Scholar] [CrossRef]
- Bekhor, E.; Carr, J.; Bs, M.H.; Sullivan, B.; Solomon, D.; Leigh, N.; Bolton, N.; Golas, B.; Sarpel, U.; Labow, D.; et al. The Safety of Iterative Cytoreductive Surgery and HIPEC for Peritoneal Carcinomatosis: A High Volume Center Prospectively Maintained Database Analysis. Ann. Surg. Oncol. 2019, 27, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Ihemelandu, C.; Bijelic, L.; Sugarbaker, P.H. Iterative Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Recurrent or Progressive Diffuse Malignant Peritoneal Mesothelioma: Clinicopathologic Characteristics and Survival Outcome. Ann. Surg. Oncol. 2014, 22, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Welten, V.M.; Fields, A.C.; Malizia, R.A.; Yoo, J.; Irani, J.L.; Goldberg, J.E.; Bleday, R.; Melnitchouk, N. Survival Outcomes for Malignant Peritoneal Mesothelioma at Academic Versus Community Hospitals. J. Gastrointest. Surg. 2021, 26, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Blackham, A.U.; Shen, P.; Stewart, J.H.; Russell, G.B.; Levine, E.A. Cytoreductive Surgery with Intraperitoneal Hyperthermic Chemotherapy for Malignant Peritoneal Mesothelioma: Mitomycin Versus Cisplatin. Ann. Surg. Oncol. 2010, 17, 2720–2727. [Google Scholar] [CrossRef] [PubMed]
- Malgras, B.; Network, O.B.O.T.R.; Gayat, E.; Aoun, O.; Dico, R.L.; Eveno, C.; Pautrat, K.; Delhorme, J.-B.; Passot, G.; Marchal, F.; et al. Impact of Combination Chemotherapy in Peritoneal Mesothelioma Hyperthermic Intraperitoneal Chemotherapy (HIPEC): The RENAPE Study. Ann. Surg. Oncol. 2018, 25, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Kepenekian, V.; Péron, J.; You, B.; Bonnefoy, I.; Villeneuve, L.; Alyami, M.; Bakrin, N.; Rousset, P.; Benzerdjeb, N.; Glehen, O. ASO Visual Abstract: Non-resectable Malignant Peritoneal Mesothelioma Treated with Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Plus Systemic Chemotherapy Could Lead to Secondary Complete Cytoreductive Surgery: A Cohort Study. Ann. Surg. Oncol. 2022, 29, 2116–2117. [Google Scholar] [CrossRef]
- Jänne, P.A.; Wozniak, A.J.; Belani, C.P.; Keohan, M.-L.; Ross, H.J.; Polikoff, J.A.; Mintzer, D.M.; Taylor, L.; Ashland, J.; Ye, Z.; et al. Open-Label Study of Pemetrexed Alone or in Combination with Cisplatin for the Treatment of Patients with Peritoneal Mesothelioma: Outcomes of an Expanded Access Program. Clin. Lung Cancer 2005, 7, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.R.; Verschraegen, C.F.; Jänne, P.A.; Langer, C.J.; Dowlati, A.; Gadgeel, S.M.; Kelly, K.; Kalemkerian, G.P.; Traynor, A.M.; Peng, G.; et al. Pemetrexed Plus Gemcitabine As First-Line Chemotherapy for Patients With Peritoneal Mesothelioma: Final Report of a Phase II Trial. J. Clin. Oncol. 2008, 26, 3567–3572. [Google Scholar] [CrossRef]
- Popat, S.; Baas, P.; Faivre-Finn, C.; Girard, N.; Nicholson, A.; Nowak, A.; Opitz, I.; Scherpereel, A.; Reck, M. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 33, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Raghav, K.; Liu, S.; Overman, M.J.; Willett, A.F.; Knafl, M.; Fu, S.-C.; Malpica, A.; Prasad, S.; Royal, R.E.; Scally, C.P.; et al. Efficacy, Safety, and Biomarker Analysis of Combined PD-L1 (Atezolizumab) and VEGF (Bevacizumab) Blockade in Advanced Malignant Peritoneal Mesothelioma. Cancer Discov. 2021, 11, 2738–2747. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Nemunaitis, J.J.; Patel, M.R.; Bennouna, J.; Chen, F.L.; Delord, J.P.; Dowlati, A.; Kochuparambil, S.T.; Taylor, M.H.; et al. Efficacy and Safety of Avelumab Treatment in Patients With Advanced Unresectable Mesothelioma: Phase 1b Results From the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef]
- White, M.G.; Schulte, J.J.; Xue, L.; Berger, Y.; Schuitevoerder, D.; Vining, C.C.; Kindler, H.L.; Husain, A.; Turaga, K.K.; Eng, O.S. Heterogeneity in PD-L1 expression in malignant peritoneal mesothelioma with systemic or intraperitoneal chemotherapy. Br. J. Cancer 2020, 124, 564–566. [Google Scholar] [CrossRef] [PubMed]
- de Boer, N.L.; van Kooten, J.P.; Burger, J.W.; Verhoef, C.; Aerts, J.G.; Madsen, E.V. Adjuvant dendritic cell based immunotherapy (DCBI) after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal mesothelioma, a phase II single centre open-label clinical trial: Rationale and design of the MESOPEC trial. BMJ Open 2019, 9, e026779. [Google Scholar] [PubMed]
- Adusumilli, P.S.; Zauderer, M.G.; Rivière, I.; Solomon, S.B.; Rusch, V.W.; O’Cearbhaill, R.E.; Zhu, A.; Cheema, W.; Chintala, N.K.; Halton, E.; et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti–PD-1 Agent Pembrolizumab. Cancer Discov. 2021, 11, 2748–2763. [Google Scholar] [CrossRef] [PubMed]
| Study | N | Median OS (Months) | 5-y OS | Favorable Prognostic Factors |
|---|---|---|---|---|
| Yan 2009 [19] Multi-center international | 405 | 53 | 47% | Epithelioid histology Negative LNs, Optimal CCR Use of HIPEC |
| Schaub 2012 [16] Single institution | 104 | N/A | 46% | Low PCI Histologic grade Low pre-op CA-125 |
| Baratti 2013 [15] Single institution | 108 | 63 | N/A | Low Mitotic count (Ki-67) Epithelioid histology, Optimal CCR |
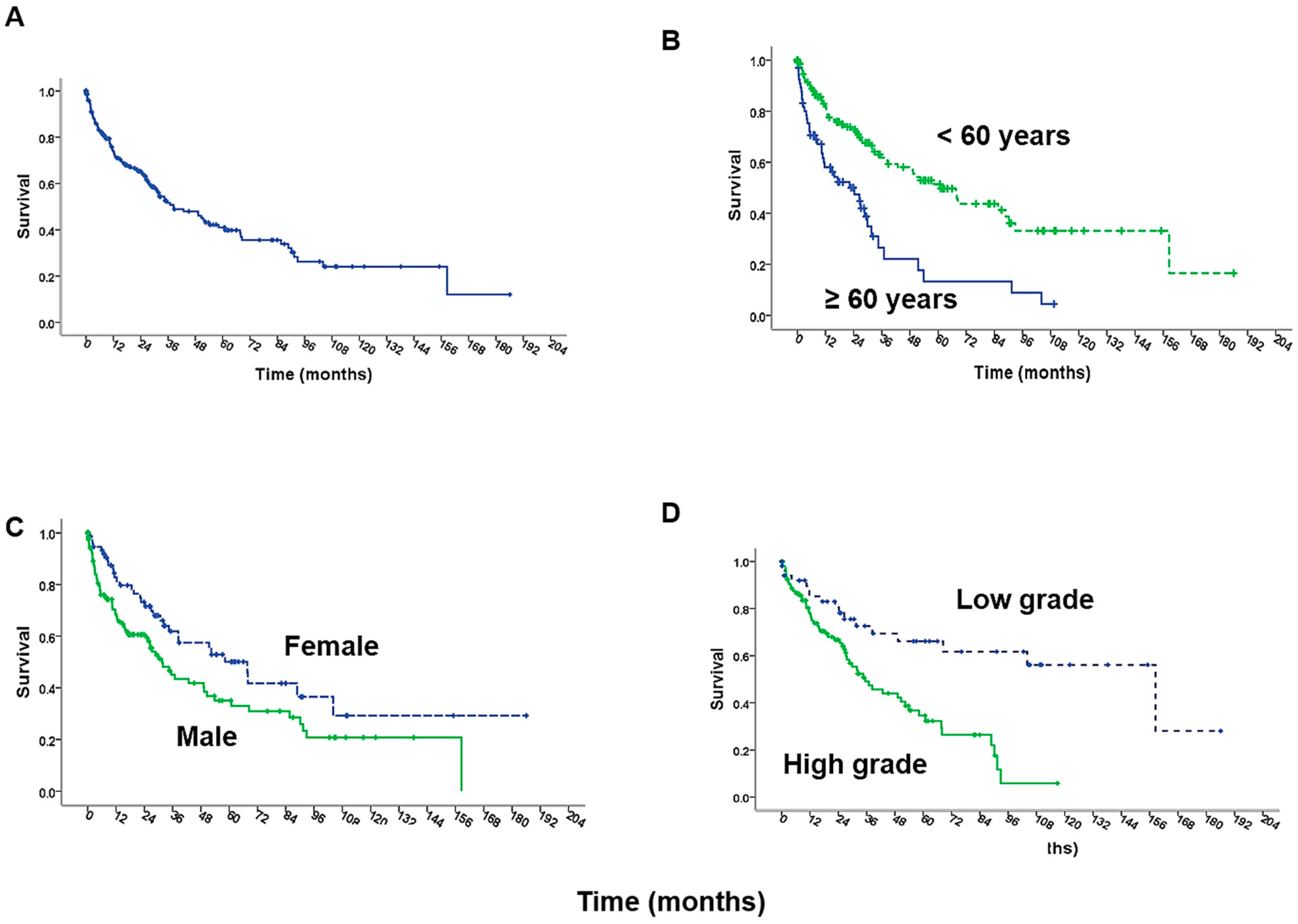
| Alexander 2013 [20] Multi-center U.S. | 211 | 38 | 41% | Histologic grade, Optimal CCR Age < 60 years Use of Cisplatin Female gender |
| Helm 2014 [21] Miura 2014 [8] SEER database | 1047 1591 | N/A 38 | 42% N/A | Use of Surgery Use of Cisplatin Use of EPIC |
| Kepenekian 2016 [22] RENAPE | 126 | 61 | 53% | PCI < 30, ASA Score ≤ 2 CCR 0/1 No change OS with neoadjuvant chemo |
| Li 2017 [23] Single institution | 100 | 33 | 36% | Thrombocytosis (−) Optimal CCR, PCI ≤ 20 |
| Naffouje 2018 [24] NCDB | 1740 | 52–57 | N/A | No change in OS with surgery alone v neoadjuvant v adjuvant chemotherapy. OS: Surgery > chemotherapy > BSC |
| Bijelic 2020 [7] NCDB | 1756 | 38 | N/A | Increasing age, female sex, no comorbidity, epithelioid histology, surgery, chemotherapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.Y.; Kennedy, T.; Alexander, H.R. Treatment of Patients with Malignant Peritoneal Mesothelioma. J. Clin. Med. 2022, 11, 1891. https://doi.org/10.3390/jcm11071891
Li CY, Kennedy T, Alexander HR. Treatment of Patients with Malignant Peritoneal Mesothelioma. Journal of Clinical Medicine. 2022; 11(7):1891. https://doi.org/10.3390/jcm11071891
Chicago/Turabian StyleLi, Claire Y., Timothy Kennedy, and Henry Richard Alexander. 2022. "Treatment of Patients with Malignant Peritoneal Mesothelioma" Journal of Clinical Medicine 11, no. 7: 1891. https://doi.org/10.3390/jcm11071891
APA StyleLi, C. Y., Kennedy, T., & Alexander, H. R. (2022). Treatment of Patients with Malignant Peritoneal Mesothelioma. Journal of Clinical Medicine, 11(7), 1891. https://doi.org/10.3390/jcm11071891






