Malignant Transformation Rate of Oral Submucous Fibrosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Keywords Selection
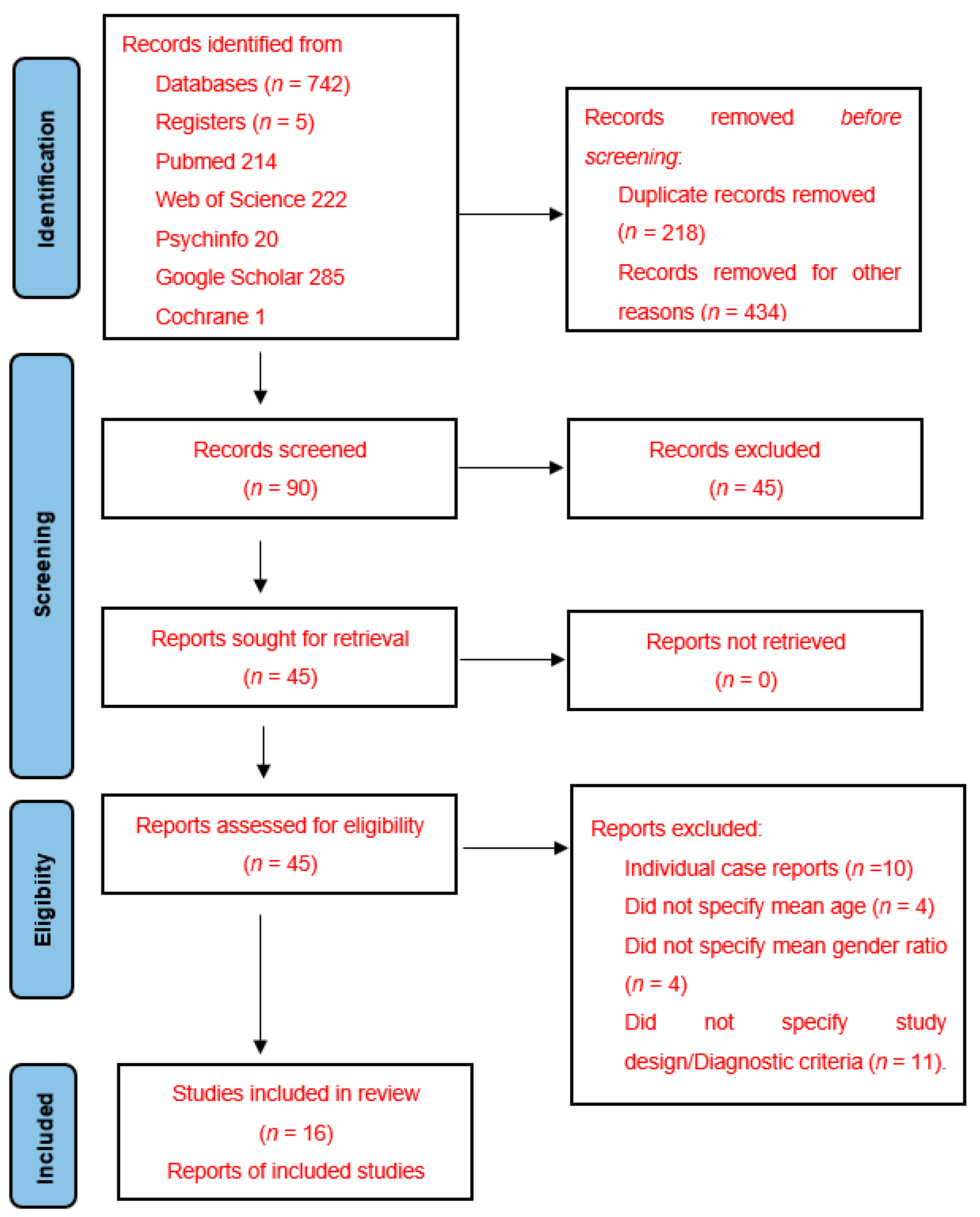
2.3. Studies Selection and Data Extraction Process
2.4. Risk of Bias Assessment
2.5. Summary Measures and Methods of Analysis
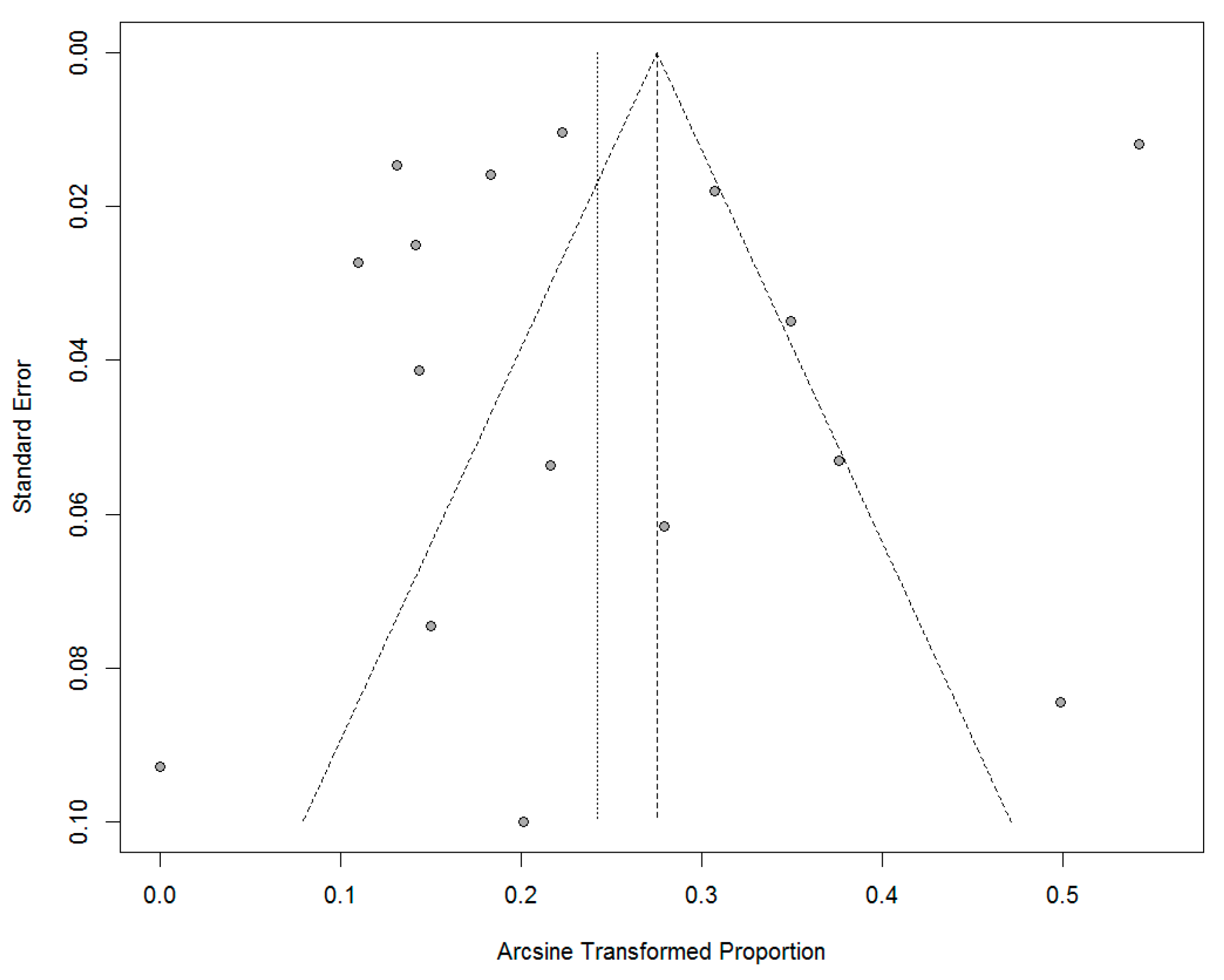
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paymaster, J.C. Cancer of the buccal mucosa; a clinical study of 650 cases in Indian patients. Cancer 1956, 9, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Mhaske, S.; Ragavendra, R.I. Oral Submucous Fibrosis—Current Concepts in Etiopathogenesis. People’s J. Sci. Res. 2008, 1, 39–44. [Google Scholar]
- Tilakaratne, W.M.; Klinikowski, M.F.; Takashi, S.; Peters, T.J.; Warnakulasuriya, S. Oral submucous fibrosis: Review on etiology and pathogenesis. Oral Oncol. 2006, 42, 561–568. [Google Scholar] [CrossRef]
- Auluck, A.; Rosin, M.P.; Zhang, L.; Sumanth, K.N. Oral submucous fibrosis, a clinically benign but potentially malignant disease: Report of 3 cases and review of the literature. J. Can. Dent. Assoc. 2008, 74, 735–740. [Google Scholar] [PubMed]
- Angadi, P.V.; Rao, S.S. Management of Oral Submucous Fibrosis: An overview. Oral Maxillofac. Surg. 2010, 14, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Arakeri, G.; Hunasgi, S.; Colbert, S.; Merkx, M.A.; Brennan, P.A. Role of drinking water copper in pathogenesis of oral submucous fibrosis: A prospective case control study. Br. J. Oral Maxillofac. Surg. 2014, 52, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Pindborg, J.J.; Chawla, T.N.; Srivastava, A.N.; Gupta, D.; Mehrotra, M.L. Clinical aspects of oral submucous fibrosis. Acta Odontol. Scand. 1964, 22, 679–691. [Google Scholar] [PubMed]
- Pindborg, J.J.; Murti, P.R.; Bhonsle, R.B.; Gupta, P.C.; Daftary, D.K.; Mehta, F.S. Oral submucous fibrosis as a precancerous condition. Scand. J. Dent. Res. 1984, 92, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Murti, P.R.; Bhonsle, R.B.; Pindborg, J.J.; Daftary, D.K.; Gupta, P.C.; Mehta, F.S. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent. Oral Epidemiol. 1985, 13, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; Mello, F.W.; Warnakulasuriya, S. Malignant transformation of oral submucous fibrosis: A systematic review and meta-analysis. Oral Dis. 2021, 27, 1936–1946. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341, Correction in Int. J. Surg. 2010, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.A.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 21 October 2021).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–635. [Google Scholar] [CrossRef]
- Shiau, Y.Y.; Kwan, H.W. Submucous Fibrosis in Taiwan. Oral Surg. Oral Med. Oral Pathol. 1979, 47, 453–457. [Google Scholar] [CrossRef]
- Liu, S.F.; Jian, X.C.; Shen, Z.H. A study of oral submucous fibrosis. J. Clin. Stomatol. 1988, 4, 81–83. [Google Scholar]
- Jian, X.C.; Liu, S.F.; Shen, Z.H.; Cheng, H.B. A clinical study of oral submucous fibrosis. Clin. J. Stomatol. 1989, 24, 299–302. [Google Scholar]
- Tang, J.G.; Jian, X.F.; Gao, M.L.; Ling, T.Y.; Zhang, K.H. Epidemiological survey of oral submucous fibrosis in Xiangtan City, Hunan Province, China. Community Dent. Oral Epidemiol. 1997, 25, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.C.; Peng, J.Y.; Tang, Z.G.; Shen, Q.; Su, T. Three cases of oral cancer associated with oral submucous fibrosis. West China J. Stomatol. 2000, 18, 130–131. [Google Scholar]
- Gao, Y.J.; Ling, T.Y.; Yin, X.M.; Yao, Z.G.; Tang, J.Q. A retrospective study of malignant transformation of oral submucous fibrosis. J. Clin. Stomatol. 2005, 21, 119–120. [Google Scholar]
- Hazarey, V.K.; Erlewad, D.M.; Mundhe, K.A.; Ughade, S.N. Oral Submucous Fibrosis: Study of 1000 cases from central India. J. Oral Pathol. Med. 2007, 36, 12–17. [Google Scholar] [CrossRef]
- Hsue, S.S.; Wang, W.C.; Chen, C.H.; Lin, C.C.; Chen, Y.K.; Lin, L.M. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: A follow-up study based in a Taiwanese hospital. J. Oral Pathol. Med. 2007, 36, 252–259. [Google Scholar] [CrossRef]
- Angadi, P.V.; Rao, S.S. Areca nut in pathogenesis of oral submucous fibrosis: Revisited. Oral Maxillofac. Surg. 2011, 15, 1–9. [Google Scholar] [PubMed]
- Mohiuddin, S.; Fatima, N.; Hosein, S.; Fatima, N. High risk of malignant transformation of oral submucous fibrosis in Pakistani females: A potential national disaster. J. Pak. Med. Assoc. 2016, 66, 1362–1366. [Google Scholar] [PubMed]
- Yang, P.Y.; Chen, Y.T.; Wang, Y.H.; Su, N.Y.; Yu, H.C.; Chang, Y.C. Malignant transformation of oral submucous fibrosis in Taiwan: A nationwide population-based retrospective cohort study. J. Oral Pathol. Med. 2017, 46, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.L.; Wang, C.P.; Chen, M.K.; Su, W.W.; Su, C.W.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; Yen, A.M. Malignant transformation to oral cancer by subtype of oral potentially malignant disorder: A prospective cohort study of Taiwanese nationwide oral cancer screening program. Oral Oncol. 2018, 87, 58–63. [Google Scholar] [CrossRef]
- Chiang, W.F.; Liu, S.Y.; Lin, J.F.; Chiu, S.F.; Gou, S.B.; Chiou, C.T.; Chang, C.H. Malignant development in patients with oral potentially malignant disorders detected through nationwide screening: Outcomes of 5-year follow-up at a single hospital. Head Neck 2020, 42, 67–76. [Google Scholar] [CrossRef]
- Angadi, P.V.; Rekha, K.P. Oral Submucous Fibrosis: A clinicopathologic review of 205 cases in Indians. Oral Maxillofac. Surg. 2011, 15, 15–19. [Google Scholar] [CrossRef]
- Yang, Y.H.; Lien, Y.C.; Ho, P.S.; Chen, C.H.; Chang, J.S.; Cheng, T.C.; Shieh, T.I. The effects of chewing areca/betel quid with and without cigarette smoking on oral submucous fibrosis and oral mucosal lesions. Oral Dis. 2005, 11, 88–94. [Google Scholar] [CrossRef]
- Reddy, V.; Wanjari, P.V.; Banda, R.N.; Reddy, P. Oral Submucous Fibrosis: Correlation of Clinical Grading to various habit factors. Int. J. Dent. Clin. 2011, 3, 21–34. [Google Scholar]
- Zhang, X.; Reichart, P.A. A review of betel quid chewing, oral cancer and precancer in Mainland China. Oral Oncol. 2007, 43, 424–430. [Google Scholar] [CrossRef]
- Liu, S.F.; Shen, Z.H.; Tang, Z.G.; Su, H.B.; Luo, C.F. On treatment of oral submucous fibrosis with glucosidorum tripterygii totorum. Beijing J. Stomatol. 1999, 7, 167–169. [Google Scholar]
- Wu, M.S.; Tang, L.D. Interference of betel quid chewing with the therapy of duodenal ulcer. China J. Mod. Med. 1998, 8, 45–47. [Google Scholar]
- Lei, R.C.; Jian, X.C. The habit of chewing betel and oral submucous fibrosis. Chin. J. Clin. Rehabil. 2005, 9, 24–95. [Google Scholar]
- Shah, N.; Sharma, P.P. Role of chewing and smoking habits in the etiology of oral submucous fibrosis—A case control study. J. Oral Pathol. Med. 1988, 8, 475–479. [Google Scholar] [CrossRef]
- Zhang, S.S.; Li, W.H.; Gao, Y.J.; Liu, Z.W.; Liu, L.; Tang, J.Q.; Ling, T.Y. Betel-quid and oral submucous fibrosis: A cross-sectional study in Hunan province, China. J. Oral Pathol. Med. 2012, 41, 748–754. [Google Scholar] [CrossRef]
- Gupta, V.K.; Malhotra, S.; Patil, R.; Tripathi, A. Oral submucous fibrosis at pediatric age, now time to think: Series of two cases. Indian J. Med. Paediatr. Oncol. 2013, 34, 107–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef] [PubMed]
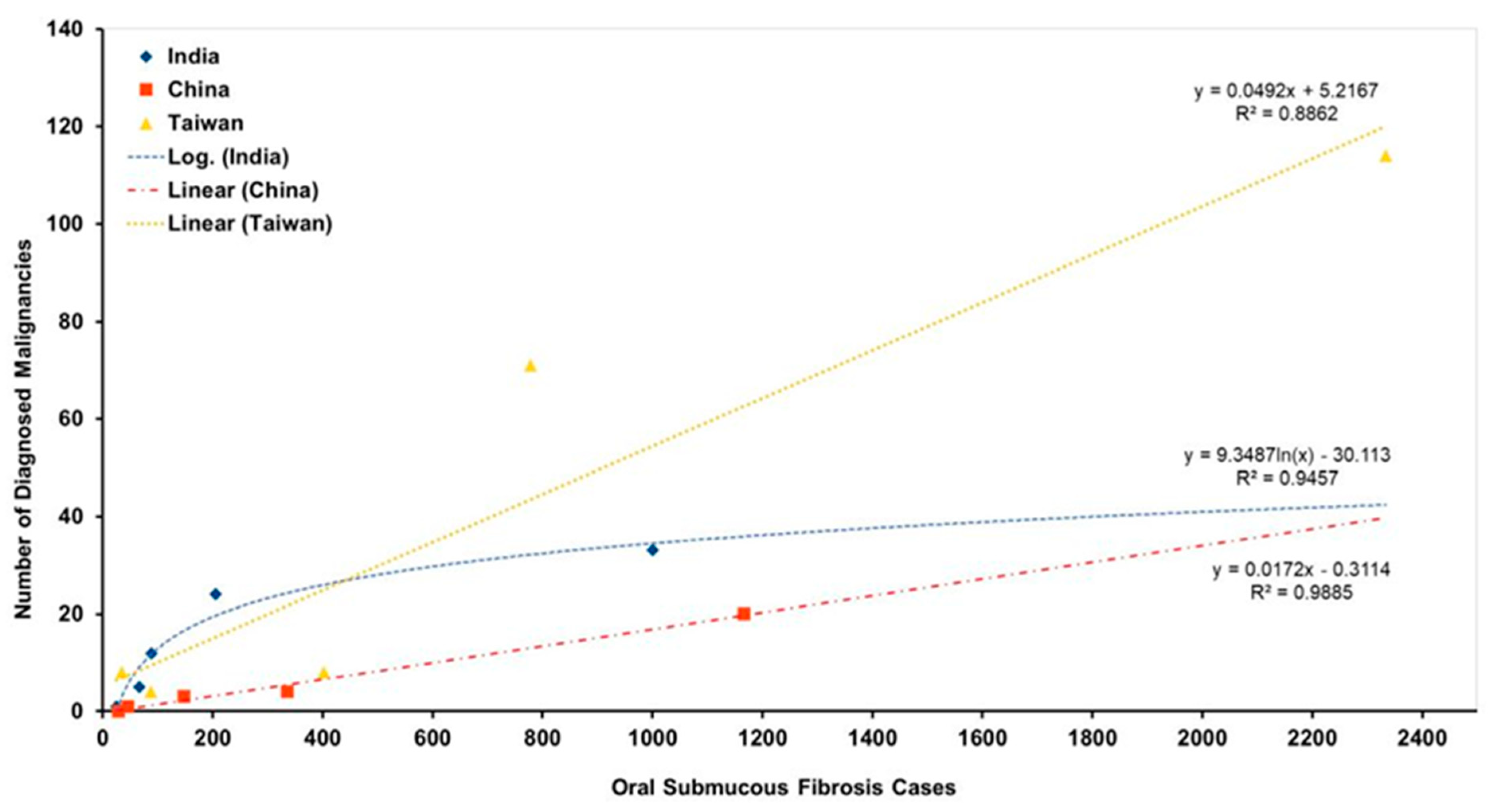
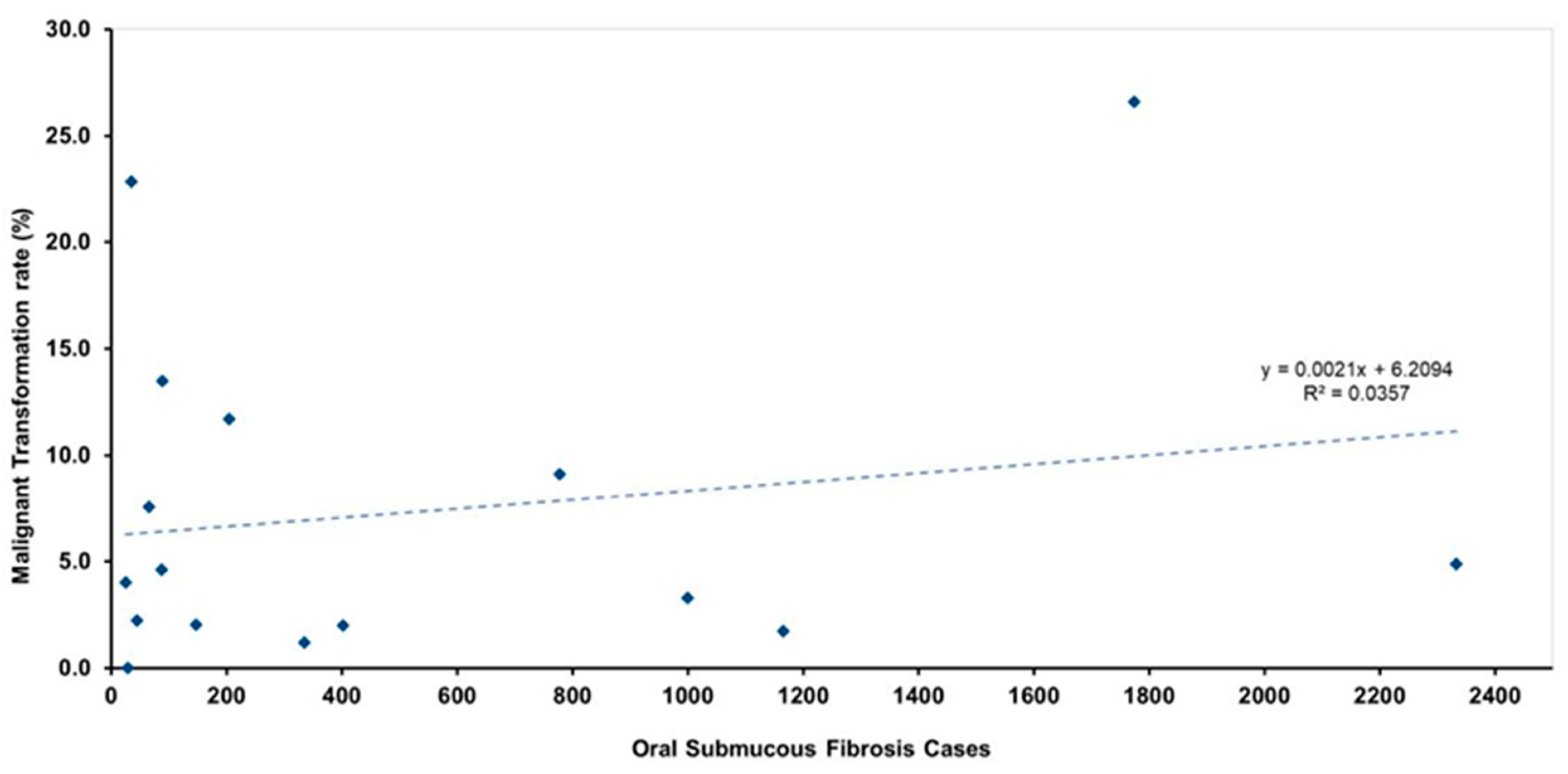
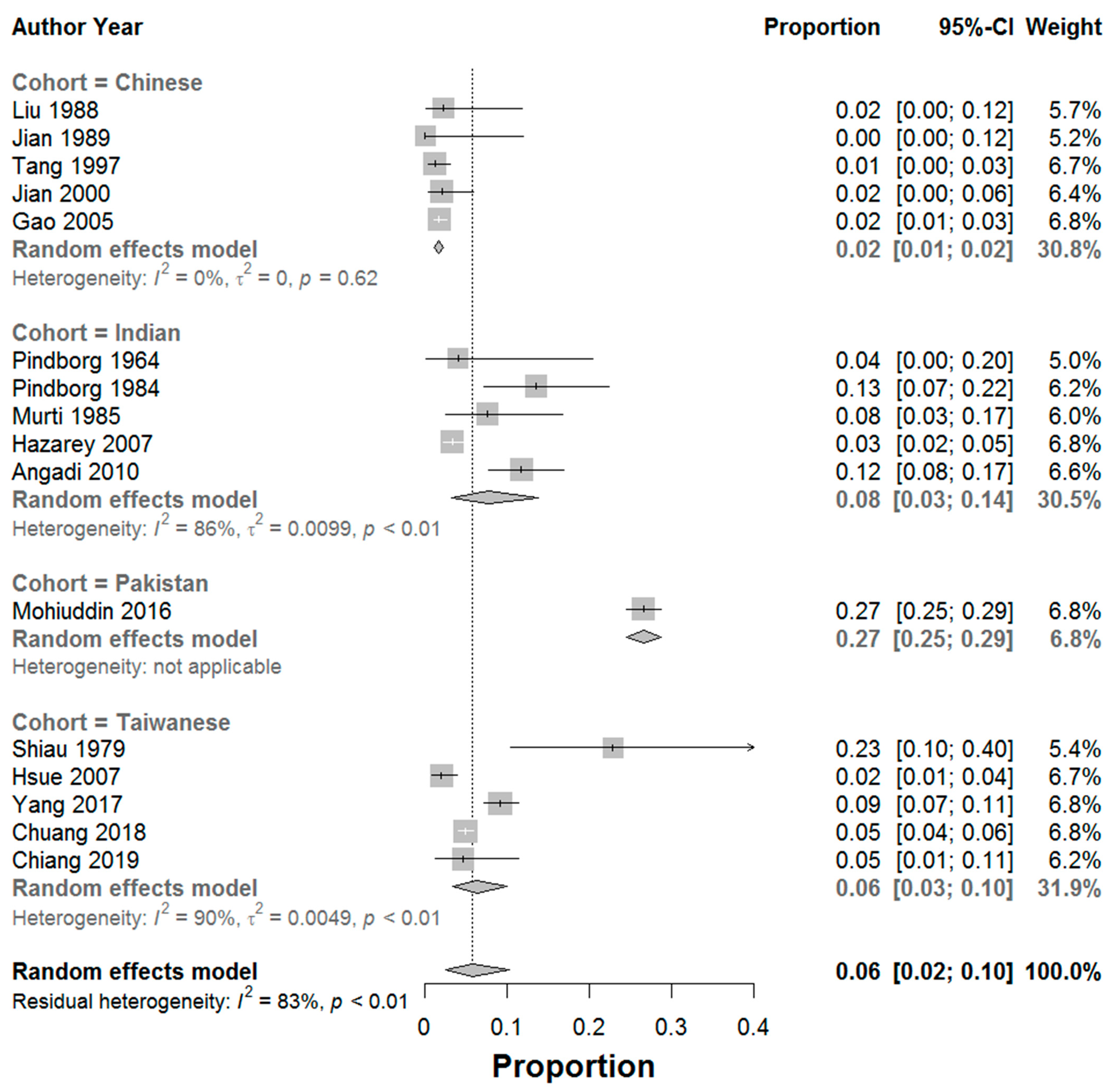
| Study, Year of Study | Cohort Ethnicity [Study Location] | Number of Patients | Number of Patients Diagnosed with Cancer | Mean Age | F:M | Follow Up Period [Years] | Calculated MTR | Reported MTR | Calculated Annual MTR (%) [Reported Annual MTR (%)] | Type of Study |
|---|---|---|---|---|---|---|---|---|---|---|
| Pindborg et al. [7], 1964 | Indian [India] | 25 | 1 | 41.7 | 1.5:1 | NR | 4.0 | 2.8 | NR [NR] | Pros |
| Pindborg et al. [8], 1984 | Indian [India] | 89 | 12 | NR | NR | 8 | 13.5 | 4.5 | 1.7 [0.6] | Ret |
| Murti et al. [9], 1985 | Indian [India] | 66 | 5 | NR | NR | 15 | 7.6 | 4.5 | 0.5 [0.3] | Ret |
| Shiau & Kwan [14], 1979 | Taiwanese [Taiwan] | 35 | 8 | 40.5 | 1:34 | NR | 22.9 | 23.0 | NR [NR] | Ret |
| Liu et al. [15], 1988 | Chinese [China] | 45 | 1 | NR | 1:1.5 | NR | 2.2 | 2.2 | NR [NR] | Pros |
| Jian et al. [16], 1989 | Chinese [China] | 29 | 0 | 40.2 | 1:2.5 | NR | 0.0 | 0.0 | NR [NR] | Pros |
| Tang et al. [17], 1997 | Chinese [China] | 335 | 4 | 38.6 | 1:3 | NR | 1.2 | 1.2 | NR [NR] | Pros |
| Jian et al. [18], 2000 | Chinese [China] | 147 | 3 | NR | 1:5.7 | NR | 2.0 | 2.0 | NR [NR] | Pros |
| Gao et al. [19], 2005 | Chinese [China] | 1166 | 20 | 37.6 | 1:5.4 | NR | 1.7 | 1.7 | NR [NR] | Pros |
| Hazarey et al. [20], 2007 | Indian [India] | 1000 | 33 | NR | NR | NR | 3.3 | NR | NR [NR] | Pros |
| Hsue et al. [21], 2007 | Taiwanese [Taiwan] | 402 | 8 | 47.5 | NR | 10 | 2.0 | 1.9 | 0.2 [0.2] | Ret |
| Angadi & Rekha [22], 2011 | Indian [India] | 205 | 24 | 46 | 1:11 | NR | 11.7 | 11.7 | NR [NR] | Ret |
| Mohiuddin et al. [23], 2016 | Pakistan [Pakistan] | 1774 | 472 | NR | 3:1 | NR | 26.6 | 26.6 | NR [NR] | Ret |
| Yang et al. [24], 2017 | Taiwanese [Taiwan] | 778 | 71 | 41.8 | 1:6.7 | 6 | 9.1 | 9.1 | 1.5 [NR] | Ret |
| Chuang et al. [25], 2018 | Taiwanese [Taiwan] | 2333 | 114 | 45 | NR | 5.7 | 4.9 | 0.9 | 0.9 [0.9] | Pros |
| Chiang et al. [26], 2020 | Taiwanese [Taiwan] | 87 | 4 | NR | NR | 6.7 | 4.6 | 4.6 | 0.7 [NR] | Pros |
| Study/Year | Selection (Score) | Comparability (Score) | Exposure (Score) | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representatives of the Expo Sed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Was not Present at Start of Study | Based on the Design or Analysis | Assessment of Outcome | Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-UP of Cohorts | ||
| Pindborg et al. [7], 1964 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 |
| Pindborg et al. [8], 1984 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 4 |
| Murti et al. [9], 1985 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 4 |
| Shiau & Kwan [14], 1979 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 |
| Liu et al. [15], 1988 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Jian et al. [16], 1989 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Tang et al. [17], 1997 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Jian et al. [18], 2000 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Gao et al. [19], 2005 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Hazarey et al. [20], 2007 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Hsue et al. [21], 2007 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Angadi & Rekha [22], 2011 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Mohiuddin et al. [23], 2016 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| Yang et al. [24], 2019 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Chuang et al. [25], 2018 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Chiang et al. [26], 2020 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| India | China |
|---|---|
| Smokeless tobacco | Betel fruit (husk and leaf) |
| Betel leaves | Peppermint |
| Spices | Gelatine |
| Molasses | Lime |
| Catechu | Calcium carbonate |
| Slaked lime | Calcium hydroxide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murthy, V.; Mylonas, P.; Carey, B.; Yogarajah, S.; Farnell, D.; Addison, O.; Cook, R.; Escudier, M.; Diniz-Freitas, M.; Limeres, J.; et al. Malignant Transformation Rate of Oral Submucous Fibrosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1793. https://doi.org/10.3390/jcm11071793
Murthy V, Mylonas P, Carey B, Yogarajah S, Farnell D, Addison O, Cook R, Escudier M, Diniz-Freitas M, Limeres J, et al. Malignant Transformation Rate of Oral Submucous Fibrosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(7):1793. https://doi.org/10.3390/jcm11071793
Chicago/Turabian StyleMurthy, Vignesh, Petros Mylonas, Barbara Carey, Sangeetha Yogarajah, Damian Farnell, Owen Addison, Richard Cook, Michael Escudier, Marcio Diniz-Freitas, Jacobo Limeres, and et al. 2022. "Malignant Transformation Rate of Oral Submucous Fibrosis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 7: 1793. https://doi.org/10.3390/jcm11071793
APA StyleMurthy, V., Mylonas, P., Carey, B., Yogarajah, S., Farnell, D., Addison, O., Cook, R., Escudier, M., Diniz-Freitas, M., Limeres, J., Monteiro, L., Silva, L., Fricain, J.-C., Catros, S., Fenelon, M., Lodi, G., Lombardi, N., Brailo, V., Ariyaratnam, R., ... Albuquerque, R. (2022). Malignant Transformation Rate of Oral Submucous Fibrosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(7), 1793. https://doi.org/10.3390/jcm11071793










