Effects of Foam Rolling vs. Manual Therapy in Patients with Tension-Type Headache: A Randomized Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Randomization and Masking
2.4. Intervention
- (a)
- MTG. A protocol composed of six MT techniques was applied. In total, each MT session lasted 40 min. After MT treatment, all groups rested for 10 min in a supine position with neutral ranges of neck flexion, extension, lateral flexion and rotation [13,27]. The treatment consisted of:
- (1)
- Compression of the fourth ventricle (CV-4). The participant lay down in a supine position. The physiotherapist, situated behind the participant’s head, slightly approximated the occipital squama lateral angles toward the posterior occipital convexity while taking the cranium into extension. The compression traction was maintained until an inactive state was perceived in the cranial pulse and released when the perception of movement was noted. The duration of this technique was 10 min [15].
- (2)
- Suboccipital inhibitory pressure: suboccipital musculature was palpated until contact was made with the posterior arch of the atlas, and progressive and deep gliding pressure was applied, pushing the atlas anteriorly. The occiput rested on the hands of the physiotherapist while fingertips supported the atlas. Finger pressure was maintained for 10 min to produce the proposed therapeutic effect of inhibiting the suboccipital soft tissues [12].
- (3)
- Trigger point release (TPR) was applied bilaterally in the trapezius to relax the trapezius, since tension may influence the occipital region due to the trapezius insertion. Myofascial trigger points were identified using published criteria [28]. Trapezius was palpated for a tender nodule along with taut bands. Force was progressively applied to the nodule, with the patient instructed to verbally indicate whether they felt pain locally or referred pain. The physiotherapist applied a pincer grip of sufficient force to elicit referred pain (or 6 on a 10-point scale) to the identified site. The duration was until the participant verbally reported dissipation of referred pain, the physiotherapist detected a physical softening in the trigger points, or a maximum of 60 s had elapsed. Up to five compressions were performed at each site with a 10 s rest between contractions [16]. In total, this technique lasted 6 min.
- (4)
- Occiput-atlas-axis technique. This was applied bilaterally and performed on a vertical axis, passing through the odontoid process of the axis without extension or flexion and very little side bending. The technique was used in two stages. First, the physiotherapist performed a light decompression and then made a small circumduction. Then, the appropriate joint barrier was sought by selective tension, and high-velocity rotation manipulation was performed in a cranial helical motion without raising the participant’s head [15,17]. This technique lasted 2 min. All of these manual osteopathic treatments are believed to improve circulation, release joint restrictions, reduce tension in muscles, fascia and dura, decrease nociceptive input and promote a normalizing or calming effect on the CNS according to Hanten et al. [15].
- (5)
- Lumbar-sacral technique. Since the relationship between a lack of mobility of cranial bones, sacrum and iliac bones, and TTH has been reported [18], three lumbar-sacral mobilizations were performed, and the appropriate joint barrier was sought by selective tension. In lateral decubitus, the patient placed the lower leg with knee extension, the upper portion with hip and knee flexion, setting the foot at the level of the popliteal fossa of the opposite leg. Then, a trunk rotation manipulation was performed, and the appropriate joint barrier was sought by selective tension. The technique was performed bilaterally and lasted 2 min.
- (6)
- Cranial massage: classic massage techniques were applied based on the study conducted by Weerapong et al. [29]. Methods used included soft and shallow effleurage and digital petrissage, and tapotement, a superficial massage composed of gentle rubbing and kneading, was performed in the skull and cervical region for 10 min (5 min prone and 5 min supine).
- (b)
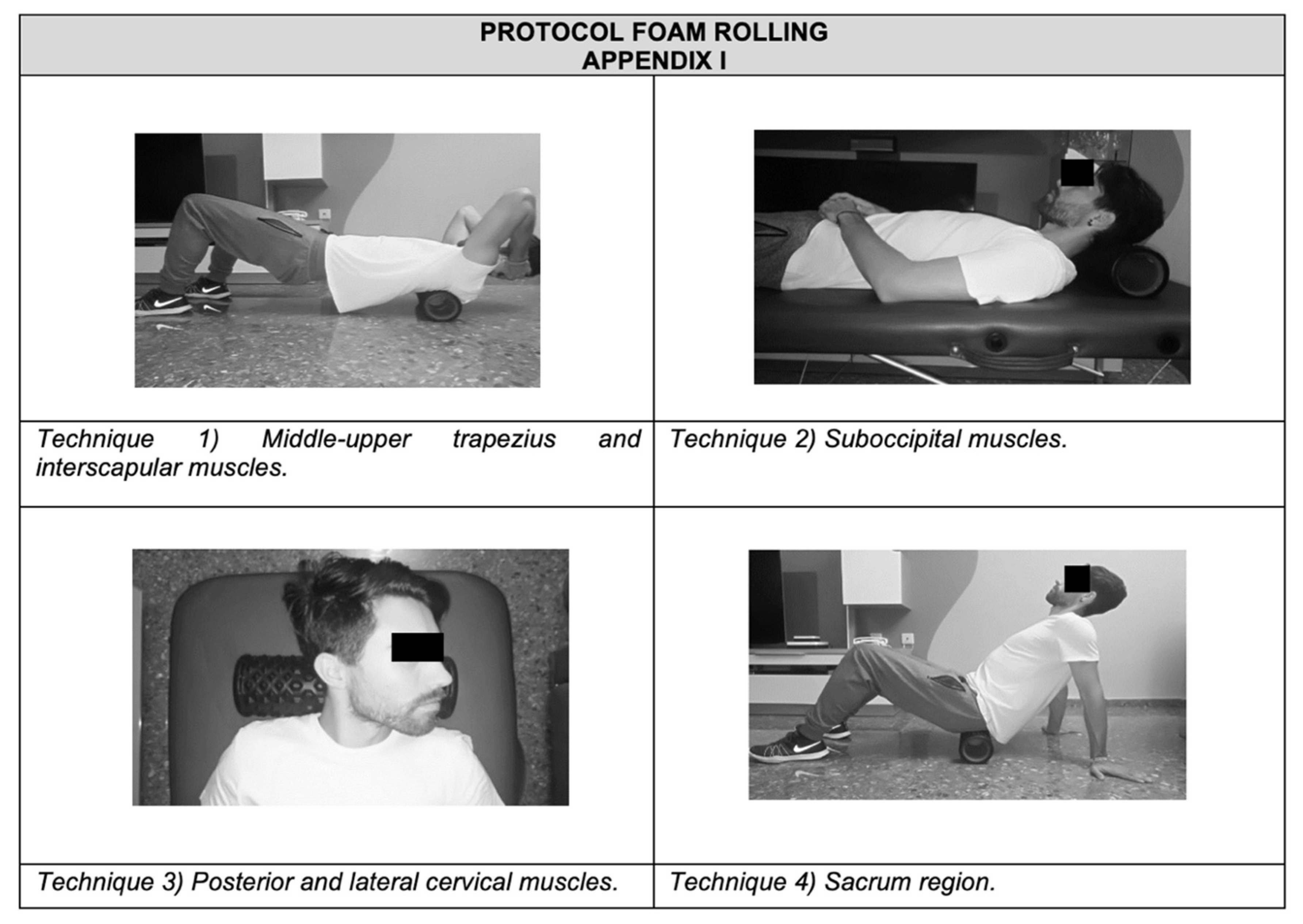
- FRG. Participants were given a brief introduction to the FR procedure. A foam roller with a length of 38 cm and a diameter of 12.5 cm was used (Domyos, Paris, France). An exercise protocol focused on the back’s fascia (suboccipital, cervical and sacral region) was developed [30]. Participants performed four FR exercises under supervision. All exercises were repeated for 1 min, rested for 30 s, and then the procedure was repeated for another minute. Participants rolled out the muscles three times during each minute of FR [31]. Each technique lasted 6 min. In total, the FR treatment lasted 25 min.The rolling frequency was standardized using a metronome set at 60 beats per minute (bpm). Participants were instructed to roll at a velocity of 2 metronome beats (thus, 2 s) for each rolling direction, resulting in 15 complete rolling cycles in 60 s (0.25 Hz). The intensity of pressure was controlled with a target Numerical Rating Scale rating of 7/10 (0 = no discomfort, 10 = maximal discomfort) during the intervention. The treatment was as follows (see Appendix A):
- (1)
- Middle-upper trapezius and interscapular muscles: participants were instructed to roll the FR up using flexion extension in the supine position, with the FR at the interscapular muscles until the FR was just above the upper trapezius. Then, participants were told to roll the FR back to the initial position in one fluid motion.
- (2)
- Suboccipital muscles: in the supine position, with the FR at the suboccipital region, suboccipital flexion was required until the FR was just above the upper trapezius. Then, participants were told to roll the FR back to the initial position.
- (3)
- Posterior and lateral cervical muscles: in the supine position, with the FR at the posterior cervical muscles, participants were asked to rotate the neck and then roll the FR back to the initial work, bilaterally.
- (4)
- Sacrum region: participants were in the supine position, with the FR at the proximal part of the sacrum and their body supported by the hands placed behind them. From this position, they were instructed to roll the FR down using flexion extension of the knees until the foam roller was just above the distal region of the sacrum. Then, participants were told to move the FR back to the initial position.
- (c)
2.5. Outcome Measures
2.5.1. Primary Outcome
2.5.2. Secondary Outcomes
2.6. Sample Size
2.7. Statistical Analysis
2.8. Role of the Funding Source
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Stovner, L.; Hagen, K.; Jensen, R.; Katsarava, Z.; Lipton, R.; Scher, A.; Steiner, T.; Zwart, J.A. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007, 27, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stovner, L.J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; Ruiz de la Torre, E.; Tassorelli, C.; Barré, J.; et al. The impact of headache in Europe: Principal results of the Eurolight project. J. Headache Pain 2014, 15, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barré, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; et al. The cost of headache disorders in Europe: The Eurolight project. Eur. J. Neurol. 2012, 19, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L. Central sensitization in tension-type headache—Possible pathophysiological mechanisms. Cephalalgia 2000, 20, 486–508. [Google Scholar] [CrossRef]
- Bendtsen, L.; Ashina, S.; Moore, A.; Steiner, T.J. Muscles and their role in episodic tension-type headache: Implications for treatment. Eur. J. Pain 2016, 20, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Alonso-Blanco, C.; Cuadrado, M.L.; Pareja, J.A. Myofascial trigger points in the suboccipital muscles in episodic tension-type headache. Man. Ther. 2006, 11, 225–230. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Cuadrado, M.L.; Arendt-Nielsen, L.; Simons, D.G.; Pareja, J.A. Myofascial trigger points and sensitization: An updated pain model for tension-type headache. Cephalalgia 2007, 27, 383–393. [Google Scholar] [CrossRef]
- Cumplido-Trasmonte, C.; Fernández-González, P.; Alguacil-Diego, I.M.; Molina-Rueda, F. Manual therapy in adults with tension-type headache: A systematic review. Neurologia 2021, 36, 537–547. [Google Scholar] [CrossRef]
- Kamonseki, D.H.; Lopes, E.P.; van der Meer, H.A.; Calixtre, L.B. Effectiveness of manual therapy in patients with tension-type headache. A systematic review and meta-analysis. Disabil. Rehabil. 2020, 1–10. [Google Scholar] [CrossRef]
- Castien, R.F.; van der Windt, D.A.; Grooten, A.; Dekker, J. Effectiveness of manual therapy for chronic tension-type headache: A pragmatic, randomised, clinical trial. Cephalalgia 2011, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Espí-López, G.V.; Rodríguez-Blanco, C.; Oliva-Pascual-Vaca, A.; Benítez-Martínez, J.C.; Lluch, E.; Falla, D. Effect of manual therapy techniques on headache disability in patients with tension-type headache. Randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2014, 50, 641–647. [Google Scholar] [PubMed]
- Espí-López, G.V.; Rodríguez-Blanco, C.; Oliva-Pascual-Vaca, A.; Molina-Martínez, F.; Falla, D. Do manual therapy techniques have a positive effect on quality of life in people with tension-type headache? A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 447–456. [Google Scholar] [PubMed]
- Lozano López, C.; Mesa Jiménez, J.; de la Hoz Aizpurúa, J.L.; Pareja Grande, J.; Fernández de Las Peñas, C. Efficacy of manual therapy in the treatment of tension-type headache. A systematic review from 2000–2013. Neurologia 2016, 31, 357–369. [Google Scholar] [CrossRef]
- Hanten, W.P.; Olson, S.L.; Hodson, J.L.; Imler, V.L.; Knab, V.M.; Magee, J.L. The Effectiveness of CV-4 and Resting Position Techniques on Subjects with Tension-Type Headaches. J. Man. Manip. Ther. 1999, 7, 64–70. [Google Scholar] [CrossRef]
- Espí-López, G.V.; Ruescas-Nicolau, M.A.; Nova-Redondo, C.; Benítez-Martínez, J.C.; Dugailly, P.M.; Falla, D. Effect of Soft Tissue Techniques on Headache Impact, Disability, and Quality of Life in Migraine Sufferers: A Pilot Study. J. Altern. Complement. Med. 2018, 24, 1099–1107. [Google Scholar] [CrossRef]
- Espí-López, G.V.; Zurriaga-Llorens, R.; Monzani, L.; Falla, D. The effect of manipulation plus massage therapy versus massage therapy alone in people with tension-type headache. A randomized controlled clinical trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 606–617. [Google Scholar]
- Anderson, R.E.; Seniscal, C. A comparison of selected osteopathic treatment and relaxation for tension-type headaches. Headache 2006, 46, 1273–1280. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Kolber, M.J.; Cain, M.; Lee, M. The effects of self-myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: A systematic review. Int. J. Sports Phys. Ther. 2015, 10, 827–838. [Google Scholar]
- MacDonald, G.Z.; Penney, M.D.; Mullaley, M.E.; Cuconato, A.L.; Drake, C.D.; Behm, D.G.; Button, D.C. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J. Strength Cond. Res. 2013, 27, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Acute effects of self-myofascial release using a foam roller on arterial function. J. Strength Cond. Res. 2014, 28, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.M.; Silvey, D.B.; Button, D.C.; Behm, D.G. Roller-massager application to the hamstrings increases sit-and-reach range of motion within five to ten seconds without performance impairments. Int. J. Sports Phys. Ther. 2013, 8, 228–236. [Google Scholar] [PubMed]
- Macdonald, G.Z.; Button, D.C.; Drinkwater, E.J.; Behm, D.G. Foam rolling as a recovery tool after an intense bout of physical activity. Med. Sci. Sports Exerc. 2014, 46, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearcey, G.E.; Bradbury-Squires, D.J.; Kawamoto, J.E.; Drinkwater, E.J.; Behm, D.G.; Button, D.C. Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. J. Athl. Train. 2015, 50, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Healey, K.C.; Hatfield, D.L.; Blanpied, P.; Dorfman, L.R.; Riebe, D. The effects of myofascial release with foam rolling on performance. J. Strength Cond. Res. 2014, 28, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.; Keene, D.; Dyson, C.; Harvey, L.; Pruvey, C.; Phillips, R. Is cervical spine rotation, as used in the standard vertebrobasilar insufficiency test, associated with a measureable change in intracranial vertebral artery blood flow? Man. Ther. 2004, 9, 220–227. [Google Scholar] [CrossRef]
- Espí-López, G.V.; Gómez-Conesa, A. Efficacy of manual and manipulative therapy in the perception of pain and cervical motion in patients with tension-type headache: A randomized, controlled clinical trial. J. Chiropr. Med. 2014, 13, 4–13. [Google Scholar]
- Simons, D.G.; Travell, J.G.; LS, S. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; Williams & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Weerapong, P.; Hume, P.A.; Kolt, G.S. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005, 35, 235–256. [Google Scholar] [CrossRef]
- Griefahn, A.; Oehlmann, J.; Zalpour, C.; von Piekartz, H. Do exercises with the Foam Roller have a short-term impact on the thoracolumbar fascia?—A randomized controlled trial. J. Bodyw. Mov. Ther. 2017, 21, 186–193. [Google Scholar] [CrossRef]
- Bushell, J.E.; Dawson, S.M.; Webster, M.M. Clinical Relevance of Foam Rolling on Hip Extension Angle in a Functional Lunge Position. J. Strength Cond. Res. 2015, 29, 2397–2403. [Google Scholar] [CrossRef]
- Cerritelli, F.; Ginevri, L.; Messi, G.; Caprari, E.; Di Vincenzo, M.; Renzetti, C.; Cozzolino, V.; Barlafante, G.; Foschi, N.; Provinciali, L. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-Armed randomized controlled trial. Complement. Ther. Med. 2015, 23, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Lamba, A.K.; Faraz, F.; Tandon, S.; Makker, K. Comparison of anxiety and pain perceived with conventional and computerized local anesthesia delivery systems for different stages of anesthesia delivery in maxillary and mandibular nerve blocks. J. Dent. Anesth. Pain Med. 2018, 18, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, C.W.; Park, S.B.; Kim, M.J.; Jang, S.H. Reliability and usefulness of the pressure pain threshold measurement in patients with myofascial pain. Ann. Rehabil. Med. 2011, 35, 412–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, G.P.; Ramadan, N.M.; Norris, L.; Newman, C.W. Headache disability inventory (HDI): Short-term test-retest reliability and spouse perceptions. Headache 1995, 35, 534–539. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Kosinski, M.; Dewey, J.E.; Gandek, B. How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8 Health Survey; With a supplement on the SF-6 Health Survey; QualityMetric, Inc.: Lincoln, RI, USA, 2001. [Google Scholar]
- Martin, M.; Blaisdell, B.; Kwong, J.W.; Bjorner, J.B. The Short-Form Headache Impact Test (HIT-6) was psychometrically equivalent in nine languages. J. Clin. Epidemiol. 2004, 57, 1271–1278. [Google Scholar] [CrossRef]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef]
- Bodes-Pardo, G.; Pecos-Martín, D.; Gallego-Izquierdo, T.; Salom-Moreno, J.; Fernández-de-Las-Peñas, C.; Ortega-Santiago, R. Manual treatment for cervicogenic headache and active trigger point in the sternocleidomastoid muscle: A pilot randomized clinical trial. J. Manip. Physiol. Ther. 2013, 36, 403–411. [Google Scholar] [CrossRef]
- Ferragut-Garcías, A.; Plaza-Manzano, G.; Rodríguez-Blanco, C.; Velasco-Roldán, O.; Pecos-Martín, D.; Oliva-Pascual-Vaca, J.; Llabrés-Bennasar, B.; Oliva-Pascual-Vaca, Á. Effectiveness of a Treatment Involving Soft Tissue Techniques and/or Neural Mobilization Techniques in the Management of Tension-Type Headache: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 211–219.e2. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Sim, J.; Wright, C.C.; Foster, N.E. Interrater Reliability of Algometry in Measuring Pressure Pain Thresholds in Healthy Humans, Using Multiple Raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef]
- Moraska, A.F.; Stenerson, L.; Butryn, N.; Krutsch, J.P.; Schmiege, S.J.; Mann, J.D. Myofascial trigger point-focused head and neck massage for recurrent tension-type headache: A randomized, placebo-controlled clinical trial. Clin. J. Pain 2015, 31, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Ceca, D.; Elvira, L.; Guzmán, J.F.; Pablos, A. Benefits of a self-myofascial release program on health-related quality of life in people with fibromyalgia: A randomized controlled trial. J. Sports Med. Phys. Fit. 2017, 57, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Castien, R.F.; Blankenstein, A.H.; Windt, D.A.v.d.; Dekker, J. Minimal clinically important change on the Headache Impact Test-6 questionnaire in patients with chronic tension-type headache. Cephalalgia 2012, 32, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Kaptchuk, T.J.; Kelley, J.M.; Conboy, L.A.; Davis, R.B.; Kerr, C.E.; Jacobson, E.E.; Kirsch, I.; Schyner, R.N.; Nam, B.H.; Nguyen, L.T.; et al. Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008, 336, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Benz, L.N.; Flynn, T.W. Placebo, nocebo, and expectations: Leveraging positive outcomes. J. Orthop. Sports Phys. Ther. 2013, 43, 439–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, C.; Hardy, J.; Spruyt, O.; Currow, D.C. Placebo and nocebo effects in randomized controlled trials: The implications for research and practice. J. Pain Symptom Manag. 2013, 46, 722–730. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| FRG (n = 13) | MTG (n = 13) | CG (n = 12) | Differences among Groups | |
|---|---|---|---|---|
| Demographic variables | ||||
| Age (years) | 31.08 (9.75) | 29.62 (10.08) | 35.58 (13.46) | F(2,35) = 0.96; p = 0.39 |
| BMI (kg/m2) | 25.28 (3.61) | 25.50 (3.87) | 28.50 (5.30) | F(2,35) = 2.16; p = 0.13 |
| Gender, n (M/F) | 3/10 | 4/9 | 2/10 | χ2(2)= 0.69; p = 0.71 |
| History | ||||
| Evolution of headache (years) | 7.85 (7.94) | 8.00 (7.23) | 15.92 (12.16) | F(2,35) = 3.05; p = 0.06 |
| Frequency, n (FETTH/CTTH) | 9/4 | 9/4 | 8/4 | χ2(2) = 0.03; p = 0.99 |
| Severity of disorder, n (mild/moderate/severe) | 1/12/0 | 0/11/2 | 0/7/5 | χ2(4) = 8.90; p = 0.06 |
| Intensity (VAS) | 6.38 (0.65) | 5.92 (1.50) | 5.92 (1.31) | F(2,35) = 0.64; p = 0.54 |
| Pain profile (triggers) | ||||
| Location of pain, n (front/parietal/occipital pain) | 4/5/4 | 8/2/3 | 6/3/3 | χ2(4) = 2.81; p = 0.59 |
| Location bilateral pain, n (no/yes) | 4/9 | 3/10 | 4/8 | χ2(2) = 0.35; p = 0.84 |
| Pressing or tightening (non-pulsating), n (no/yes) | 3/10 | 6/7 | 4/8 | χ2(2) = 1.54; p = 0.46 |
| No pain increases with physical activity, n (no/yes) | 9/4 | 10/3 | 7/5 | χ2(2) = 1.00; p = 0.61 |
| Time when the pain begins, n (morning/day/night/indifferent) | 1/5/4/3 | 4/4/4/1 | 2/6/2/2 | χ2(6) = 4.10; p = 0.66 |
| Associated factors | ||||
| Photophobia, n (no/yes) | 6/7 | 6/7 | 3/9 | χ2(2) = 1.54; p = 0.46 |
| Pericranial sensitivity, n (no/yes) | 9/4 | 7/6 | 6/6 | χ2(2) = 1.08; p = 0.58 |
| Time Factor | Interaction Group × Time | |
|---|---|---|
| Pain (VAS) | p = 0.005; ηp2 = 0.14 | p = 0.047; ηp2 = 0.13 |
| Pressure pain threshold, right suboccipital (algometry, kg/cm2) | p = 0.005; ηp2 = 0.14 | p < 0.001; ηp2 = 0.30 |
| Pressure pain threshold, left suboccipital (algometry, kg/cm2) | p = 0.045; ηp2 = 0.09 | p = 0.002; ηp2 = 0.21) |
| Emotional disability (emotional HDI) | p < 0.001; ηp2 = 0.30 | * |
| Functional disability (functional HDI) | p < 0.001; ηp2 = 0.43 | * |
| Overall disability (total HDI) | p < 0.001; ηp2 = 0.41 | * |
| Impact of headache (HIT-6) | p < 0.001; ηp2 = 0.55 | p = 0.010; ηp2 = 0.17 |
| Pre-Treatment | Post-Treatment | Follow-Up | Pre vs. Post p [95% CI]; d | Pre vs. Follow-Up p [95% CI]; d | |
|---|---|---|---|---|---|
| Pain (VAS) | |||||
| FRG (n = 13) | 6.38 (0.65) | 5.38 (1.19) | 6.00 (0.91) | 0.010 [0.20:1.80]; 1.00 | * |
| MTG (n = 13) | 5.92 (1.50) | 4.69 (0.86) | 5.08 (1.75) | 0.001 [0.43:2.03]; 0.97 | * |
| CG (n = 12) | 5.92 (1.31) | 5.92 (1.62) | 6.83 (2.04) | * | * |
| FRG vs. MTG | * | * | * | ||
| FRG vs. CG | * | * | * | ||
| MTG vs. CG | * | * | 0.032 [−3.39:−0.12]; 0.90 | ||
| Pressure pain threshold, right suboccipital (algometry, kg/cm2) | |||||
| FRG (n = 13) | 2.19 (0.81) | 2.52 (0.62) | 2.42 (0.66) | 0.045 [−0.66:−0.01]; 0.44 | * |
| MTG (n = 13) | 2.01 (0.69) | 2.68 (0.74) | 2.17 (0.35) | <0.001 [−0.99:−0.35]; 0.90 | * |
| CG (n = 12) | 2.13 (0.79) | 1.87 (0.53) | 2.03 (0.58) | * | * |
| FRG vs. MTG | * | * | * | ||
| FRG vs. CG | * | 0.044 [0.01:1.30]; 1.10 | * | ||
| MTG vs. CG | * | 0.009 [0.17:1.45]; 1.21 | * | ||
| Pressure pain threshold, left suboccipital (algometry, kg/cm2) | |||||
| FRG (n = 13) | 2.30 (0.72) | 2.62 (0.64) | 2.47 (0.60) | 0.015 [−0.60:−0.05]; 0.45 | * |
| MTG (n = 13) | 2.19 (0.58) | 2.48 (0.58) | 2.40 (0.34) | 0.032 [−0.56:−0.02]; 0.48 | * |
| CG (n = 12) | 2.07 (0.63) | 1.83 (0.47) | 2.10 (0.58) | * | * |
| FRG vs. MTG | * | * | * | ||
| FRG vs. CG | * | 0.004 [0.22:1.36]; 1.35 | * | ||
| MTG vs. CG | * | 0.021 [0.08:1.22]; 1.20 | * | ||
| Pre-Treatment | Post-Treatment | Follow-Up | Pre vs. Post p [95% CI]; d | Pre vs. Follow-Up p [95% CI]; d | |
|---|---|---|---|---|---|
| Impact of headache (HIT 6) | |||||
| FRG (n = 13) | 63.31 (3.50) | 55.85 (5.08) | 57.62 (6.15) | <0.001 [4.05:10.87]; 1.64 | 0.002 [1.93:9.46]; 1.09 |
| MTG (n = 13) | 63.23 (3.61) | 52.38 (7.43) | 57.38 (7.19) | <0.001 [7.44:14.25]; 1.78 | 0.001 [2.08:9.61]; 0.98 |
| CG (n = 12) | 64.67 (4.03) | 60.58 (6.23) | 58.42 (6.60) | 0.019 [0.54:7.63]; 0.74 | 0.001 [2.33:10.17]; 1.09 |
| FRG vs. MTG | * | * | * | ||
| FRG vs. CG | * | * | * | ||
| MTG vs. CG | * | 0.008 [−14.56:−1.83]; 1.15 | * | ||
| Functional disability (functional HDI) | |||||
| FRG (n = 13) | 26.62 (8.22) | 17.23 (8.58) | 17.62 (9.43) | <0.001 [4.79:13.98]; 1.07 | 0.002 [3.02:14.98]; 0.97 |
| MTG (n = 13) | 25.38 (9.71) | 14.77 (7.85) | 17.23 (9.33) | <0.001 [6.02:15.21]; 1.15 | 0.005 [2.17:14.14]; 0.82 |
| CG (n = 12) | 27.33 (7.30) | 21.67 (9.57) | 21.17 (12.61) | 0.016 [0.89:10.45]; 0.63 | * |
| FRG vs. MTG | * | * | * | ||
| FRG vs. CG | * | * | * | ||
| MTG vs. CG | * | * | * | ||
| Emotional disability (emotional HDI) | |||||
| FRG (n = 13) | 17.54 (10.11) | 11.85 (9.07) | 11.08 (8.78) | 0.007 [1.33:10.05]; 0.57 | * |
| MTG (n = 13) | 17.69 (7.78) | 9.69 (6.47) | 13.08 (8.97) | <0.001 [3.64:12.36]; 1.07 | * |
| CG (n = 12) | 23.50 (10.89) | 17.50 (9.27) | 19.17 (12.66) | 0.006 [1.46:10.54]; 0.57 | * |
| FRG vs. MTG | * | * | * | ||
| FRG vs. CG | * | * | * | ||
| MTG vs. CG | * | * | * | ||
| Overall disability (total HDI) | |||||
| FRG (n = 13) | 44.15 (17.60) | 29.08 (16.71) | 28.69 (17.56) | <0.001 [7.29:22.86]; 0.84 | 0.007 [3.70:27.22]; 0.84 |
| MTG (n = 13) | 43.08 (16.94) | 24.46 (12.76) | 30.31 (17.34) | <0.001 [10.83:26.40]; 1.19 | 0.030 [1.01:24.53]; 0.71 |
| CG (n = 12) | 50.83 (17.28) | 39.17 (17.13) | 40.33 (24.63) | 0.003 [3.57:19.77]; 0.65 | * |
| FRG vs. MTG | * | * | * | ||
| FRG vs. CG | * | * | * | ||
| MTG vs. CG | * | * | * | ||
| Frequency (HDI) | Severity (HDI) | ||||||
|---|---|---|---|---|---|---|---|
| <1 Episode/Month | 1 to 4 Episodes/Month | >1 Episode/Week | Mild | Moderate | Severe | ||
| FRG | Pre-treatment | 0 | 7 | 6 | 1 | 11 | 1 |
| Post-treatment | 2 | 11 | 0 | 7 | 5 | 1 | |
| Follow-up | 1 | 11 | 1 | 5 | 7 | 1 | |
| χ2 analysis | χ2 (4) = 12.0; p = 0.011 | χ2 (4) =6.7; p = 0.167 | |||||
| MTG | Pre-treatment | 0 | 6 | 7 | 0 | 11 | 2 |
| Post-treatment | 4 | 6 | 3 | 5 | 7 | 1 | |
| Follow-up | 0 | 8 | 5 | 3 | 8 | 2 | |
| χ2 analysis | χ2 (4) = 10.0, p = 0.042 | χ2 (4) =6.2; p = 0.199 | |||||
| CG | Pre-treatment | 0 | 2 | 10 | 0 | 9 | 3 |
| Post-treatment | 1 | 5 | 6 | 0 | 10 | 2 | |
| Follow-up | 3 | 6 | 3 | 2 | 7 | 3 | |
| χ2 analysis | χ2 (4) = 9.4, p = 0.052 | χ2 (4) =4.8; p = 0.419 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espi-Lopez, G.V.; Ingles, M.; Carrasco-Fernandez, J.J.; Serra-Añó, P.; Copete-Fajardo, L.; Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Marques-Sule, E. Effects of Foam Rolling vs. Manual Therapy in Patients with Tension-Type Headache: A Randomized Pilot Study. J. Clin. Med. 2022, 11, 1778. https://doi.org/10.3390/jcm11071778
Espi-Lopez GV, Ingles M, Carrasco-Fernandez JJ, Serra-Añó P, Copete-Fajardo L, Gonzalez-Gerez JJ, Saavedra-Hernandez M, Marques-Sule E. Effects of Foam Rolling vs. Manual Therapy in Patients with Tension-Type Headache: A Randomized Pilot Study. Journal of Clinical Medicine. 2022; 11(7):1778. https://doi.org/10.3390/jcm11071778
Chicago/Turabian StyleEspi-Lopez, Gemma V., Marta Ingles, Juan J. Carrasco-Fernandez, Pilar Serra-Añó, Luis Copete-Fajardo, Juan Jose Gonzalez-Gerez, Manuel Saavedra-Hernandez, and Elena Marques-Sule. 2022. "Effects of Foam Rolling vs. Manual Therapy in Patients with Tension-Type Headache: A Randomized Pilot Study" Journal of Clinical Medicine 11, no. 7: 1778. https://doi.org/10.3390/jcm11071778
APA StyleEspi-Lopez, G. V., Ingles, M., Carrasco-Fernandez, J. J., Serra-Añó, P., Copete-Fajardo, L., Gonzalez-Gerez, J. J., Saavedra-Hernandez, M., & Marques-Sule, E. (2022). Effects of Foam Rolling vs. Manual Therapy in Patients with Tension-Type Headache: A Randomized Pilot Study. Journal of Clinical Medicine, 11(7), 1778. https://doi.org/10.3390/jcm11071778









