Hearing Therapy Improves Tinnitus-Related Distress in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss: A Randomized-Controlled Cross-Over Design
Abstract
1. Introduction
- Compared to a waiting, delayed-intervention group (DIG), an immediate intervention group (IIG) shows higher reductions in tinnitus-related distress and psychological distress following Terzo© (Sonneberg, Germany) hearing therapy;
- Given the primarily audiological–cognitive focus of the intervention, treatment-related change may be most strongly reflected in TFI (vs. TQ or THI) scores;
- Any observed effects will be stable at a 70-day follow-up.
2. Materials and Methods
2.1. Participants
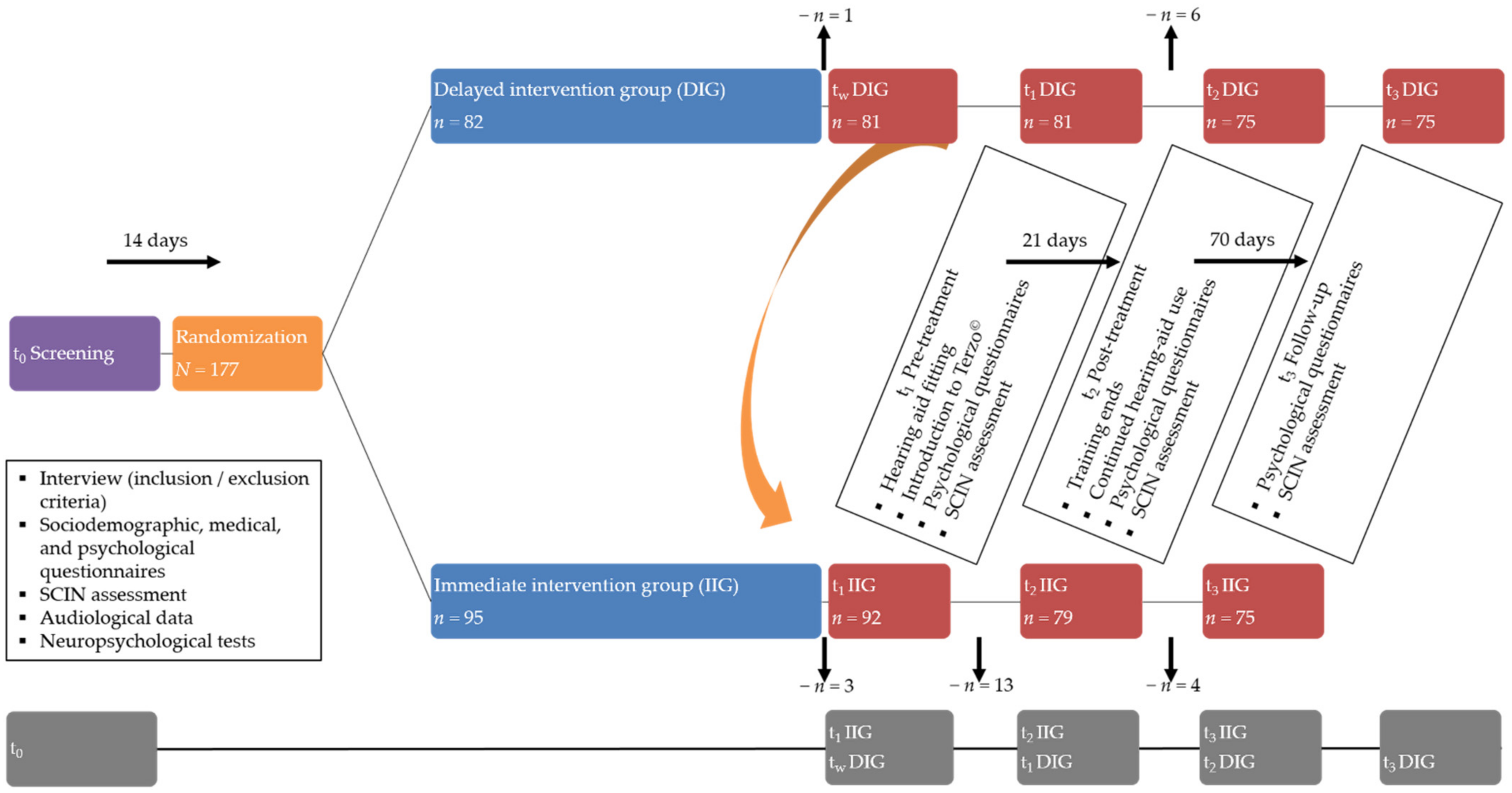
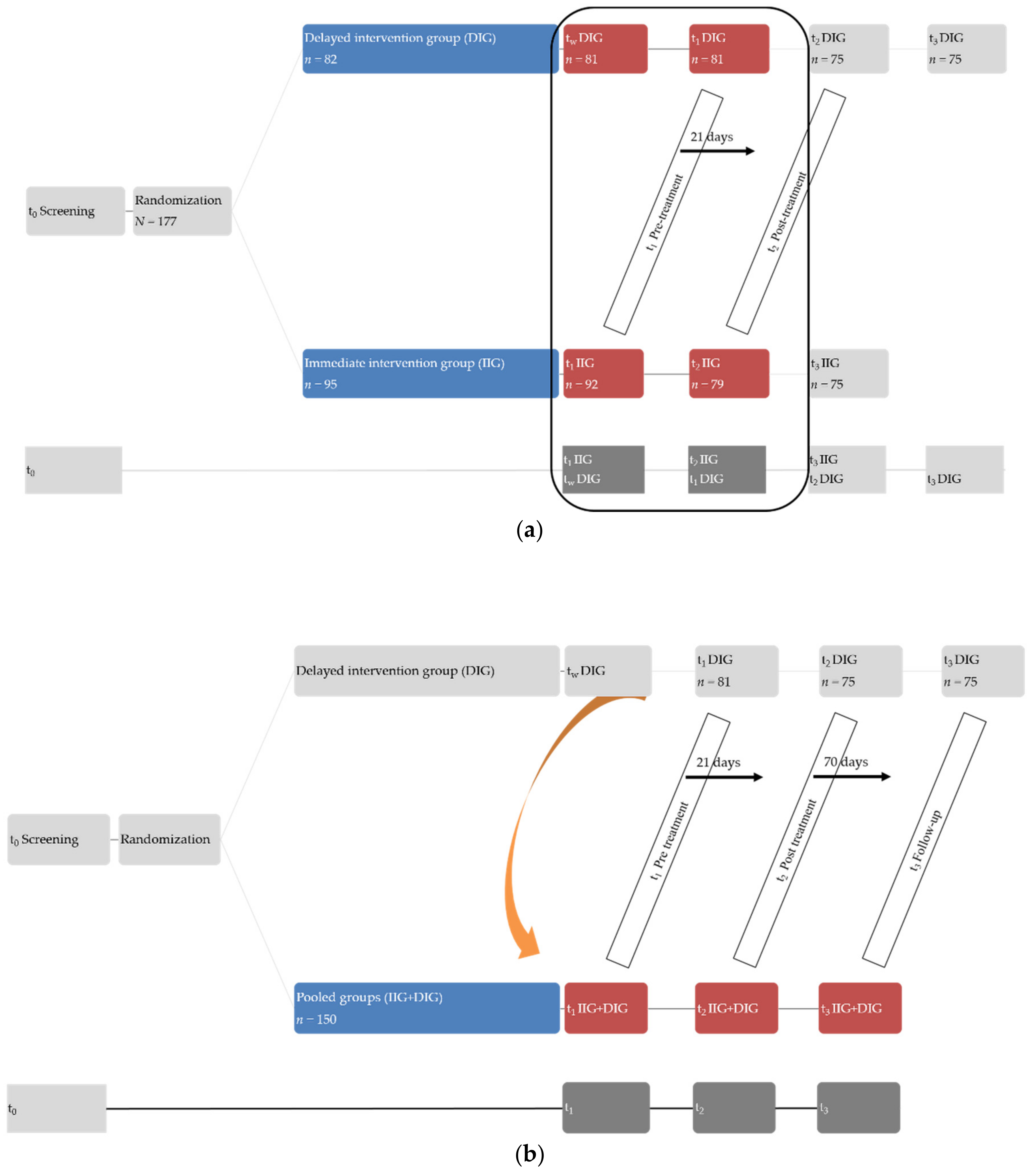
2.2. Procedure
2.3. Study Protocol
2.4. Hearing Ability
2.5. Terzo© Hearing Therapy
2.6. Measures
2.6.1. Tinnitus-Related Distress
2.6.2. Perceived Stress
2.6.3. Psychological Epiphenomena
2.7. Statistical Analyses
3. Results
3.1. Psychological Variables
3.2. Effects of Terzo© Hearing Therapy on Tinnitus-Related Distress, Perceived Stress, and Psychological Epiphenomena: Immediate Intervention Group vs. Wait (Delayed Intervention Group)
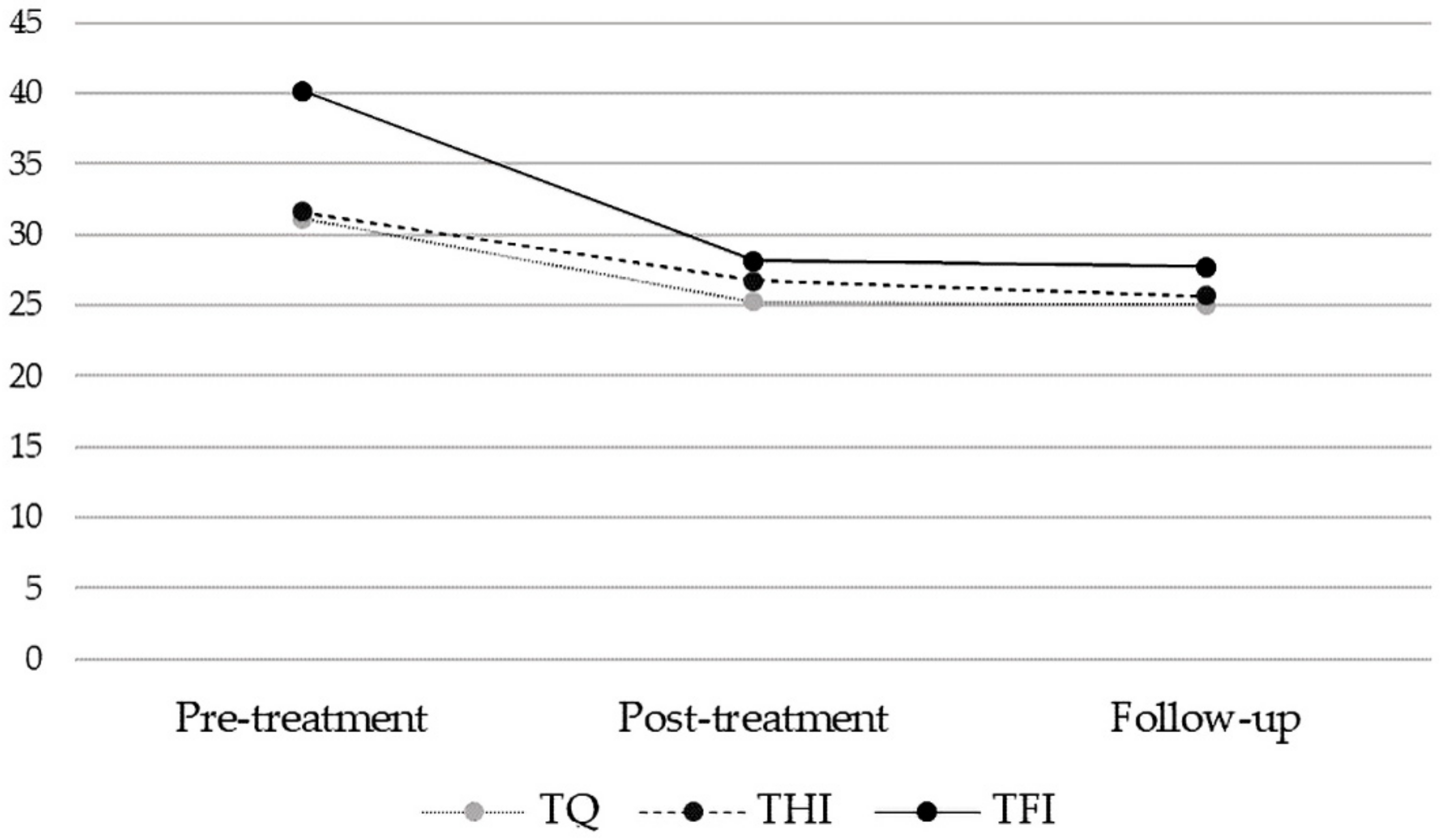
3.3. Stability of Treatment Effects [Pooled Sample]
3.4. Exploratory Analyses: Uncontrolled Effects from Pre-Treatment to Follow-Up [Pooled Sample]
3.5. Exploratory Analyses: Hearing Ability and [Pre-to-Follow-Up Change in] Psychological Variables [Pooled Sample]
4. Discussion
4.1. Limitations
4.2. Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Biswas, R.; Hall, D.A. Prevalence, Incidence, and Risk Factors for Tinnitus; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–28. [Google Scholar]
- Henry, J.A.; Dennis, K.C.; Schechter, M.A. General Review of Tinnitus. J. Speech Lang. Hear. Res. 2005, 48, 1204–1235. [Google Scholar] [CrossRef]
- Gallus, S.; Lugo, A.; Garavello, W.; Bosetti, C.; Santoro, E.; Colombo, P.; Perin, P.; La Vecchia, C.; Langguth, B. Prevalence and Determinants of Tinnitus in the Italian Adult Population. Neuroepidemiology 2015, 45, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ramage-Morin, P.L.; Banks, R.; Pineault, D.; Atrach, M. Tinnitus in Canada. Public Health Rep. 2019, 30, 3–11. [Google Scholar]
- Boecking, B.; Brueggemann, P.; Kleinjung, T.; Mazurek, B. All for One and One for All?–Examining Convergent Validity and Responsiveness of the German Versions of the Tinnitus Ques-tionnaire (TQ), Tinnitus Handicap Inventory (THI), and Tinnitus Functional Index (TFI). Front. Psychol. 2021, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Nondahl, D.M.; Cruickshanks, K.J.; Huang, G.-H.; Klein, B.E.K.; Klein, R.; Nieto, F.J.; Tweed, T.S. Tinnitus and its risk factors in the Beaver Dam Offspring Study. Int. J. Audiol. 2011, 50, 313–320. [Google Scholar] [CrossRef]
- Mazurek, B.; Olze, H.; Haupt, H.; Szczepek, A.J. The More the Worse: The Grade of Noise-Induced Hearing Loss Associates with the Severity of Tinnitus. Int. J. Environ. Res. Public Health 2010, 7, 3071–3079. [Google Scholar] [CrossRef]
- Jayarajan, V.; Bartlett, J.; Ratnayake, S.A.B. Could an underlying hearing loss be a significant factor in the handicap caused by tinnitus? Noise Health 2009, 11, 156–160. [Google Scholar] [CrossRef]
- Oosterloo, B.C.; Homans, N.C.; Goedegebure, A. Tinnitus Affects Speech in Noise Comprehension in Individuals With Hearing Loss. Otol. Neurotol. 2020, 41, e1074–e1081. [Google Scholar] [CrossRef]
- Peelle, J.E.; Troiani, V.; Grossman, M.; Wingfield, A. Hearing Loss in Older Adults Affects Neural Systems Supporting Speech Comprehension. J. Neurosci. 2011, 31, 12638–12643. [Google Scholar] [CrossRef]
- Quist-Hanssen, S.; Thorud, E.; Aasand, G. Noise-Induced Hearing Loss and the Comprehension of Speech in Noise. Acta Oto-Laryngol. 1978, 86, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ivansic, D.; Guntinas-Lichius, O.; Müller, B.; Volk, G.F.; Schneider, G.; Dobel, C. Impairments of Speech Comprehension in Patients with Tinnitus—A Review. Front. Aging Neurosci. 2017, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Noreña, A.; Hoare, D.J. A multidisciplinary European guideline for tinnitus: Diagnostics, assessment, and treatment. HNO 2019, 67, 10–42. [Google Scholar] [CrossRef]
- De Ronde-Brons, I.; Soede, W.; Dreschler, W. Systematic Evaluation of Self-Reported Hearing Ability in Six Dimensions Before and After a Hearing Aid Trial. J. Speech Lang. Hear. Res. 2019, 62, 4150–4164. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Poveda, E.A.; Johannesen, P.T.; Pérez-González, P.; Blanco, J.L.; Kalluri, S.; Edwards, B. Predictors of Hearing-Aid Outcomes. Trends Hear. 2017, 21, 2331216517730526. [Google Scholar] [CrossRef]
- Folmer, R.L.; Carroll, J.R. Long-Term Effectiveness of Ear-Level Devices for Tinnitus. Otolaryngol. Neck Surg. 2006, 134, 132–137. [Google Scholar] [CrossRef]
- McNeill, C.; Tavora-Vieira, D.; Alnafjan, F.; Searchfield, G.D.; Welch, D. Tinnitus pitch, masking, and the effectiveness of hearing aids for tinnitus therapy. Int. J. Audiol. 2012, 51, 914–919. [Google Scholar] [CrossRef]
- Searchfield, G.D.; Kaur, M.; Martin, W.H. Hearing aids as an adjunct to counseling: Tinnitus patients who choose amplification do better than those that don’t. Int. J. Audiol. 2010, 49, 574–579. [Google Scholar] [CrossRef]
- Trotter, M.I.; Donaldson, I. Hearing aids and tinnitus therapy: A 25-year experience. J. Laryngol. Otol. 2008, 122, 1052–1056. [Google Scholar] [CrossRef]
- Del Bo, L.; Ambrosetti, U. Hearing aids for the treatment of tinnitus. Prog. Brain Res. 2007, 166, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Eriksson-Mangold, M.; Carlsson, S. Psychological and somatic distress in relation to perceived hearing disability, hearing handicap, and hearing measurements. J. Psychosom. Res. 1991, 35, 729–740. [Google Scholar] [CrossRef]
- Jayakody, D.M.; Almeida, O.P.; Speelman, C.; Bennett, R.J.; Moyle, T.C.; Yiannos, J.M.; Friedland, P.L. Association between speech and high-frequency hearing loss and depression, anxiety and stress in older adults. Maturitas 2018, 110, 86–91. [Google Scholar] [CrossRef]
- Langguth, B.; Landgrebe, M.; Kleinjung, T.; Sand, P.; Hajak, G. Tinnitus and depression. World J. Biol. Psychiatry 2011, 12, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Lee, S.H.; Koo, J.-W.; Park, H.Y.; Lee, K.Y.; Choi, Y.S.; Oh, K.W.; Lee, A.; Yang, J.-E.; Woo, S.-Y.; et al. Prevalence and Associated Factors of Tinnitus: Data From the Korean National Health and Nutrition Examination Survey 2009–2011. J. Epidemiol. 2014, 24, 417–426. [Google Scholar] [CrossRef]
- Trevis, K.J.; McLachlan, N.M.; Wilson, S.J. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin. Psychol. Rev. 2018, 60, 62–86. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Reed, N.S.; Brewster, K.K.; Huang, A.; Rebok, G.; Rutherford, B.R.; Lin, F.R. Association of Hearing Loss With Psychological Distress and Utilization of Mental Health Services Among Adults in the United States. JAMA Netw. Open 2020, 3, e2010986. [Google Scholar] [CrossRef]
- Gil, D.; Iório, M.C.M. Formal auditory training in adult hearing aid users. Clincs 2010, 65, 165–174. [Google Scholar] [CrossRef][Green Version]
- Olson, A.D.; Preminger, J.E.; Shinn, J.B. The Effect of LACE DVD Training in New and Experienced Hearing Aid Users. J. Am. Acad. Audiol. 2013, 24, 214–230. [Google Scholar] [CrossRef]
- Song, J.H.; Skoe, E.; Banai, K.; Kraus, N. Training to Improve Hearing Speech in Noise: Biological Mechanisms. Cereb. Cortex 2011, 22, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Naumann, H.H.; Pfaltz, C.R. Hals-Nasen-Ohren-Heilkunde, 4th ed.; Thieme: Stuttgart, Germany, 1989. [Google Scholar]
- Neff, P.; Simões, J.; Psatha, S.; Nyamaa, A.; Boecking, B.; Rausch, L.; Dettling-Papargyris, J.; Funk, C.; Brueggemann, P.; Mazurek, B. The impact of tinnitus distress on cognition. Sci. Rep. 2021, 11, 2243. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Wohlfeil, J.; Jauch, E.; Sorg, R. Manual der terzo Gehörtherapie. 2008; Internal Document. [Google Scholar]
- Moodie, S.T.F.; Scollie, S.D.; Bagatto, M.P.; Keene, K.; Canada, T.N.O.P.A.O. Fit-to-Targets for the Desired Sensation Level Version 5.0a Hearing Aid Prescription Method for Children. Am. J. Audiol. 2017, 26, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Scollie, S.; Seewald, R.; Cornelisse, L.; Moodie, S.; Bagatto, M.; Laurnagaray, D.; Beaulac, S.; Pumford, J. The Desired Sensation Level Multistage Input/Output Algorithm. Trends Amplif. 2005, 9, 159–197. [Google Scholar] [CrossRef]
- Goebel, G.; Hiller, W. Tinnitus-Fragebogen:(TF); ein Instrument zur Erfassung von Belastung und Schweregrad bei Tinnitus; Handanweisung; Hogrefe, Verlag für Psychologie: Göttingen, Germany, 1998. [Google Scholar]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kleinjung, T.; Fischer, B.; Langguth, B.; Sand, P.G.; Hajak, G.; Dvorakova, J.; Eichhammer, P. Validierung einer deutschsprachigen Version des „Tinnitus Handicap Inventory”. Psychiatr. Prax. 2007, 34, 140–142. [Google Scholar] [CrossRef]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Myers, P.J.; Newman, C.W.; Sandridge, S.; Turk, D.C.; et al. The Tinnitus Functional Index. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef]
- Brüggemann, P.; Szczepek, A.J.; Kleinjung, T.; Ojo, M.; Mazurek, B. Validierung der deutschen Version des Tinnitus Functional Index (TFI). Laryngo-Rhino-Otologie 2017, 96, 615–619. [Google Scholar] [CrossRef]
- Fliege, H.; Rose, M.; Arck, P.; Walter, O.B.; Kocalevent, R.-D.; Weber, C.; Klapp, B.F. The Perceived Stress Questionnaire (PSQ) Reconsidered: Validation and Reference Values From Different Clinical and Healthy Adult Samples. Psychosom. Med. 2005, 67, 78–88. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Herrmann, C.; Buss, U. Vorstellung Validierung einer deutschen Version der ‚Hospital Anxiety and Depression Scale‘ (HAD-Skala). Ein Fragebogen zur Erfassung des psychischen Befindens bei Patienten mit körperlichen Beschwerden. Description and validation of a German version of the Hospital Anxiety and Depression Scale (HADS): A questionnaire for identifying emotional disorders in physically ill patients. Diagnostica 1994, 40, 143–154. [Google Scholar]
- Tritt, K.; Von Heymann, F.; Zaudig, M.; Zacharias, I.; Söllner, W.; Loew, T. Entwicklung des Fragebogens »ICD-10-Symptom-Rating«(ISR). Z. Psychosom. Med. Psychother. 2008, 54, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Yakunina, N.; Lee, W.H.; Ryu, Y.-J.; Nam, E.-C. Tinnitus Suppression Effect of Hearing Aids in Patients With High-frequency Hearing Loss: A Randomized Double-blind Controlled Trial. Otol. Neurotol. 2019, 40, 865–871. [Google Scholar] [CrossRef]
- Hoare, D.J.; Stacey, P.; Hall, D. The Efficacy of Auditory Perceptual Training for Tinnitus: A Systematic Review. Ann. Behav. Med. 2010, 40, 313–324. [Google Scholar] [CrossRef]
- Kochkin, S.; Tyler, R. Tinnitus treatment and the effectiveness of hearing aids: Hearing care professional perceptions. Hear. Rev. 2008, 15, 14–18. [Google Scholar]
- Ivansic, D.; Dobel, C.; Volk, G.F.; Reinhardt, D.; Müller, B.; Smolenski, U.C.; Guntinas-Lichius, O. Results of an Interdisciplinary Day Care Approach for Chronic Tinnitus Treatment: A Prospective Study Introducing the Jena Interdisciplinary Treatment for Tinnitus. Front. Aging Neurosci. 2017, 9, 192. [Google Scholar] [CrossRef]
- Timmer, B.; Hickson, L.; Launer, S. Hearing Aid Use and Mild Hearing Impairment: Learnings from Big Data. J. Am. Acad. Audiol. 2017, 28, 731–741. [Google Scholar] [CrossRef]
- Solheim, J.; Gay, C.; Hickson, L. Older adults’ experiences and issues with hearing aids in the first six months after hearing aid fitting. Int. J. Audiol. 2018, 57, 31–39. [Google Scholar] [CrossRef]
- Jacquemin, L.; Mertens, G.; Van de Heyning, P.; Vanderveken, O.M.; Topsakal, V.; De Hertogh, W.; Michiels, S.; Van Rompaey, V.; Gilles, A. Sensitivity to change and convergent validity of the Tinnitus Functional Index (TFI) and the Tinnitus Questionnaire (TQ): Clinical and research perspectives. Hear. Res. 2019, 382, 107796. [Google Scholar] [CrossRef]
- Blazer, D.G.; Tucci, D.L. Hearing loss and psychiatric disorders: A review. Psychol. Med. 2019, 49, 891–897. [Google Scholar] [CrossRef]
- Hoare, D.J.; Kowalkowski, V.L.; Kang, S.; Hall, D. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope 2011, 121, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, L. t-Test and ANOVA for data with ceiling and/or floor effects. Behav. Res. Methods 2021, 53, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Arrindell, W. Changes in waiting-list patients over time: Data on some commonly-used measures. Beware! Behav. Res. Ther. 2001, 39, 1227–1247. [Google Scholar] [CrossRef]
- Hesser, H.; Weise, C.; Rief, W.; Andersson, G. The effect of waiting: A meta-analysis of wait-list control groups in trials for tinnitus distress. J. Psychosom. Res. 2011, 70, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Steinert, C.; Stadter, K.; Stark, R.; Leichsenring, F. The Effects of Waiting for Treatment: A Meta-Analysis of Waitlist Control Groups in Randomized Controlled Trials for Social Anxiety Disorder. Clin. Psychol. Psychother. 2016, 24, 649–660. [Google Scholar] [CrossRef]
- Dawes, P.; Emsley, R.; Cruickshanks, K.J.; Moore, D.R.; Fortnum, H.; Edmondson-Jones, M.; McCormack, A.; Munro, K.J. Hearing Loss and Cognition: The Role of Hearing Aids, Social Isolation and Depression. PLoS ONE 2015, 10, e0119616. [Google Scholar] [CrossRef]
- Scinicariello, F.; Przybyla, J.; Carroll, Y.; Eichwald, J.; Decker, J.; Breysse, P.N. Age and sex differences in hearing loss association with depressive symptoms: Analyses of NHANES 2011–2012. Psychol. Med. 2019, 49, 962–968. [Google Scholar] [CrossRef]
- Chou, K.-L. Combined effect of vision and hearing impairment on depression in older adults: Evidence from the English Longitudinal Study of Ageing. J. Affect. Disord. 2008, 106, 191–196. [Google Scholar] [CrossRef]
- Kiely, K.M.; Anstey, K.J.; Luszcz, M.A. Dual Sensory Loss and Depressive Symptoms: The Importance of Hearing, Daily Functioning, and Activity Engagement. Front. Hum. Neurosci. 2013, 7, 837. [Google Scholar] [CrossRef]
- Castiglione, A.; Benatti, A.; Velardita, C.; Favaro, D.; Padoan, E.; Severi, D.; Pagliaro, M.; Bovo, R.; Vallesi, A.; Gabelli, C.; et al. Aging, Cognitive Decline and Hearing Loss: Effects of Auditory Rehabilitation and Training with Hearing Aids and Cochlear Implants on Cognitive Function and Depression among Older Adults. Audiol. Neurotol. 2016, 21, 21–28. [Google Scholar] [CrossRef]
- Nkyekyer, J.; Meyer, D.; Pipingas, A.; Reed, N.S. The cognitive and psychosocial effects of auditory training and hearing aids in adults with hearing loss. Clin. Interv. Aging 2019, 14, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Dawes, P.; Cruickshanks, K.J.; Fischer, M.E.; Klein, B.E.; Klein, R.; Nondahl, D.M. Hearing-aid use and long-term health outcomes: Hearing handicap, mental health, social engagement, cognitive function, physical health, and mortality. Int. J. Audiol. 2015, 54, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Köhler-Forsberg, O.; Moss-Morris, R.; Mehnert, A.; Miranda, J.J.; Bullinger, M.; Steptoe, A.; Whooley, M.A.; Otte, C. Comorbid depression in medical diseases. Nat. Rev. Dis. Prim. 2020, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-S.; Eaton, W.W.; Gallo, J.J.; Nestadt, G. Understanding the heterogeneity of depression through the triad of symptoms, course and risk factors: A longitudinal, population-based study. J. Affect. Disord. 2000, 59, 1–11. [Google Scholar] [CrossRef]
- Ketterer, M.C.; Knopke, S.; Häußler, S.M.; Hildenbrand, T.; Becker, C.; Gräbel, S.; Olze, H. Asymmetric hearing loss and the benefit of cochlear implantation regarding speech perception, tinnitus burden and psychological comorbidities: A prospective follow-up study. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2683–2693. [Google Scholar] [CrossRef]
- Ketterer, M.C.; Häussler, S.-M.; Hildenbrand, T.; Speck, I.; Peus, D.; Rosner, B.; Knopke, S.; Graebel, S.; Olze, H. Binaural Hearing Rehabilitation Improves Speech Perception, Quality of Life, Tinnitus Distress, and Psychological Comorbidities. Otol. Neurotol. 2020, 41, e563–e574. [Google Scholar] [CrossRef]
- Knopke, S.; Szczepek, A.J.; Häussler, S.M.; Gräbel, S.; Olze, H. Cochlear implantation of bilaterally deafened patients with tinnitus induces sustained decrease of tinnitus-related distress. Front. Neurol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Olze, H.; Szczepek, A.J.; Haupt, H.; Förster, U.; Zirke, N.; Gräbel, S.; Mazurek, B. Cochlear implantation has a positive influence on quality of life, tinnitus, and psychological comorbidity. Laryngoscope 2011, 121, 2220–2227. [Google Scholar] [CrossRef]
- Zarenoe, R.; Hallgren, M.; Andersson, G.; Ledin, T. Working Memory, Sleep, and Hearing Problems in Patients with Tinnitus and Hearing Loss Fitted with Hearing Aids. J. Am. Acad. Audiol. 2017, 28, 141–151. [Google Scholar] [CrossRef]
| n | % | ||
|---|---|---|---|
| Education | |||
| Completed junior apprenticeship | 72 | 40.7 | |
| Completed senior apprenticeship | 40 | 22.6 | |
| University degree | 60 | 33.9 | |
| Other | 4 | 2.3 | |
| Employment ‘yes’ | 105 | 59.3 | |
| Relationship status | |||
| Single | 25 | 14.1 | |
| Married | 114 | 64.4 | |
| Divorced | 27 | 15.3 | |
| Widowed | 10 | 5.6 | |
| Duration of tinnitus | |||
| <0.5 year | 5 | 2.8 | |
| 0.5–1 year | 9 | 5.1 | |
| 1–2 years | 23 | 13.0 | |
| 2–5 years | 24 | 13.6 | |
| >5 years | 107 | 60.5 | |
| Tinnitus onset | |||
| gradual | 92 | 52.0 | |
| sudden | 73 | 41.2 | |
| Frequency | |||
| very high | 37 | 20.9 | |
| high | 104 | 58.8 | |
| middle | 32 | 18.1 | |
| low | 3 | 1.7 | |
| Past psychotherapy ‘yes’ | 53 | 29.9 | |
| Use of hearing aid ‘yes’ | 53 | 31.5 | |
| Group | Timepoint | Group × Time Interaction Effect | d | Main Effect of Time | d | ||||
|---|---|---|---|---|---|---|---|---|---|
| nIIG = 79 nDIG = 81 | t1IIG; twDIG | t2IIG; t1DIG | |||||||
| Measure | M | SD | M | SD | |||||
| TQ | IIG | 31.11 | 15.72 | 24.71 | 15.46 | Fgroup × time(1, 158) = 21.21, p < 0.001 | −0.304 | ||
| DIG | 33.05 | 17.09 | 31.67 | 16.14 | |||||
| THI | IIG | 30.61 | 22.09 | 26.20 | 21.41 | Fgroup × time(1, 158) = 5.02, p = 0.026 | −0.137 | ||
| DIG | 34.17 | 23.68 | 32.91 | 22.06 | |||||
| TFI | IIG | 40.38 | 21.46 | 27.85 | 21.61 | Fgroup × time(1, 158) = 34.40, p < 0.001 | −0.525 | ||
| DIG | 41.87 | 21.83 | 40.75 | 21.68 | |||||
| PSQ_total | IIG | 28.12 | 19.00 | 26.60 | 18.85 | ||||
| DIG | 30.07 | 19.59 | 29.42 | 19.66 | |||||
| PSQ_w | IIG | 23.46 | 19.91 | 20.59 | 20.18 | ||||
| DIG | 26.75 | 23.39 | 26.58 | 23.18 | |||||
| PSQ_t | IIG | 34.01 | 25.41 | 30.72 | 23.98 | ||||
| DIG | 35.12 | 26.08 | 34.40 | 23.70 | |||||
| PSQ_j | IIG | 57.05 | 27.67 | 57.62 | 26.18 | ||||
| DIG | 57.12 | 27.37 | 56.21 | 28.44 | |||||
| PSQ_d | IIG | 31.98 | 21.60 | 31.05 | 22.39 | ||||
| DIG | 32.67 | 22.45 | 33.00 | 24.40 | |||||
| HADS_a | IIG | 6.09 | 4.06 | 5.30 | 4.10 | Ftime(1,158) = 7.98, p = 0.005 | −0.114 | ||
| DIG | 6.40 | 4.55 | 6.20 | 4.18 | |||||
| HADS_d | IIG | 5.19 | 4.44 | 4.94 | 4.46 | ||||
| DIG | 5.73 | 5.07 | 5.86 | 4.91 | |||||
| ISR_total | IIG | 0.59 | 0.49 | 0.54 | 0.52 | Ftime(1,158) = 8.67, p = 0.004 | −0.094 | ||
| DIG | 0.66 | 0.62 | 0.61 | 0.57 | |||||
| ISR_ds | IIG | 0.88 | 0.95 | 0.82 | 0.96 | ||||
| DIG | 0.98 | 1.01 | 0.96 | 0.97 | |||||
| ISR_as | IIG | 0.90 | 0.84 | 0.68 | 0.73 | Ftime(1,158) = 13.83, p < 0.001 | −0.186 | ||
| DIG | 0.84 | 0.91 | 0.75 | 0.81 | |||||
| ISR_ocd | IIG | 0.49 | 0.70 | 0.54 | 0.85 | ||||
| DIG | 0.64 | 0.84 | 0.58 | 0.77 | |||||
| ISR_sds | IIG | 0.33 | 0.51 | 0.27 | 0.52 | ||||
| DIG | 0.48 | 0.70 | 0.40 | 0.67 | |||||
| ISR_eds | IIG | 0.51 | 0.60 | 0.54 | 0.68 | ||||
| DIG | 0.48 | 0.73 | 0.47 | 0.73 | |||||
| ISR_sup | IIG | 0.52 | 0.44 | 0.47 | 0.42 | Ftime(1,157) = 9.21, p = 0.003 | −0.098 | ||
| DIG | 0.62 | 0.59 | 0.58 | 0.56 | |||||
| Group n = 150 | Timepoint Pre | Post | Follow-Up | Paired Samples t-Tests | |||||
|---|---|---|---|---|---|---|---|---|---|
| t1IIG and DIG | t2IIG and DIG | t3IIG and DIG | t2- t3 | t1- t3 | d | ||||
| Measure | M | SD | M | SD | M | SD | |||
| TQ [a] | 31.09 | 16.16 | 25.27 | 15.80 | 25.07 | 17.02 | t(149) = 7.67, p < 0.001 | −0.363 | |
| THI [a] | 31.64 | 22.36 | 26.75 | 22.03 | 25.65 | 22.42 | t(149) = 5.50, p < 0.001 | −0.268 | |
| TFI [a] | 40.18 | 21.67 | 28.14 | 21.22 | 27.69 | 22.16 | t(149) = 10.02, p < 0.001 | −0.570 | |
| PSQ_total [b] | 28.80 | 19.85 | 27.12 | 19.95 | 25.84 | 19.16 | t(149) = 3.13, p < 0.01 | −0.152 | |
| PSQ_w | 25.20 | 22.14 | 23.16 | 22.32 | 23.24 | 22.73 | |||
| PSQ_t [b] | 34.31 | 25.16 | 30.80 | 23.79 | 30.18 | 25.01 | t(149) = 2.60, p < 0.05 | −0.165 | |
| PSQ_j 2[b] | 57.20 | 28.49 | 59.19 | 28.49 | 60.31 | 28.29 | |||
| PSQ_d 1[b] | 32.36 | 23.36 | 31.29 | 23.58 | 29.56 | 21.97 | t(149) = 2.21, p < 0.05 | −0.122 | |
| HADS_a [b] | 6.04 | 4.19 | 5.58 | 4.42 | 5.55 | 4.69 | t(149) = 2.35, p < 0.05 | −0.110 | |
| HADS_d [b] | 5.47 | 4.78 | 5.02 | 4.85 | 5.09 | 4.71 | t(149) = 2.15, p < 0.05 | −0.080 | |
| ISR_total 2[b] | 0.60 | 0.54 | 0.56 | 0.56 | 0.54 | 0.56 | t(149) = 2.79, p < 0.01 | −0.109 | |
| ISR_ds [b] | 0.93 | 0.97 | 0.87 | 0.98 | 0.82 | 0.94 | t(149) = 2.45, p < 0.05 | −0.115 | |
| ISR_as 2[b] | 0.83 | 0.84 | 0.67 | 0.75 | 0.69 | 0.82 | |||
| ISR_ocd | 0.51 | 0.73 | 0.53 | 0.81 | 0.49 | 0.75 | |||
| ISR_sds | 0.35 | 0.58 | 0.34 | 0.58 | 0.30 | 0.58 | |||
| ISR_eds | 0.48 | 0.68 | 0.47 | 0.71 | 0.48 | 0.74 | |||
| ISR_sup 2[b] | 0.55 | 0.52 | 0.51 | 0.54 | 0.50 | 0.53 | t(149) = 2.62, p < 0.05 | −0.100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boecking, B.; Rausch, L.; Psatha, S.; Nyamaa, A.; Dettling-Papargyris, J.; Funk, C.; Brueggemann, P.; Rose, M.; Mazurek, B. Hearing Therapy Improves Tinnitus-Related Distress in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss: A Randomized-Controlled Cross-Over Design. J. Clin. Med. 2022, 11, 1764. https://doi.org/10.3390/jcm11071764
Boecking B, Rausch L, Psatha S, Nyamaa A, Dettling-Papargyris J, Funk C, Brueggemann P, Rose M, Mazurek B. Hearing Therapy Improves Tinnitus-Related Distress in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss: A Randomized-Controlled Cross-Over Design. Journal of Clinical Medicine. 2022; 11(7):1764. https://doi.org/10.3390/jcm11071764
Chicago/Turabian StyleBoecking, Benjamin, Leonie Rausch, Stamatina Psatha, Amarjargal Nyamaa, Juliane Dettling-Papargyris, Christine Funk, Petra Brueggemann, Matthias Rose, and Birgit Mazurek. 2022. "Hearing Therapy Improves Tinnitus-Related Distress in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss: A Randomized-Controlled Cross-Over Design" Journal of Clinical Medicine 11, no. 7: 1764. https://doi.org/10.3390/jcm11071764
APA StyleBoecking, B., Rausch, L., Psatha, S., Nyamaa, A., Dettling-Papargyris, J., Funk, C., Brueggemann, P., Rose, M., & Mazurek, B. (2022). Hearing Therapy Improves Tinnitus-Related Distress in Mildly Distressed Patients with Chronic Tinnitus and Mild-to-Moderate Hearing Loss: A Randomized-Controlled Cross-Over Design. Journal of Clinical Medicine, 11(7), 1764. https://doi.org/10.3390/jcm11071764









