Expression of Calbindin, a Marker of Gamma-Aminobutyric Acid Neurons, Is Reduced in the Amygdala of Oestrogen Receptor β-Deficient Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation
2.3. Immunohistochemistry
2.4. Controls
2.5. Counts and Measurements
2.6. Statistics
3. Results
3.1. Neuronal Deficits in the Amygdala of ERβ−/− Mice
3.2. Calcium-Binding Proteins in the Amygdala of ERβ−/− Mice
3.3. Somatostatin in the Amygdala of ERβ−/− Mice
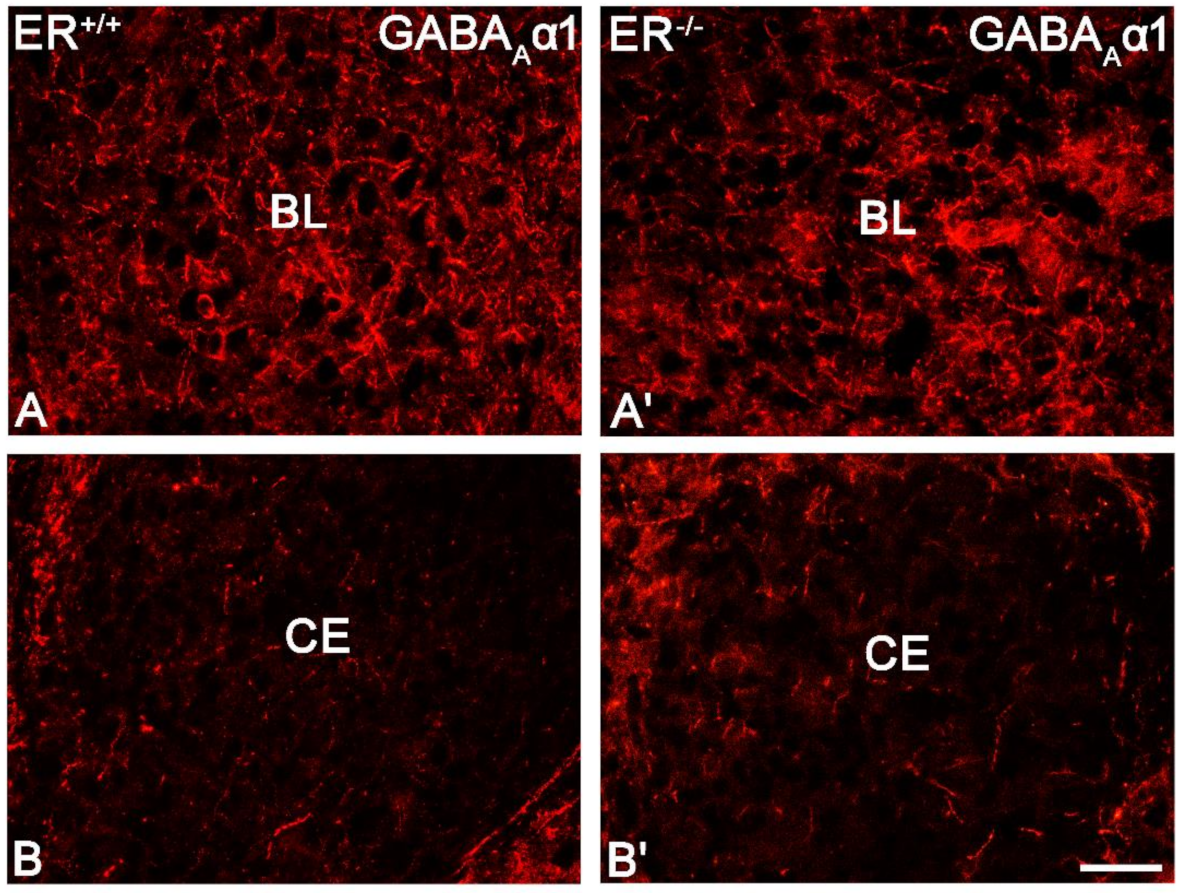
3.4. GABA Type A Receptor with α1 Subunit in the Amygdala of ERβ−/− Mice
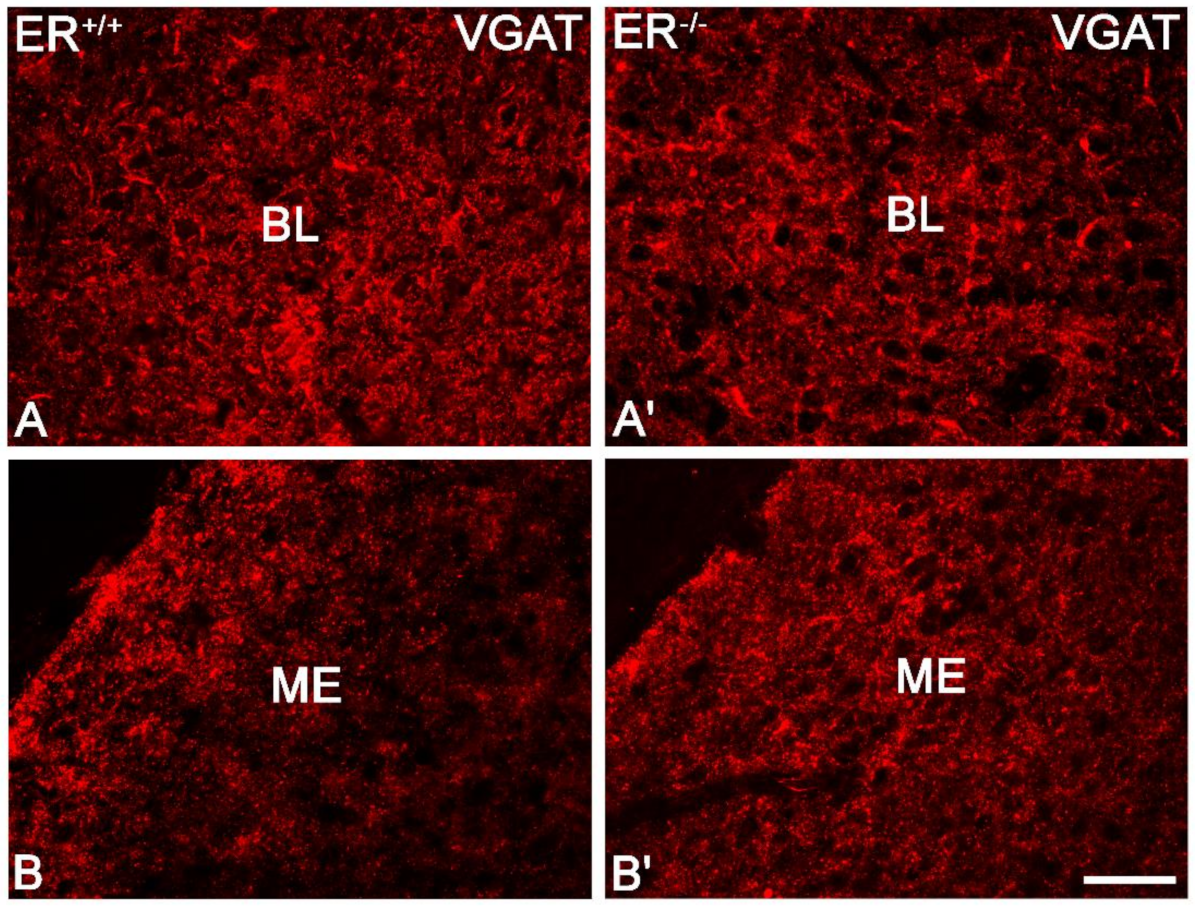
3.5. Vesicular GABA Transporter in the Amygdala of ERβ−/− Mice
3.6. Overexpression of Glial Fibrillary Acidic Protein in the Amygdala of ERβ−/− Mice
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, R.C.; Angermeyer, M.; Anthony, J.C.; DE Graaf, R.; Demyttenaere, K.; Gasquet, I.; DE Girolamo, G.; Gluzman, S.; Gureje, O.; Haro, J.M.; et al. Lifetime Prevalence and Age-of-Onset Distributions of Mental Disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 2007, 6, 168–176. [Google Scholar] [PubMed]
- Steel, Z.; Marnane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The Global Prevalence of Common Mental Disorders: A Systematic Review and Meta-Analysis 1980–2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Craske, M.G.; Stein, M.B. Anxiety. Lancet 2016, 388, 3048–3059. [Google Scholar] [CrossRef]
- Nomura, M.; Durbak, L.; Chan, J.; Smithies, O.; Gustafsson, J.-Å.; Korach, K.S.; Pfaff, D.W.; Ogawa, S. Genotype/Age Interactions on Aggressive Behavior in Gonadally Intact Estrogen Receptor β Knockout (ΒERKO) Male Mice. Horm. Behav. 2002, 41, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global Prevalence of Anxiety Disorders: A Systematic Review and Meta-Regression. Psychol. Med. 2013, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar]
- Ritchie, H.; Roser, M. Mental Health. Our World Data. 2018. Available online: https://ourworldindata.org/mental-health (accessed on 17 March 2021).
- Locke, A.; Kirst, N.; Shultz, C.G. Diagnosis and Management of Generalized Anxiety Disorder and Panic Disorder in Adults. AFP 2015, 91, 617–624. [Google Scholar]
- LeDoux, J. The Emotional Brain, Fear, and the Amygdala. Cell. Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Prater, K.E.; Schatzberg, A.F.; Menon, V.; Greicius, M.D. Disrupted Amygdalar Subregion Functional Connectivity and Evidence of a Compensatory Network in Generalized Anxiety Disorder. Arch. Gen. Psychiatry 2009, 66, 1361–1372. [Google Scholar] [CrossRef]
- Ramasubbu, R.; Konduru, N.; Cortese, F.; Bray, S.; Gaxiola, I.; Goodyear, B. Reduced Intrinsic Connectivity of Amygdala in Adults with Major Depressive Disorder. Front. Psychiatry 2014, 5, 17. [Google Scholar] [CrossRef]
- Cullen, K.R.; Vizueta, N.; Thomas, K.M.; Han, G.J.; Lim, K.O.; Camchong, J.; Mueller, B.A.; Bell, C.H.; Heller, M.D.; Schulz, S.C. Amygdala Functional Connectivity in Young Women with Borderline Personality Disorder. Brain Connect. 2011, 1, 61. [Google Scholar] [CrossRef]
- Buffalari, D.M.; See, R.E. Amygdala Mechanisms of Pavlovian Psychostimulant Conditioning and Relapse. Curr. Top. Behav. Neurosci. 2010, 3, 73–99. [Google Scholar] [CrossRef]
- Swaab, D.F.; Chung, W.C.J.; Kruijver, F.P.M.; Hofman, M.A.; Hestiantoro, A. Sex Differences in the Hypothalamus in the Different Stages of Human Life. Neurobiol. Aging 2003, 24 (Suppl. 1), S1–S16; discussion S17–S19. [Google Scholar] [CrossRef]
- Riecher-Rössler, A. Prospects for the Classification of Mental Disorders in Women. Eur. Psychiatry 2010, 25, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Dalal, P.K.; Agarwal, M. Postmenopausal Syndrome. Indian J. Psychiatry 2015, 57, S222–S232. [Google Scholar] [CrossRef] [PubMed]
- Marsh, W.K.; Bromberger, J.T.; Crawford, S.L.; Leung, K.; Kravitz, H.M.; Randolph, J.F.; Joffe, H.; Soares, C.N. Lifelong Estradiol Exposure and Risk of Depressive Symptoms during the Transition to Menopause and Postmenopause. Menopause 2017, 24, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.; Cohen, L.S. Estrogen, Serotonin, and Mood Disturbance: Where Is the Therapeutic Bridge? Biol.. Psychiatry 1998, 44, 798–811. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Palav, A.; McCaffrey, R.J. A Review of Symptoms Commonly Associated with Menopause: Implications for Clinical Neuropsychologists and Other Health Care Providers. Neuropsychol. Rev. 2003, 13, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N.; Almeida, O.P.; Joffe, H.; Cohen, L.S. Efficacy of Estradiol for the Treatment of Depressive Disorders in Perimenopausal Women: A Double-Blind, Randomized, Placebo-Controlled Trial. Arch. Gen. Psychiatry 2001, 58, 529–534. [Google Scholar] [CrossRef]
- Bromberger, J.T.; Kravitz, H.M.; Chang, Y.; Randolph, J.F.; Avis, N.E.; Gold, E.B.; Matthews, K.A. Does Risk for Anxiety Increase during the Menopausal Transition? Study of Women’s Health Across the Nation. Menopause J. N. Am. Menopause Soc. 2013, 20, 488. [Google Scholar] [CrossRef]
- Hoyt, L.T.; Falconi, A. Puberty and Perimenopause: Reproductive Transitions and Their Implications for Women’s Health. Soc. Sci. Med. 2015, 132, 103–112. [Google Scholar] [CrossRef]
- Gupta, R.R.; Sen, S.; Diepenhorst, L.L.; Rudick, C.N.; Maren, S. Estrogen Modulates Sexually Dimorphic Contextual Fear Conditioning and Hippocampal Long-Term Potentiation (LTP) in Rats(1). Brain Res. 2001, 888, 356–365. [Google Scholar] [CrossRef]
- Krȩżel, W.; Dupont, S.; Krust, A.; Chambon, P.; Chapman, P.F. Increased Anxiety and Synaptic Plasticity in Estrogen Receptor β-Deficient Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 12278–12282. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.D.; Rovis, T.; Chung, W.C.J.; Handa, R.J. Novel Actions of Estrogen Receptor-β on Anxiety-Related Behaviors. Endocrinology 2005, 146, 797–807. [Google Scholar] [CrossRef]
- Oyola, M.G.; Portillo, W.; Reyna, A.; Foradori, C.D.; Kudwa, A.; Hinds, L.; Handa, R.J.; Mani, S.K. Anxiolytic Effects and Neuroanatomical Targets of Estrogen Receptor-β (ERβ) Activation by a Selective ERβ Agonist in Female Mice. Endocrinology 2012, 153, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Andersson, S.; Warner, M.; Gustafsson, J.-A. Morphological Abnormalities in the Brains of Estrogen Receptor Knockout Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2792–2796. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Tan, L.; Wang, Y.; Lu, C.; Chen, R.; Zhang, S.; Gao, Y.; Liu, Y.; Yin, Y.; et al. Correcting MiR92a-VGAT-Mediated GABAergic Dysfunctions Rescues Human Tau-Induced Anxiety in Mice. Mol. Ther. 2017, 25, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Kubo, C.; Rhee, J.-S.; Akaike, N. Presynaptic Serotonergic Inhibition of GABAergic Synaptic Transmission in Mechanically Dissociated Rat Basolateral Amygdala Neurons. J. Physiol. 1999, 518, 525–538. [Google Scholar] [CrossRef]
- Sibille, E.; Pavlides, C.; Benke, D.; Toth, M. Genetic Inactivation of the Serotonin 1A Receptor in Mice Results in Downregulation of Major GABA A Receptor α Subunits, Reduction of GABA A Receptor Binding, and Benzodiazepine-Resistant Anxiety. J. Neurosci. 2000, 20, 2758–2765. [Google Scholar] [CrossRef]
- Stutzmann, G.E.; LeDoux, J.E. GABAergic Antagonists Block the Inhibitory Effects of Serotonin in the Lateral Amygdala: A Mechanism for Modulation of Sensory Inputs Related to Fear Conditioning. J. Neurosci. 1999, 19, RC8. [Google Scholar] [CrossRef] [PubMed]
- Gundlah, C.; Pecins-Thompson, M.; Schutzer, W.E.; Bethea, C.L. Ovarian Steroid Effects on Serotonin 1A, 2A and 2C Receptor MRNA in Macaque Hypothalamus. Mol. Brain Res. 1999, 63, 325–339. [Google Scholar] [CrossRef]
- Osterlund, M.K.; Halldin, C.; Hurd, Y.L. Effects of Chronic 17beta-Estradiol Treatment on the Serotonin 5-HT(1A) Receptor MRNA and Binding Levels in the Rat Brain. Synapse 2000, 35, 39–44. [Google Scholar] [CrossRef]
- Pitkänen, A.; Amaral, D.G. The Distribution of GABAergic Cells, Fibers, and Terminals in the Monkey Amygdaloid Complex: An Immunohistochemical and in Situ Hybridization Study. J. Neurosci. 1994, 14, 2200–2224. [Google Scholar] [CrossRef] [PubMed]
- Capogna, M. GABAergic Cell Type Diversity in the Basolateral Amygdala. Curr. Opin. Neurobiol. 2014, 26, 110–116. [Google Scholar] [CrossRef]
- Prager, E.M.; Bergstrom, H.C.; Wynn, G.H.; Braga, M.F.M. The Basolateral Amygdala GABAergic System in Health and Disease. J. Neurosci. Res. 2016, 94, 548–567. [Google Scholar] [CrossRef]
- Kemppainen, S.; Pitkänen, A. Distribution of Parvalbumin, Calretinin, and Calbindin-D(28k) Immunoreactivity in the Rat Amygdaloid Complex and Colocalization with Gamma-Aminobutyric Acid. J. Comp. Neurol. 2000, 426, 441–467. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mascagni, F. Colocalization of Calcium-Binding Proteins and GABA in Neurons of the Rat Basolateral Amygdala. Neuroscience 2001, 105, 681–693. [Google Scholar] [CrossRef]
- Mascagni, F.; Muly, E.C.; Rainnie, D.G.; McDonald, A.J. Immunohistochemical Characterization of Parvalbumin-Containing Interneurons in the Monkey Basolateral Amygdala. Neuroscience 2009, 158, 1541–1550. [Google Scholar] [CrossRef][Green Version]
- Sorvari, H.; Soininen, H.; Paljärvi, L.; Karkola, K.; Pitkänen, A. Distribution of Parvalbumin-Immunoreactive Cells and Fibers in the Human Amygdaloid Complex. J. Comp. Neurol. 1995, 360, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Sorvari, H.; Miettinen, R.; Soininen, H.; Pitkänen, A. Parvalbumin-Immunoreactive Neurons Make Inhibitory Synapses on Pyramidal Cells in the Human Amygdala: A Light and Electron Microscopic Study. Neurosci. Lett. 1996, 217, 93–96. [Google Scholar] [CrossRef]
- Woodruff, A.R.; Sah, P. Inhibition and Synchronization of Basal Amygdala Principal Neuron Spiking by Parvalbumin-Positive Interneurons. J. Neurophysiol. 2007, 98, 2956–2961. [Google Scholar] [CrossRef]
- Muller, J.F.; Mascagni, F.; McDonald, A.J. Postsynaptic Targets of Somatostatin-Containing Interneurons in the Rat Basolateral Amygdala. J. Comp. Neurol. 2007, 500, 513–529. [Google Scholar] [CrossRef]
- Gulyás, A.I.; Hájos, N.; Freund, T.F. Interneurons Containing Calretinin Are Specialized to Control Other Interneurons in the Rat Hippocampus. J. Neurosci. 1996, 16, 3397–3411. [Google Scholar] [CrossRef]
- Melchitzky, D.S.; Lewis, D.A. Dendritic-Targeting GABA Neurons in Monkey Prefrontal Cortex: Comparison of Somatostatin- and Calretinin-Immunoreactive Axon Terminals. Synapse 2008, 62, 456–465. [Google Scholar] [CrossRef]
- Meskenaite, V. Calretinin-Immunoreactive Local Circuit Neurons in Area 17 of the Cynomolgus Monkey, Macaca Fascicularis. J. Comp. Neurol. 1997, 379, 113–132. [Google Scholar] [CrossRef]
- Gonchar, Y.; Burkhalter, A. Connectivity of GABAergic Calretinin-Immunoreactive Neurons in Rat Primary Visual Cortex. Cereb. Cortex 1999, 9, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Kritzer, M.F. Regional, Laminar and Cellular Distribution of Immunoreactivity for ER in the Cerebral Cortex of Hormonally Intact, Postnatally Developing Male and Female Rats. Cereb. Cortex 2005, 16, 1181–1192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Równiak, M. The Neurons Expressing Calcium-Binding Proteins in the Amygdala of the Guinea Pig: Precisely Designed Interface for Sex Hormones. Brain Struct. Funct. 2017, 222, 3775–3793. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Y.; Zeng, P.; Qu, N.; Ning, L.-N.; Chu, J.; Zhang, T.; Zhou, X.-W.; Tian, Q. Evidence of Altered Depression and Dementia-Related Proteins in the Brains of Young Rats after Ovariectomy. J. Neurochem. 2018, 146, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Koonce, C.J.; Frye, C.A. Estradiol or Diarylpropionitrile Decrease Anxiety-like Behavior of Wildtype, but Not Estrogen Receptor Beta Knockout, Mice. Behav. Neurosci. 2008, 122, 974–981. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Mascagni, F. Immunohistochemical Characterization of Somatostatin Containing Interneurons in the Rat Basolateral Amygdala. Brain Res. 2002, 943, 237–244. [Google Scholar] [CrossRef]
- Dombret, C.; Naulé, L.; Trouillet, A.-C.; Parmentier, C.; Hardin-Pouzet, H.; Mhaouty-Kodja, S. Effects of Neural Estrogen Receptor Beta Deletion on Social and Mood-Related Behaviors and Underlying Mechanisms in Male Mice. Sci. Rep. 2020, 10, 6242. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.E.; Vandenput, L.; Tivesten, Å.; Norlén, A.-K.; Lagerquist, M.K.; Windahl, S.H.; Börjesson, A.E.; Farman, H.H.; Poutanen, M.; Benrick, A.; et al. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology 2015, 156, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Równiak, M.; Bogus-Nowakowska, K.; Robak, A. The Densities of Calbindin and Parvalbumin, but Not Calretinin Neurons, Are Sexually Dimorphic in the Amygdala of the Guinea Pig. Brain Res. 2015, 1604, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Bentea, E.; Van der Perren, A.; Van Liefferinge, J.; El Arfani, A.; Albertini, G.; Demuyser, T.; Merckx, E.; Michotte, Y.; Smolders, I.; Baekelandt, V.; et al. Nigral Proteasome Inhibition in Mice Leads to Motor and Non-Motor Deficits and Increased Expression of Ser129 Phosphorylated α-Synuclein. Front. Behav. Neurosci. 2015, 9, 68. [Google Scholar] [CrossRef]
- Du, T.; Chen, Y.; Shi, L.; Liu, D.; Liu, Y.; Yuan, T.; Zhang, X.; Zhu, G.; Zhang, J. Deep Brain Stimulation of the Anterior Nuclei of the Thalamus Relieves Basal Ganglia Dysfunction in Monkeys with Temporal Lobe Epilepsy. CNS Neurosci. Ther. 2021, 27, 341–351. [Google Scholar] [CrossRef]
- Deng, J.V.; Wan, Y.; Wang, X.; Cohen, S.; Wetsel, W.C.; Greenberg, M.E.; Kenny, P.J.; Calakos, N.; West, A.E. MeCP2 Phosphorylation Limits Psychostimulant-Induced Behavioral and Neuronal Plasticity. J. Neurosci. 2014, 34, 4519–4527. [Google Scholar] [CrossRef]
- Molgaard, S.; Ulrichsen, M.; Boggild, S.; Holm, M.-L.; Vaegter, C.; Nyengaard, J.; Glerup, S. Immunofluorescent Visualization of Mouse Interneuron Subtypes. F1000Res 2014, 3, 242. [Google Scholar] [CrossRef]
- Równiak, M.; Kolenkiewicz, M.; Kozłowska, A. Parvalbumin, but Not Calretinin, Neurons Express High Levels of A1-Containing GABAA Receptors, A7-Containing Nicotinic Acetylcholine Receptors and D2-Dopamine Receptors in the Basolateral Amygdala of the Rat. J. Chem. Neuroanat. 2017, 86, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Ren, S.; Gao, P.; Wan, D.; Rong, S.; Li, X.; Liu, S.; Xu, S.; Sun, K.; Guo, B.; et al. ALG13 Participates in Epileptogenesis via Regulation of GABAA Receptors in Mouse Models. Cell Death Discov. 2020, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiao, Y.-Y.; Sun, Q.-Q. Developmental Maturation of Excitation and Inhibition Balance in Principal Neurons across Four Layers of Somatosensory Cortex. Neuroscience 2011, 174, 10–25. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Gulf Professional Publishing: Oxford, UK, 2004; ISBN 978-0-12-547640-9. [Google Scholar]
- Sathyanesan, A.; Ogura, T.; Lin, W. Automated Measurement of Nerve Fiber Density Using Line Intensity Scan Analysis. J. Neurosci. Methods 2012, 206, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Meijering, E. FeatureJ: A Java Package for Image Feature Extraction. Available online: https://imagescience.org/meijering/software/featurej/ (accessed on 17 March 2021).
- McDonald, A.J.; Mascagni, F. Parvalbumin-Containing Interneurons in the Basolateral Amygdala Express High Levels of the ?1 Subunit of the GABAA Receptor. J. Comp. Neurol. 2004, 473, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.A.; Humpel, C.; Alheid, G.F.; Marksteiner, J. Compartmentation of Alpha 1 and Alpha 2 GABAA Receptor Subunits within Rat Extended Amygdala: Implications for Benzodiazepine Action. Brain Res. 2003, 964, 91–99. [Google Scholar] [CrossRef]
- Hörtnagl, H.; Tasan, R.O.; Wieselthaler, A.; Kirchmair, E.; Sieghart, W.; Sperk, G. Patterns of MRNA and Protein Expression for 12 GABAA Receptor Subunits in the Mouse Brain. Neuroscience 2013, 236, 345–372. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; McGrath, J.J.; Reynolds, G.P. Neuronal Calcium-Binding Proteins and Schizophrenia. Schizophr. Res. 2002, 57, 27–34. [Google Scholar] [CrossRef]
- Köhr, G.; Lambert, C.E.; Mody, I. Calbindin-D28K (CaBP) Levels and Calcium Currents in Acutely Dissociated Epileptic Neurons. Exp. Brain Res. 1991, 85, 543–551. [Google Scholar] [CrossRef]
- Chard, P.S.; Jordan, J.; Marcuccilli, C.J.; Miller, R.J.; Leiden, J.M.; Roos, R.P.; Ghadge, G.D. Regulation of Excitatory Transmission at Hippocampal Synapses by Calbindin D28k. Proc. Natl. Acad. Sci. USA 1995, 92, 5144–5148. [Google Scholar] [CrossRef]
- Harris, E.P.; Abel, J.M.; Tejada, L.D.; Rissman, E.F. Calbindin Knockout Alters Sex-Specific Regulation of Behavior and Gene Expression in Amygdala and Prefrontal Cortex. Endocrinology 2016, 157, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Benes, F.M.; Kwok, E.W.; Vincent, S.L.; Todtenkopf, M.S. A Reduction of Nonpyramidal Cells in Sector CA2 of Schizophrenics and Manic Depressives. Biol. Psychiatry 1998, 44, 88–97. [Google Scholar] [CrossRef]
- Kaalund, S.S.; Riise, J.; Broberg, B.V.; Fabricius, K.; Karlsen, A.S.; Secher, T.; Plath, N.; Pakkenberg, B. Differential Expression of Parvalbumin in Neonatal Phencyclidine-Treated Rats and Socially Isolated Rats. J. Neurochem. 2013, 124, 548–557. [Google Scholar] [CrossRef]
- Schwaller, B. The Continuing Disappearance of “Pure” Ca2+ Buffers. Cell. Mol. Life Sci. 2009, 66, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, L.; Christel, C.J.; Benveniste, M.; Schwaller, B.; Lee, A. Compensatory Regulation of Cav2.1 Ca2+ Channels in Cerebellar Purkinje Neurons Lacking Parvalbumin and Calbindin D-28k. J. Neurophysiol 2010, 103, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, B. Emerging Functions of the “Ca2+ Buffers” Parvalbumin, Calbindin D-28k and Calretinin in the Brain. In Handbook of Neurochemistry and Molecular Neurobiology: Neural Protein Metabolism and Function; Lajtha, A., Banik, N., Eds.; Springer: Boston, MA, USA, 2007; pp. 197–221. ISBN 978-0-387-30379-6. [Google Scholar]
- Rozov, A.; Burnashev, N.; Sakmann, B.; Neher, E. Transmitter Release Modulation by Intracellular Ca2+ Buffers in Facilitating and Depressing Nerve Terminals of Pyramidal Cells in Layer 2/3 of the Rat Neocortex Indicates a Target Cell-Specific Difference in Presynaptic Calcium Dynamics. J. Physiol. 2001, 531, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Blatow, M.; Caputi, A.; Burnashev, N.; Monyer, H.; Rozov, A. Ca2+ Buffer Saturation Underlies Paired Pulse Facilitation in Calbindin-D28k-Containing Terminals. Neuron 2003, 38, 79–88. [Google Scholar] [CrossRef]
- Tasan, R.O.; Bukovac, A.; Peterschmitt, Y.N.; Sartori, S.B.; Landgraf, R.; Singewald, N.; Sperk, G. Altered GABA Transmission in a Mouse Model of Increased Trait Anxiety. Neuroscience 2011, 183, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.; Vollmayr, B.; Gebicke-Haerter, P.J.; Henn, F.A. Reduced Expression of GABA Transporter GAT3 in Helpless Rats, an Animal Model of Depression. Neurochem. Res. 2009, 34, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Sun, Y.-X.; Liu, X.; Wang, H.; Ma, Y.-N.; Su, Y.-A.; Li, J.-T.; Si, T.-M. Adolescent Stress Increases Depression-like Behaviors and Alters the Excitatory-Inhibitory Balance in Aged Mice. Chin. Med. J. 2019, 132, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Xu, A.; Cui, S.; Sun, M.-R.; Xue, Y.-C.; Wang, J.-H. Impaired GABA Synthesis, Uptake and Release Are Associated with Depression-like Behaviors Induced by Chronic Mild Stress. Transl. Psychiatry 2016, 6, e910. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Prakash, R.; Kim, S.-Y.; Fenno, L.E.; Grosenick, L.; Zarabi, H.; Thompson, K.R.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Amygdala Circuitry Mediating Reversible and Bidirectional Control of Anxiety. Nature 2011, 471, 358–362. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Wang, K.; Chen, L.; Jiang, P. Neuroprotective Effects of Vitamin D and 17ß-Estradiol against Ovariectomy-Induced Neuroinflammation and Depressive-like State: Role of the AMPK/NF-ΚB Pathway. Int. Immunopharmacol. 2020, 86, 106734. [Google Scholar] [CrossRef]
- Gadea, A.; López-Colomé, A.M. Glial Transporters for Glutamate, Glycine, and GABA: II. GABA Transporters. J. Neurosci. Res. 2001, 63, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Chazalon, M.; Paredes-Rodriguez, E.; Morin, S.; Martinez, A.; Cristóvão-Ferreira, S.; Vaz, S.; Sebastiao, A.; Panatier, A.; Boué-Grabot, E.; Miguelez, C.; et al. GAT-3 Dysfunction Generates Tonic Inhibition in External Globus Pallidus Neurons in Parkinsonian Rodents. Cell Rep. 2018, 23, 1678–1690. [Google Scholar] [CrossRef]
- Patrone, C.; Andersson, S.; Korhonen, L.; Lindholm, D. Estrogen Receptor-Dependent Regulation of Sensory Neuron Survival in Developing Dorsal Root Ganglion. Proc. Natl. Acad. Sci. USA 1999, 96, 10905–10910. [Google Scholar] [CrossRef]
- Wang, L.; Andersson, S.; Warner, M.; Gustafsson, J.-A. Estrogen Receptor (ER) Knockout Mice Reveal a Role for ER in Migration of Cortical Neurons in the Developing Brain. Proc. Natl. Acad. Sci. 2003, 100, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Vargas, K.G.; Milic, J.; Zaciragic, A.; Wen, K.; Jaspers, L.; Nano, J.; Dhana, K.; Bramer, W.M.; Kraja, B.; van Beeck, E.; et al. The Functions of Estrogen Receptor Beta in the Female Brain: A Systematic Review. Maturitas 2016, 93, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Christakos, S. Regulation by Estrogen through the 5’-Flanking Region of the Mouse Calbindin-D28k Gene. Mol. Endocrinol. 1995, 9, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Shortall, S.E.; Brown, A.M.; Newton-Mann, E.; Dawe-Lane, E.; Evans, C.; Fowler, M.; King, M.V. Calbindin Deficits May Underlie Dissociable Effects of 5-HT6 and MGlu7 Antagonists on Glutamate and Cognition in a Dual-Hit Neurodevelopmental Model for Schizophrenia. Mol. Neurobiol. 2020, 57, 3439–3457. [Google Scholar] [CrossRef] [PubMed]
- Helmeke, C.; Ovtscharoff, W.; Poeggel, G.; Braun, K. Imbalance of Immunohistochemically Characterized Interneuron Populations in the Adolescent and Adult Rodent Medial Prefrontal Cortex after Repeated Exposure to Neonatal Separation Stress. Neuroscience 2008, 152, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Giachino, C.; Canalia, N.; Capone, F.; Fasolo, A.; Alleva, E.; Riva, M.A.; Cirulli, F.; Peretto, P. Maternal Deprivation and Early Handling Affect Density of Calcium Binding Protein-Containing Neurons in Selected Brain Regions and Emotional Behavior in Periadolescent Rats. Neuroscience 2007, 145, 568–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iritani, S.; Kuroki, N.; Ikeda, K.; Kazamatsuri, H. Calbindin Immunoreactivity in the Hippocampal Formation and Neocortex of Schizophrenics. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 409–421. [Google Scholar] [CrossRef]









| Antigen | Code | Clonality | Host Species | Dilution | Supplier | Location |
|---|---|---|---|---|---|---|
| Primary antibodies | ||||||
| NeuN | ABN78 | polyclonal | Rabbit | 1:1000 | Millipore | Temecula, CA, USA |
| CB | CB-38 | polyclonal | Rabbit | 1:4000 | SWANT | Bellinzona, Switzerland |
| PV | PV27 | polyclonal | Rabbit | 1:1000 | SWANT | Bellinzona, Switzerland |
| CR | 7697 | polyclonal | Rabbit | 1:1000 | SWANT | Bellinzona, Switzerland |
| SOM | MAB354 | monoclonal | Rat | 1:1000 | Millipore | Temecula, CA, USA |
| GABAA α1 | AB33299 | polyclonal | Rabbit | 1:2000 | Abcam | Cambridge, UK |
| VGAT | AB5062P | polyclonal | Rabbit | 1:3000 | Millipore | Temecula, CA, USA |
| GFAP | G9269 | polyclonal | Rabbit | 1:200 | Millipore | Temecula, CA, USA |
| Secondary antibodies | ||||||
| ImmPRESS HRP Universal Antibody (anti-rabbit Ig, Peroxidase) | 1:1 | Vector Laboratories | Burlingame, CA, USA | |||
| ALEXA Fluor 555 | A-31572 | polyclonal | Donkey anti-rabbit | 1:1000 | Thermo Fisher | Rockford, IL, USA |
| CY3 | 712-165-153 | polyclonal | Donkey anti-rat | 1:1000 | Jackson ImmunoResearch Laboratories | West Grove, PA, USA |
| Other reagents | ||||||
| 3,3-diaminobenzidine substrate chromogen | 3% | Dako Cytomation | Glostrup, Denmark | |||
| LA | BL | BM | ME | CE | CO | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | p | |||||||
| NeuN | t8.88 = 4.77 | 0.001057 | t9.70 = 6.88 | 0.000051 | t9.02 = 7.25 | 0.000048 | t8.98 = 6.74 | 0.000085 | t9.78 = 7.05 | 0.000039 | t6.66 = 4.86 | 0.002112 |
| CB | t7.27 = 4.74 | 0.001913 | t7.27 = 4.74 | 0.002045 | t9.55 = 4.16 | 0.004259 | t9.58 = 6.33 | 0.001030 | t9.99 = 4.46 | 0.001229 | t7.80 = 4.40 | 0.002435 |
| PV | t10 = 0.054 | 0.9581 | t10 = 0.62 | 0.5482 | t10 = 0.48 | 0.6642 | − | − | − | − | t10 = 0.46 | 0.6581 |
| CR | t10 = 0.53 | 0.6113 | t10 = 0.16 | 0.8787 | t10 = −0.24 | 0.8119 | t10 = 1.24 | 0.2426 | − | − | t10 = 2.13 | 0.0594 |
| SOM | t10 = −0.22 | 0.8274 | t10 = 0.08 | 0.9344 | t10 = −0.26 | 0.8036 | t10 = 0.17 | 0.8662 | t10 = 0.63 | 0.5441 | t10 = 0.16 | 0.8732 |
| GABAAα1 | t10 = 0.35 | 0.7337 | t10 = 0.11 | 0.9178 | t10 = −0.62 | 0.5478 | t10 = 0.08 | 0.9357 | t10 = −0.19 | 0.8513 | t10 = 0.11 | 0.9106 |
| Intensity of GABAAα1 | t10 = 0.94 | 0.3674 | t10 = 0.97 | 0.3536 | t10 = 1.24 | 0.2445 | t10 = −0.82 | 0.4334 | t10 = −0.85 | 0.4133 | t10 = −0.08 | 0.9379 |
| VGAT | t10 = −0.22 | 0.8274 | t10 = 0.08 | 0.9344 | t10 = −0.26 | 0.8036 | t10 = 0.17 | 0.8662 | t10 = 0.63 | p = 0.5441 | t10 = 0.16 | 0.8732 |
| Intensity of GABAAα1 | t10 = −0.57 | 0.5829 | t10 = −1.00 | 0.3432 | t10 = −0.29 | 0.7796 | t10 = −0.31 | 0.7607 | t10 = 0.19 | p = 0.8495 | t10 = 1.07 | 0.3091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowski, D.; Bogus-Nowakowska, K.; Kozłowska, A.; Równiak, M. Expression of Calbindin, a Marker of Gamma-Aminobutyric Acid Neurons, Is Reduced in the Amygdala of Oestrogen Receptor β-Deficient Female Mice. J. Clin. Med. 2022, 11, 1760. https://doi.org/10.3390/jcm11071760
Kalinowski D, Bogus-Nowakowska K, Kozłowska A, Równiak M. Expression of Calbindin, a Marker of Gamma-Aminobutyric Acid Neurons, Is Reduced in the Amygdala of Oestrogen Receptor β-Deficient Female Mice. Journal of Clinical Medicine. 2022; 11(7):1760. https://doi.org/10.3390/jcm11071760
Chicago/Turabian StyleKalinowski, Daniel, Krystyna Bogus-Nowakowska, Anna Kozłowska, and Maciej Równiak. 2022. "Expression of Calbindin, a Marker of Gamma-Aminobutyric Acid Neurons, Is Reduced in the Amygdala of Oestrogen Receptor β-Deficient Female Mice" Journal of Clinical Medicine 11, no. 7: 1760. https://doi.org/10.3390/jcm11071760
APA StyleKalinowski, D., Bogus-Nowakowska, K., Kozłowska, A., & Równiak, M. (2022). Expression of Calbindin, a Marker of Gamma-Aminobutyric Acid Neurons, Is Reduced in the Amygdala of Oestrogen Receptor β-Deficient Female Mice. Journal of Clinical Medicine, 11(7), 1760. https://doi.org/10.3390/jcm11071760







