Characterization of Distinct Microbiota Associated with Scalp Dermatitis in Patients with Atopic Dermatitis
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. DNA Extraction
2.3. Polymerase Chain Reaction Amplification and Sequencing
2.4. Sequence Processing and Diversity Estimation
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics and Results of Sample Clustering Analysis
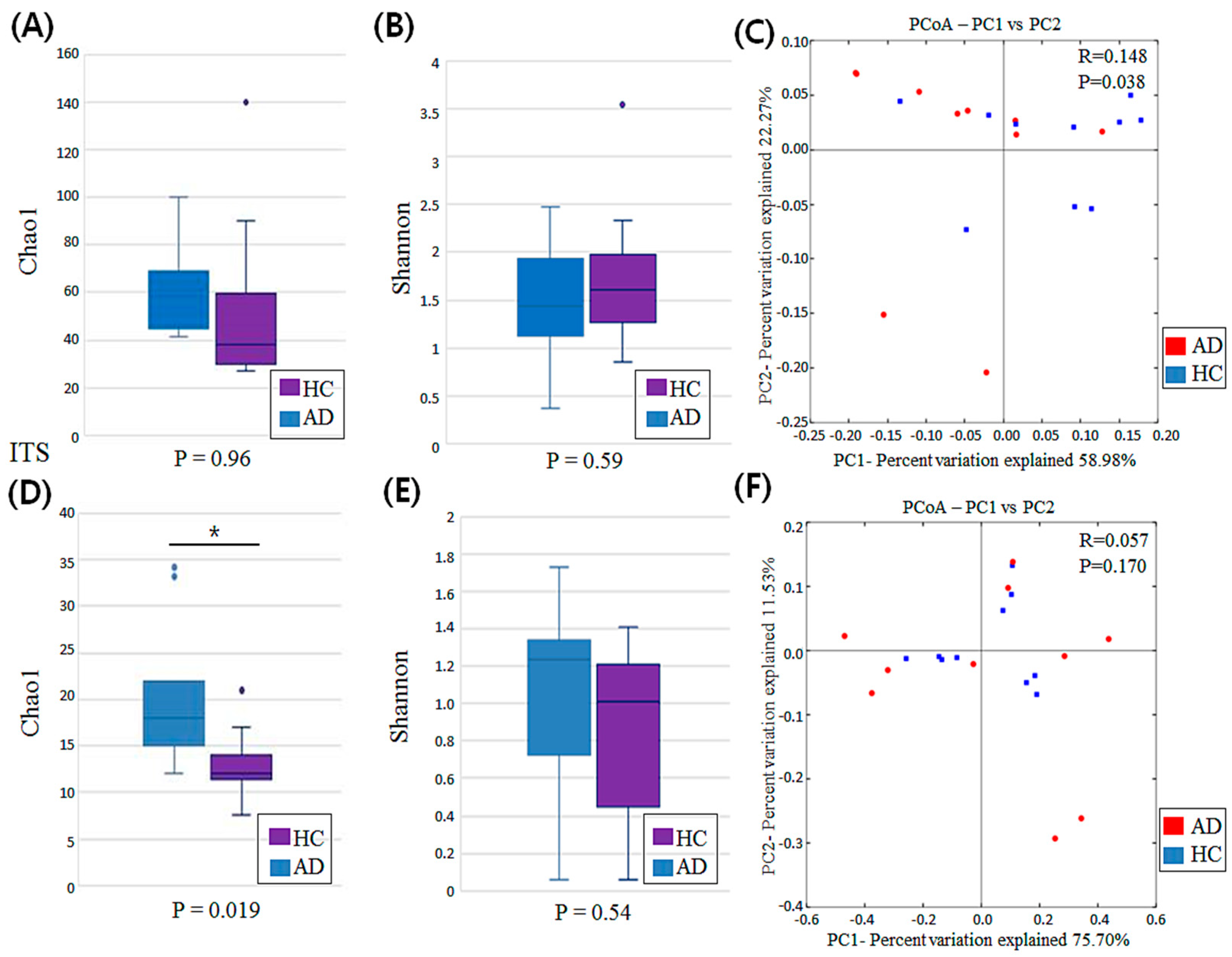
3.2. Diversity Analysis
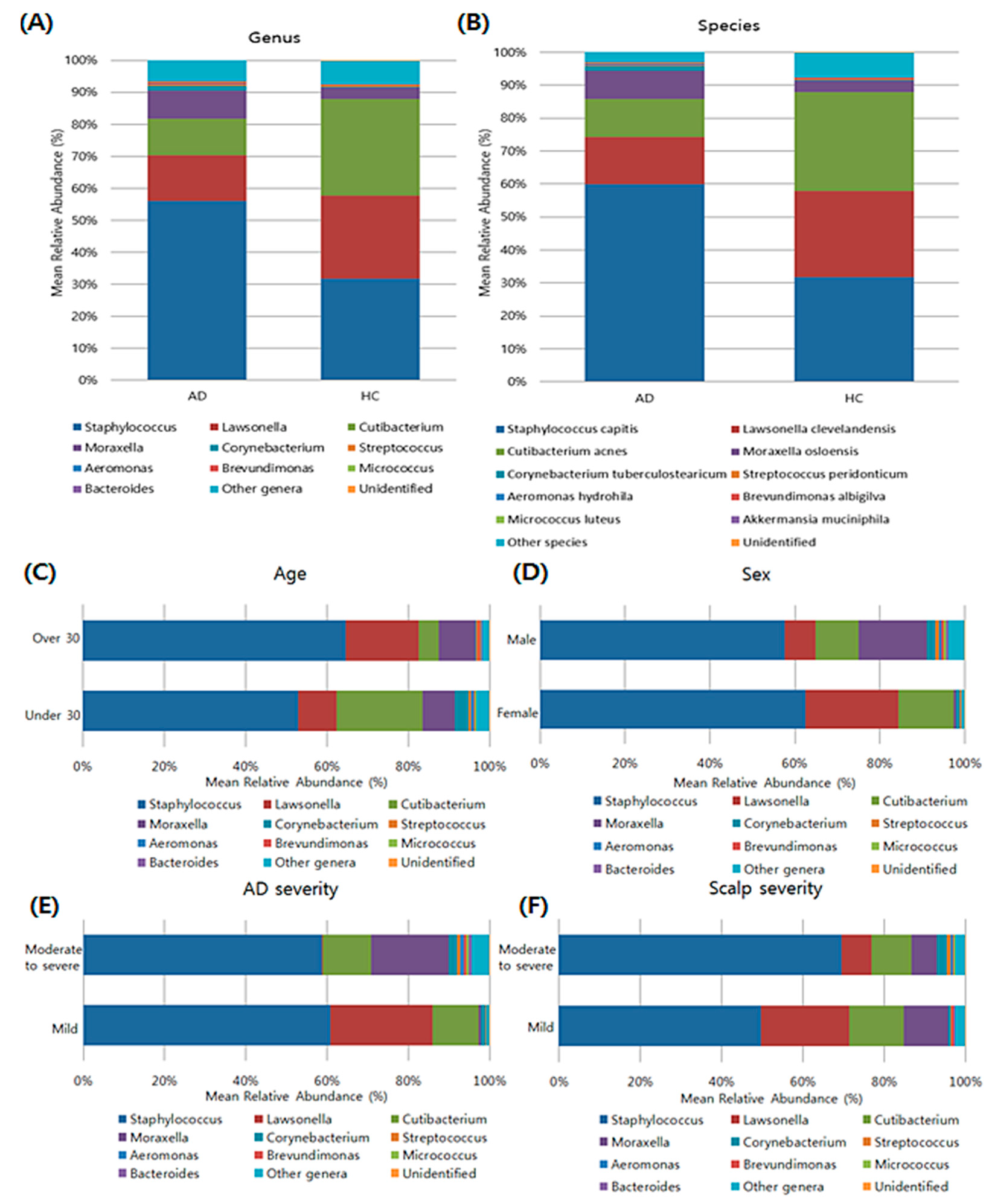
3.3. Composition of Bacterial Microbiota in the Scalp Based on Taxonomy
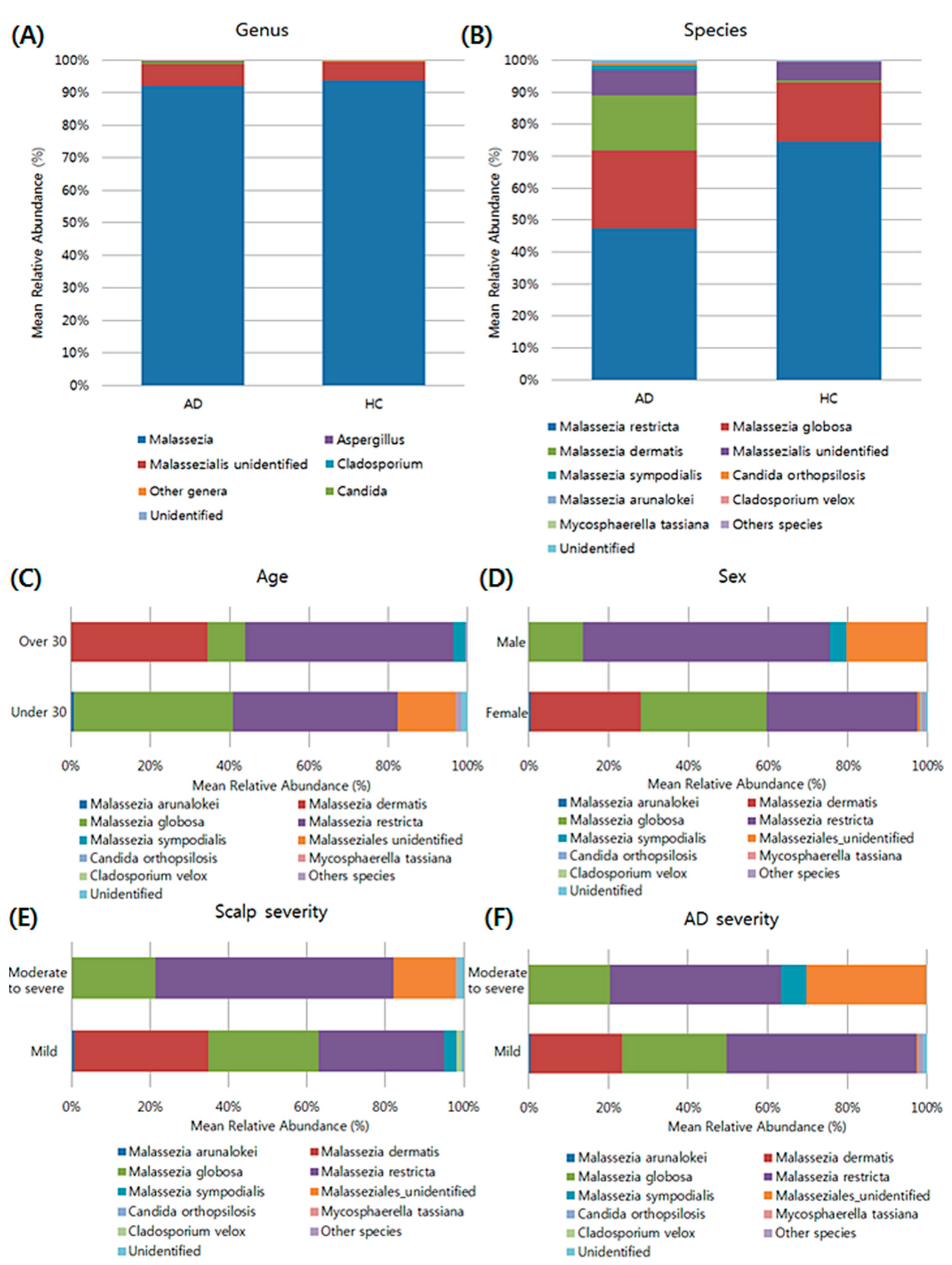
3.4. Composition of the Fungal Microbiota in the Scalp at the Taxonomic Assignment
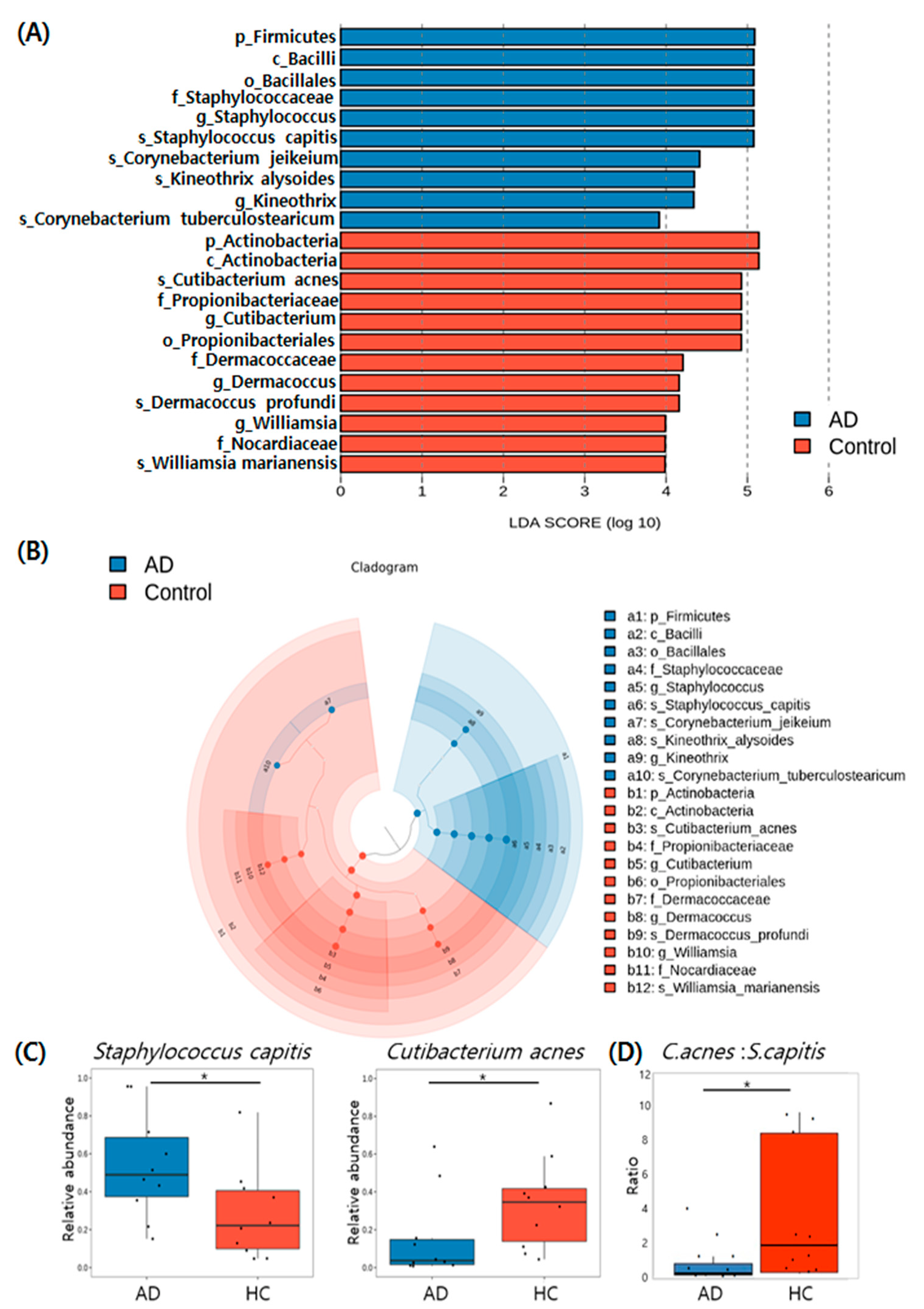
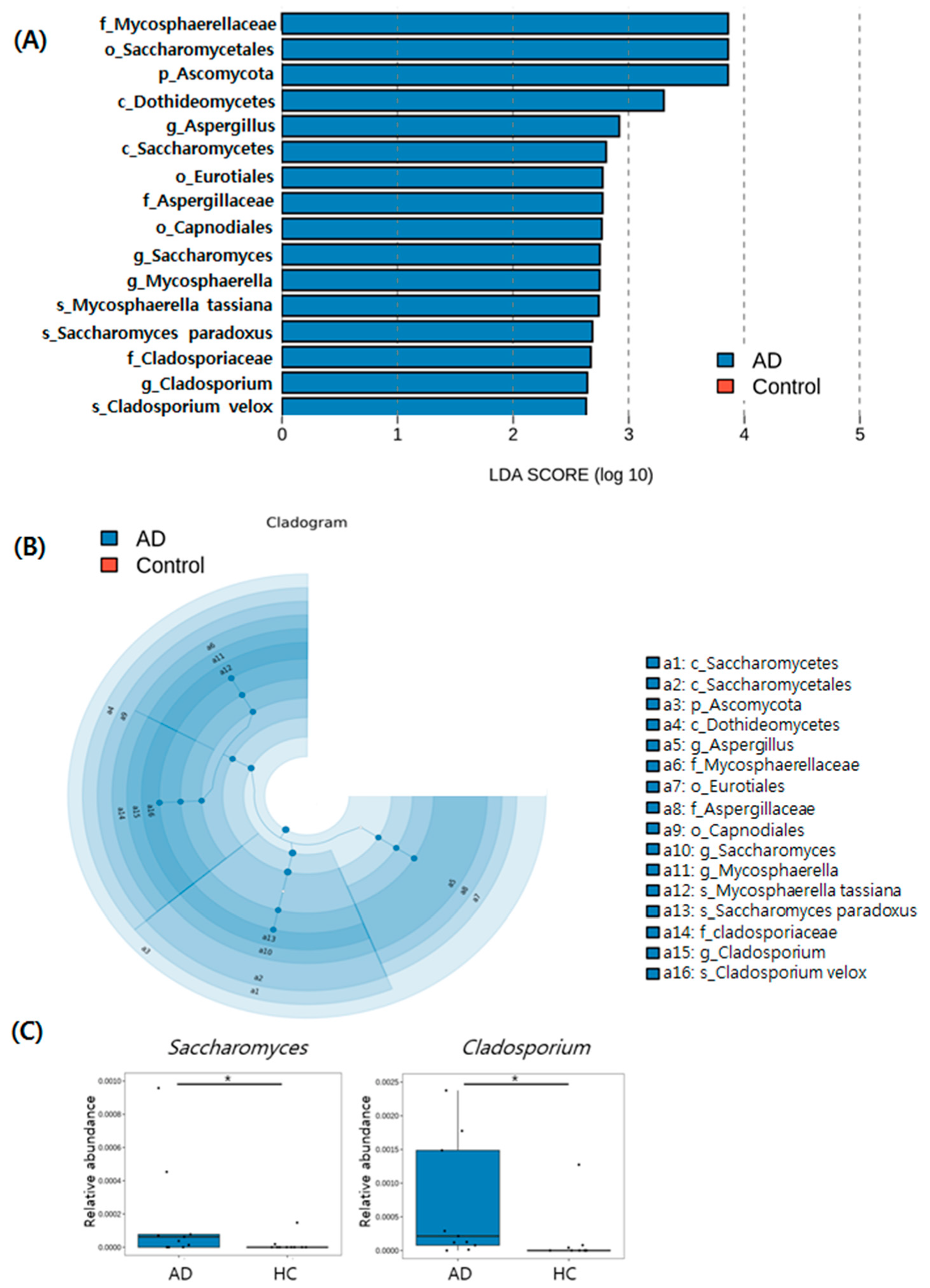
3.5. Taxonomic Biomarkers of AD and HC Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanifin, J.M.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 92, 44–47. [Google Scholar]
- Kwon, J.; Roh, K.Y.; Koh, B.K.; Kim, J.W. Clinical characteristics of adolescence and adult atopic dermatitis in Korea. Korean J. Dermatol. 2004, 42, 949–954. [Google Scholar]
- Kim, K.H.; Chung, J.H.; Park, K.C. Clinical evaluation of minor clinical features of atopic dermatitis. Ann. Dermatol. 1993, 5, 9–12. [Google Scholar] [CrossRef][Green Version]
- Shi, M.; Zhang, H.; Chen, X.; Guo, Y.; Tao, J.; Qi, H.; Gan, J.; Jiang, A.; Yu, H.; Liang, J. Clinical features of atopic dermatitis in a hospital-based setting in China. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.J.; Dhar, S.; Kaur, S. Evaluation of minor clinical features of atopic dermatitis. Pediatric Dermatol. 1991, 8, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 263–269. [Google Scholar] [CrossRef]
- Mallinckrodt, V. Colonization with superantigen-producing Staphylococcus aureus is associated with increased severity of atopic dermatitis. Clin. Exp. Allergy 2000, 30, 994–1000. [Google Scholar]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Glatz, M.; Jo, J.-H.; Kennedy, E.A.; Polley, E.C.; Segre, J.A.; Simpson, E.L.; Kong, H.H. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS ONE 2018, 13, e0192443. [Google Scholar] [CrossRef]
- Kubica, M.; Hildebrand, F.; Brinkman, B.M.; Goossens, D.; Del Favero, J.; Vercammen, K.; Cornelis, P.; Schröder, J.M.; Vandenabeele, P.; Raes, J. The skin microbiome of caspase-14-deficient mice shows mild dysbiosis. Exp. Dermatol. 2014, 23, 561–567. [Google Scholar] [CrossRef]
- Park, Y.L.; Kim, H.D.; Kim, K.H.; Kim, M.N.; Kim, J.W.; Ro, Y.S.; Park, C.W.; Lee, K.B.; Lee, A.Y.; Cho, S.H. Report from ADRG: A study on the diagnostic criteria of Korean atopic dermatitis. Korean J. Dermatol. 2006, 44, 659–663. [Google Scholar]
- Simpson, E.; Bissonnette, R.; Eichenfield, L.F.; Guttman-Yassky, E.; King, B.; Silverberg, J.I.; Beck, L.A.; Bieber, T.; Reich, K.; Kabashima, K. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): The development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J. Am. Acad. Dermatol. 2020, 83, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Li, W.; Fu, L.; Niu, B.; Wu, S.; Wooley, J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinf. 2012, 13, 656–668. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Yim, S.M.; Kim, J.Y.; Ko, J.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Molecular analysis of Malassezia microflora on the skin of the patients with atopic dermatitis. Ann. Dermatol. 2010, 22, 41–47. [Google Scholar] [CrossRef]
- Darabi, K.; Hostetler, S.G.; Bechtel, M.A.; Zirwas, M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J. Am. Acad. Dermatol. 2009, 60, 125–136. [Google Scholar] [CrossRef]
- Grimshaw, S.G.; Smith, A.M.; Arnold, D.S.; Xu, E.; Hoptroff, M.; Murphy, B. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLoS ONE 2019, 14, e0225796. [Google Scholar] [CrossRef]
- Edslev, S.M.; Olesen, C.M.; Nørreslet, L.B.; Ingham, A.C.; Iversen, S.; Lilje, B.; Clausen, M.-L.; Jensen, J.S.; Stegger, M.; Agner, T. Staphylococcal Communities on Skin Are Associated with Atopic Dermatitis and Disease Severity. Microorganisms 2021, 9, 432. [Google Scholar] [CrossRef]
- Soares, J.; Lopes, C.; Tavaria, F.; Delgado, L.; Pintado, M. A diversity profile from the staphylococcal community on atopic dermatitis skin: A molecular approach. J. Appl. Microbiol. 2013, 115, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Blicharz, L.; Rudnicka, L.; Samochocki, Z. Staphylococcus aureus: An underestimated factor in the pathogenesis of atopic dermatitis? Adv. Dermatol. Allergol. 2019, 36, 11. [Google Scholar] [CrossRef] [PubMed]
- Clavaud, C.; Jourdain, R.; Bar-Hen, A.; Tichit, M.; Bouchier, C.; Pouradier, F.; El Rawadi, C.; Guillot, J.; Ménard-Szczebara, F.; Breton, L. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS ONE 2013, 8, e58203. [Google Scholar] [CrossRef]
- Wang, L.; Clavaud, C.; Bar-Hen, A.; Cui, M.; Gao, J.; Liu, Y.; Liu, C.; Shibagaki, N.; Guéniche, A.; Jourdain, R. Characterization of the major bacterial–fungal populations colonizing dandruff scalps in Shanghai, China, shows microbial disequilibrium. Exp. Dermatol. 2015, 24, 398–400. [Google Scholar]
- Brüggemann, H.; Henne, A.; Hoster, F.; Liesegang, H.; Wiezer, A.; Strittmatter, A.; Hujer, S.; Dürre, P.; Gottschalk, G. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 2004, 305, 671–673. [Google Scholar] [CrossRef]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.-M. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef]
- Rozas, M.; Hart de Ruijter, A.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Furue, M.; Iida, K.; Imaji, M.; Nakahara, T. Microbiome analysis of forehead skin in patients with atopic dermatitis and healthy subjects: Implication of Staphylococcus and Corynebacterium. J. Dermatol. 2018, 45, 876–877. [Google Scholar] [CrossRef]
- Kobayashi, T.; Glatz, M.; Horiuchi, K.; Kawasaki, H.; Akiyama, H.; Kaplan, D.H.; Kong, H.H.; Amagai, M.; Nagao, K. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity 2015, 42, 756–766. [Google Scholar] [CrossRef]
- Totte, J.; Pardo, L.; Fieten, K.; Vos, M.; van den Broek, T.; Schuren, F.; Pasmans, S. Nasal and skin microbiomes are associated with disease severity in paediatric atopic dermatitis. Br. J. Dermatol. 2019, 181, 796–804. [Google Scholar] [CrossRef]
- Depner, M.; Ege, M.J.; Cox, M.J.; Dwyer, S.; Walker, A.W.; Birzele, L.T.; Genuneit, J.; Horak, E.; Braun-Fahrländer, C.; Danielewicz, H. Bacterial microbiota of the upper respiratory tract and childhood asthma. J. Allergy Clin. Immunol. 2017, 139, 826–834.e813. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Andersson, B.; Clavel, T.; Common, J.E.; Jackson, S.A.; Olson, N.D.; Segre, J.A.; Traidl-Hoffmann, C. Performing skin microbiome research: A method to the madness. J. Investig. Dermatol. 2017, 137, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, T.; Yun, J.I.; Lim, H.W.; Han, N.R.; Lee, S.T. Investigation of age-related changes in the skin microbiota of Korean women. Microorganisms 2020, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.H.; Song, Y.C.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Comparison of Nested PCR and RFLP for Identification and Classification of Malassezia Yeasts from Healthy Human Skin. Ann Derm. 2009, 21, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Nikkels, A.F.; Piérard, G.E. Framing the future of antifungals in atopic dermatitis. Dermatology 2003, 206, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Lintu, P.; Savolainen, J.; Kortekangas-Savolainen, O.; Kalimo, K. Systemic ketoconazole is an effective treatment of atopic dermatitis with IgE-mediated hypersensitivity to yeasts. Allergy 2001, 56, 512–517. [Google Scholar] [CrossRef]
- Soares, R.C.; Camargo-Penna, P.H.; de Moraes, V.; De Vecchi, R.; Clavaud, C.; Breton, L.; Braz, A.S.; Paulino, L.C. Dysbiotic bacterial and fungal communities not restricted to clinically affected skin sites in dandruff. Front. Cell. Infect. Microbiol. 2016, 6, 157. [Google Scholar] [CrossRef]
- Johansson, C.; Sandström, M.; Bartosik, J.; Särnhult, T.; Christiansen, J.; Zargari, A.; Bäck, O.; Wahlgren, C.; Faergemann, J.; Scheynius, A. Atopy patch test reactions to Malassezia allergens differentiate subgroups of atopic dermatitis patients. Br. J. Dermatol. 2003, 148, 479–488. [Google Scholar] [CrossRef]
- Andersson, A.; Scheynius, A.; Rasool, O. Detection of Mala f and Mala s allergen sequences within the genus Malassezia. Med. Mycol. 2003, 41, 479–485. [Google Scholar] [CrossRef][Green Version]
- Selander, C.; Zargari, A.; Möllby, R.; Rasool, O.; Scheynius, A. Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens. Allergy 2006, 61, 1002–1008. [Google Scholar] [CrossRef]
- Gehrmann, U.; Qazi, K.R.; Johansson, C.; Hultenby, K.; Karlsson, M.; Lundeberg, L.; Gabrielsson, S.; Scheynius, A. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses–novel mechanisms for host-microbe interactions in atopic eczema. PLoS ONE 2011, 6, e21480. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, T.; Hiragun, T.; Ishii, K.; Hiragun, M.; Kamegashira, A.; Hide, M. Different hypersensitivities against homologous proteins of MGL_1304 in patients with atopic dermatitis. Allergol. Int. 2018, 67, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Bjerre, R.; Bandier, J.; Skov, L.; Engstrand, L.; Johansen, J.D. The role of the skin microbiome in atopic dermatitis: A systematic review. Br. J. Dermatol. 2017, 177, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Celakovska, J.; Vankova, R.; Bukac, J.; Cermakova, E.; Andrys, C.; Krejsek, J. Atopic Dermatitis and Sensitisation to Molecular Components of Alternaria, Cladosporium, Penicillium, Aspergillus, and Malassezia—Results of Allergy Explorer ALEX 2. J. Fungi 2021, 7, 183. [Google Scholar] [CrossRef]
- Lin, Q.; Panchamukhi, A.; Li, P.; Shan, W.; Zhou, H.; Hou, L.; Chen, W. Malassezia and Staphylococcus dominate scalp microbiome for seborrheic dermatitis. Bioprocess Biosyst. Eng. 2021, 44, 965–975. [Google Scholar] [CrossRef]
- Sharma, A.; Laxman, B.; Naureckas, E.T.; Hogarth, D.K.; Sperling, A.I.; Solway, J.; Ober, C.; Gilbert, J.A.; White, S.R. Associations between fungal and bacterial microbiota of airways and asthma endotypes. J. Allergy Clin. Immunol. 2019, 144, 1214–1227.e1217. [Google Scholar] [CrossRef]
| Atopic Dermatitis (n = 10) | Healthy Control (n = 10) | |
|---|---|---|
| Age | 33.2 ± 11.80 (19–53) | 37.7 ± 10.36 (28–63) |
| Gender | ||
| Male | 5 (50%) | 5 (50%) |
| Female | 5 (50%) | 5 (50%) |
| EASI | N/A | |
| Mild (<6) | 6 (60%) | |
| Moderate to severe (≥6) | 4 (40%) | |
| vIGA-AD for scalp dermatitis | N/A | |
| Mild | 5 (50%) | |
| Moderate to severe | 5 (50%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, Y.R.; Cho, M.; Han, Y.; Lee, S.H.; Cho, S.H.; Lee, J.D.; Kim, H.S. Characterization of Distinct Microbiota Associated with Scalp Dermatitis in Patients with Atopic Dermatitis. J. Clin. Med. 2022, 11, 1735. https://doi.org/10.3390/jcm11061735
Woo YR, Cho M, Han Y, Lee SH, Cho SH, Lee JD, Kim HS. Characterization of Distinct Microbiota Associated with Scalp Dermatitis in Patients with Atopic Dermatitis. Journal of Clinical Medicine. 2022; 11(6):1735. https://doi.org/10.3390/jcm11061735
Chicago/Turabian StyleWoo, Yu Ri, Minah Cho, Yujin Han, Se Hoon Lee, Sang Hyun Cho, Jeong Deuk Lee, and Hei Sung Kim. 2022. "Characterization of Distinct Microbiota Associated with Scalp Dermatitis in Patients with Atopic Dermatitis" Journal of Clinical Medicine 11, no. 6: 1735. https://doi.org/10.3390/jcm11061735
APA StyleWoo, Y. R., Cho, M., Han, Y., Lee, S. H., Cho, S. H., Lee, J. D., & Kim, H. S. (2022). Characterization of Distinct Microbiota Associated with Scalp Dermatitis in Patients with Atopic Dermatitis. Journal of Clinical Medicine, 11(6), 1735. https://doi.org/10.3390/jcm11061735







