Analysis of Telomere Length and Its Implication in Neurocognitive Functions in Elderly Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Population
2.2. Variables
2.2.1. Telomere Measurements
2.2.2. Cognitive Functions Assessments
2.3. Statistical Procedures
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Flaxman, A.; Naghavi, M. HHS Public Access Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Li, S.-C.; Lindenberger, U.; Sikström, S. Aging cognition: From neuromodulation to representation. Trends Cogn. Sci. 2001, 5, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Heywood, R.; Gao, Q.; Nyunt, M.S.Z.; Feng, L.; Chong, M.S.; Lim, W.S.; Yap, P.; Lee, T.-S.; Yap, K.B.; Wee, S.-L.; et al. Hearing Loss and Risk of Mild Cognitive Impairment and Dementia: Findings from the Singapore Longitudinal Ageing Study. Dement. Geriatr. Cogn. Disord. 2017, 43, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell 1985, 43, 405–413. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Blasco, M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013, 29, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canela, A.; Vera, E.; Klatt, P.; Blasco, M.A. High-throughput telomere length quantification by FISH and its application to human population studies. Proc. Natl. Acad. Sci. USA 2007, 104, 5300–5305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.R.W.L.; Blackburn, E.H. Telomeres and telomerase. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Miao, K.; Wang, H.; Ding, H.; Wang, D.W. Association between telomere length and type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2013, 8, e79993. [Google Scholar] [CrossRef]
- Huzen, J.; Wong, L.S.M.; Van Veldhuisen, D.J.; Samani, N.J.; Zwinderman, A.H.; Codd, V.; Cawthon, R.M.; Benus, G.F.J.D.; Van Der Horst, I.C.C.; Navis, G.; et al. Telomere length loss due to smoking and metabolic traits. J. Intern. Med. 2014, 275, 155–163. [Google Scholar] [CrossRef]
- Best, J.R.; Davis, J.C.; Liu-Ambrose, T. Longitudinal analysis of physical performance, functional status, physical activity, and mood in relation to executive function in older adults who fall. J. Am. Geriatr. Soc. 2015, 63, 1112–1120. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Imamura, F.; Siscovick, D.S.; Jenny, N.S.; Fitzpatrick, A.L.; Mozaffarian, D. Physical Activity, Physical Fitness and Leukocyte Telomere Length: The Cardiovascular Health Study. Med. Sci. Sport Exerc. 2015, 47, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellami, M.; Bragazzi, N.; Prince, M.S.; Denham, J.; Elrayess, M. Regular, Intense Exercise Training as a Healthy Aging Lifestyle Strategy: Preventing DNA Damage, Telomere Shortening and Adverse DNA Methylation Changes Over a Lifetime. Front. Genet. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ruiz, C.; Dickinson, H.O.; Keys, B.; Rowan, E.; Kenny, R.A.; Von Zglinicki, T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann. Neurol. 2006, 60, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Lindquist, K.; Kluse, M.; Cawthon, R.; Harris, T.; Hsueh, W.-C.; Simonsick, E.M.; Kuller, L.; Li, R.; Ayonayon, H.N.; et al. Telomere length and cognitive function in community-dwelling elders: Findings from the Health ABC Study. Neurobiol. Aging 2011, 32, 2055–2060. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.L.; Lau, E.S.S.; Suen, E.W.C.; Lam, L.C.W.; Leung, P.C.; Woo, J.; Tang, N.L.S. Telomere length and cognitive function in southern Chinese community-dwelling male elders. Age Ageing 2013, 42, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Bendix, L.; Gade, M.M.; Staun, P.W.; Kimura, M.; Jeune, B.; Hjelmborg, J.V.B.; Aviv, A.; Christensen, K. Leukocyte telomere length and physical ability among Danish Twins age 70+. Mech. Ageing Dev. 2011, 132, 568–572. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.E.; Deary, I.J.; MacIntyre, A.; Lamb, K.J.; Radhakrishnan, K.; Starr, J.M.; Whalley, L.J.; Shiels, P.G. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci. Lett. 2006, 406, 260–264. [Google Scholar] [CrossRef]
- Leibel, D.K.; Shaked, D.; Moody, D.L.B.; Liu, H.B.; Weng, N.-P.; Evans, M.K.; Zonderman, A.B.; Waldstein, S.R. Telomere length and cognitive function: Differential patterns across sociodemographic groups. Neuropsychology 2020, 34, 186–188. [Google Scholar] [CrossRef]
- Theall, K.P.; Brett, Z.H.; Shirtcliff, E.A.; Dunn, E.C.; Drury, S.S. Exposure To Community Level Stress and Cellular Response. Soc. Sci. Med. 2014, 85, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Sánchez González, J.L.; Calvo Arenillas, J.I.; Sánchez Rodríguez, J.L. Programa de Revitalización Geriátrica: Efectos sobre la cognición en adultos mayores de 60 años. Fisioterapia 2019, 41, 266–274. [Google Scholar] [CrossRef]
- Sánchez-González, J.L.; Sánchez-Rodríguez, J.L.; Martín-Vallejo, J.; Martel-Martel, A.; González-Sarmiento, R. Effects of physical exercise on cognition and telomere length in healthy older women. Brain Sci. 2021, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Broer, L.; Codd, V.; Nyholt, D.R.; Deelen, J.; Mangino, M.; Willemsen, G.; Albrecht, E.; Amin, N.; Beekman, M.; de Geus, E.J.C.; et al. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 2013, 21, 1163–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haycock, P.C.; Integrative, M.R.C.; Unit, E.; Li, D.; Hunt, S.; Lin, K.; Försti, A. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Lillenes, M.S.; Rabano, A.; Støen, M.; Riaz, T.; Misaghian, D.; Møllersen, L.; Esbensen, Y.; Günther, C.C.; Selnes, P.; Stenset, V.T.V.; et al. Altered DNA base excision repair profile in brain tissue and blood in Alzheimer’s disease. Mol. Brain 2016, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedenreich, C.M.; Wang, Q.; Ting, N.S.; Brenner, D.R.; Conroy, S.M.; McIntyre, J.B.; Mickle, A.; Courneya, K.S.; Beattie, T. Effect of a 12-month exercise intervention on leukocyte telomere length: Results from the ALPHA Trial. Cancer Epidemiol. 2018, 56, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Weng, T.B.; Narayana-Kumanan, K.; Cole, R.C.; Wharff, C.; Reist, L.; DuBose, L.; Sigurdsson, G.; Mills, J.A.; Long, J.; et al. Acute exercise effects predict training change in cognition and connectivity. Med. Sci. Sports Exerc. 2021, 52, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Sanchez, M.B.H.; Pasquino, C.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G. Human Liver-Derived Stem Cells Improve Fibrosis and Inflammation Associated with Nonalcoholic Steatohepatitis. Stem Cells Int. 2019, 2019, 6351091. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Dutta, P.; Chard, N.; Wu, Y.; Chen, Q.H.; Chen, G.; Vadgama, J. A novel curcumin analog inhibits canonical and non-canonical functions of telomerase through STAT3 and NF-κB inactivation in colorectal cancer cells. Oncotarget 2019, 10, 4516–4531. [Google Scholar] [CrossRef] [Green Version]
- Lobo, A.; Ezquerra, J.; Escobar, V.; Seva Díaz, A. El Mini Examen Cognoscitivo. Un test sencillo y práctico para detectar alteraciones intelectuales en pacientes médicos. Act. Luso. Esp. Neurol. Psiquiatr. Cienc. Afines 1979, 7, 198–202. [Google Scholar]
- Benton, A.; Hamsher, K. Contributions to Neuropsychologic Assement; Oxford University Press: New York, NY, USA, 1983. [Google Scholar]
- Rey, A. Memorisation d’e une serie de 15 mots en 5 répétitions. In L´Examen Clinique en Psychologie; PUF: Paris, France, 1968. [Google Scholar]
- Bixby, W.R.; Spalding, T.W.; Haufler, A.J.; Deeny, S.P.; Mahlow, P.T.; Zimmerman, J.B.; Hatfield, B.D. The unique relation of physical activity to executive function in older men and women. Med. Sci. Sports Exerc. 2007, 39, 1408–1416. [Google Scholar] [CrossRef]
- Reitan, R. Trail Making Test. Manual for Administration and Scoring; Reitan Neuropsychology Laboratory: Tucson, AZ, USA, 1992. [Google Scholar]
- Mather, A.K.; Jorm, A.F.; Anstey, K.J.; Milburn, P.J.; Easteal, S.; Christensen, H. Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: A population study. BMC Geriatr. 2010, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, A.; Deary, I.; Gardner, J.; Kimura, M.; Lu, X.; Spector, T.; Aviv, A.; Cherkas, L. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol. Aging 2010, 31, 986–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zekry, D.; Herrmann, F.; Irminger-Finger, I.; Graf, C.; Genet, C.; Vitale, A.-M.; Michel, J.-P.; Gold, G.; Krause, K.-H. Telomere length and ApoE polymorphism in mild cognitive impairment, degenerative and vascular dementia. J. Neurol. Sci. 2010, 299, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, T.; Marksteiner, J.; Humpel, C. Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp. Gerontol. 2012, 47, 160–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movérare-Skrtic, S.; Johansson, P.; Mattsson, N.; Hansson, O.; Wallin, A.; Johansson, J.-O.; Zetterberg, H.; Blennow, K.; Svensson, J. Leukocyte Telomere Length [LTL] is reduced in stable mild cognitive impairment but low LTL is not associated with conversion to Alzheimer’s Disease: A pilot study. Exp. Gerontol. 2012, 47, 179–182. [Google Scholar] [CrossRef]
- Kaja, R.; Reyes, S.M.; Rossetti, H.C.; Brown, E.S. Association between telomere length and cognitive ability in a community-based sample. Neurobiol. Aging 2019, 75, 51–53. [Google Scholar] [CrossRef]
- Hägg, S.; NeuroCHARGE Cognitive Working Group; Zhan, Y.; Karlsson, R.; Gerritsen, L.; Ploner, A.; Van Der Lee, S.J.; Broer, L.; Deelen, J.; Marioni, R.; et al. Short telomere length is associated with impaired cognitive performance in European ancestry cohorts. Transl. Psychiatry 2017, 7, e1100. [Google Scholar] [CrossRef] [Green Version]
- Jeanclos, E.; Schork, N.J.; Kyvik, K.O.; Kimura, M.; Skurnick, J.H.; Aviv, A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 2000, 36, 195–200. [Google Scholar] [CrossRef]
- Aviv, A.; Valdes, A.; Gardner, J.P.; Swaminathan, R.; Kimura, M.; Spector, T.D. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J. Clin. Endocrinol. Metab. 2006, 91, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Evans, G.W.; Schamberg, M.A. Childhood poverty, chronic stress, and adult working memory. Proc. Natl. Acad. Sci. USA 2009, 106, 6545–6549. [Google Scholar] [CrossRef] [Green Version]
- Winkleby, M.A.; Kraemer, H.C.; Ahn, D.K.; Varady, A.N. Ethnic and Socioeconomic Differences in Cardiovascular Disease Risk Factors. JAMA 1998, 280, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
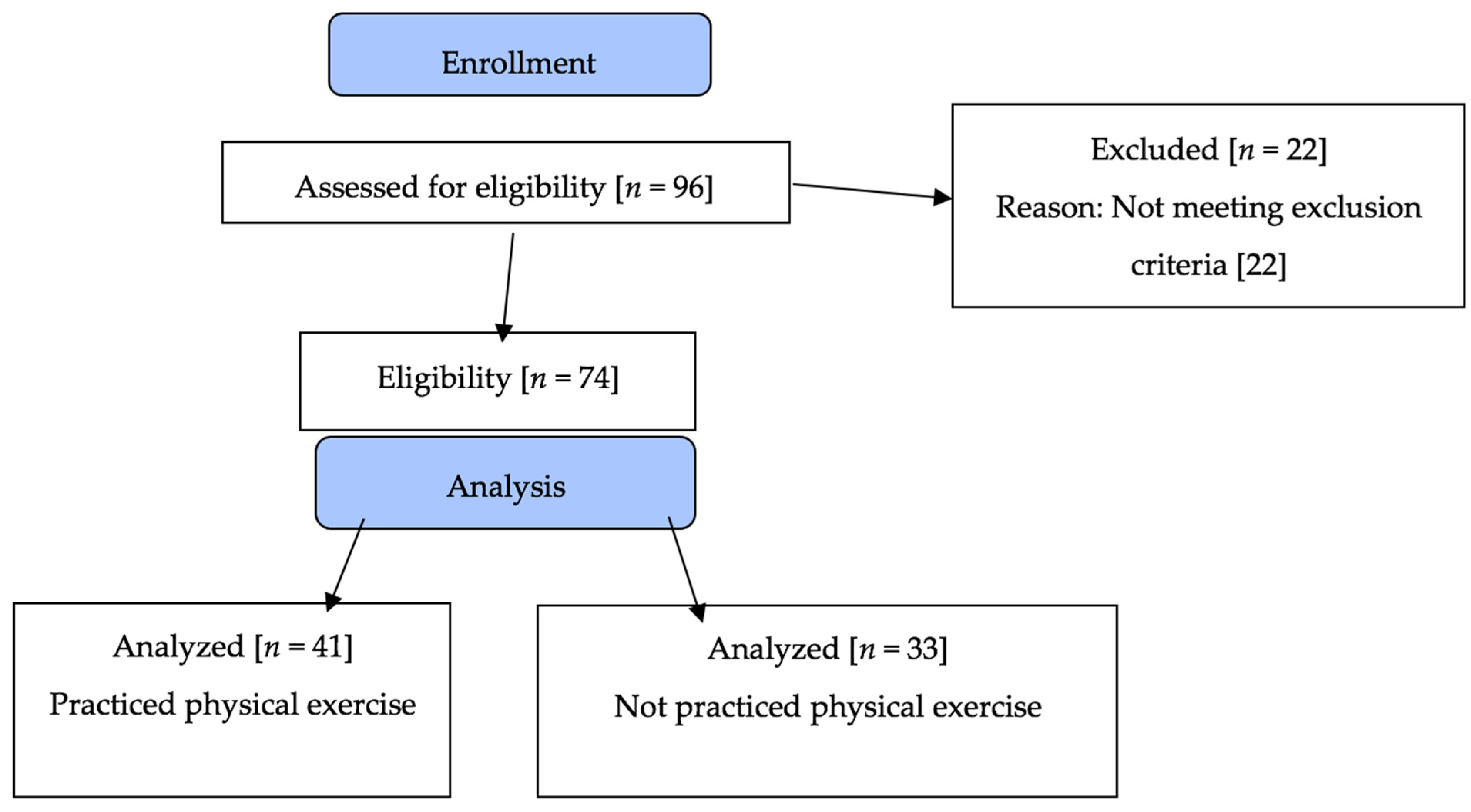
| Sociodemographic Variables | Inactive Group Mean [SD]/Counts | Active Group Mean [SD]/Counts | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 71.21 (±4.32) | 72.70 (±4.13) | 0.138 | ||||||
| Years of schooling | 8.42 (±2.56) | 8.18 (±1.55) | 0.576 | ||||||
| Marital Status | Single | Married | Widower | Single | Married | Widower | 0.238 | ||
| 9 (27.3%) | 19 (57.6%) | 5 (15.2%) | 6 (14.6%) | 30 (73.2%) | 5 (12.25%) | ||||
| Educational level | Primary/G.B.E 21 (63.6%) | Mid-Higher level 12 (36.4%) | Primary/G.B.E 20 (8.48) | Mid-Higher level 21 (51.2%) | 0.201 | ||||
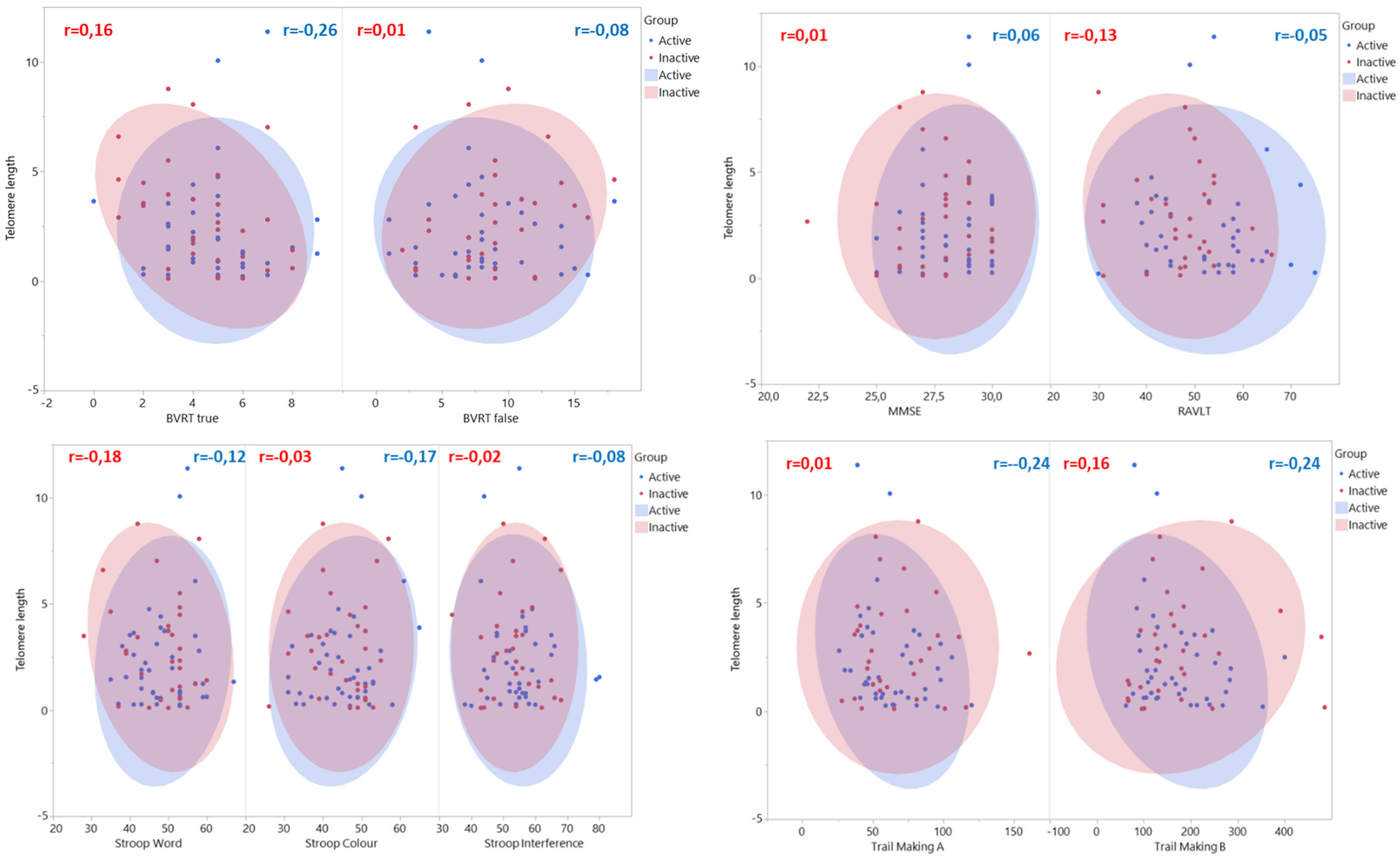
| Inactive Group (rp) | Active Group (rp) | Adjusted p-Values | |
|---|---|---|---|
| MMSE | 0.015 (n.s) | 0.057 (n.s) | 0.853 |
| RAVLT | −0.132 (n.s) | −0.022 (n.s) | 0.731 |
| BVRT true | −0.404 (n.s) | 0.078 (n.s) | 0.176 |
| BVRT false | 0.237 (n.s) | −0.153 (n.s) | 0.315 |
| Stroop word | −0.196 (n.s) | 0.119 (n.s) | 0.374 |
| Stroop colour | 0.014 (n.s) | 0.172 (n.s) | 0.731 |
| Stroop interference | 0.015 (n.s) | −0.097 (n.s) | 0.731 |
| Trail Making B | 0.194 (n.s) | −0.299 (n.s) | 0.176 |
| Trail Making A | 0.026 (n.s) | −0.274 (n.s) | 0.374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-González, J.L.; Sánchez-Rodríguez, J.L.; Juárez-Vela, R.; Ruiz de Viñaspre-Hernandez, R.; González-Sarmiento, R.; Martin-Vallejo, F.J. Analysis of Telomere Length and Its Implication in Neurocognitive Functions in Elderly Women. J. Clin. Med. 2022, 11, 1728. https://doi.org/10.3390/jcm11061728
Sánchez-González JL, Sánchez-Rodríguez JL, Juárez-Vela R, Ruiz de Viñaspre-Hernandez R, González-Sarmiento R, Martin-Vallejo FJ. Analysis of Telomere Length and Its Implication in Neurocognitive Functions in Elderly Women. Journal of Clinical Medicine. 2022; 11(6):1728. https://doi.org/10.3390/jcm11061728
Chicago/Turabian StyleSánchez-González, Juan Luis, Juan Luis Sánchez-Rodríguez, Raúl Juárez-Vela, Regina Ruiz de Viñaspre-Hernandez, Rogelio González-Sarmiento, and Francisco Javier Martin-Vallejo. 2022. "Analysis of Telomere Length and Its Implication in Neurocognitive Functions in Elderly Women" Journal of Clinical Medicine 11, no. 6: 1728. https://doi.org/10.3390/jcm11061728
APA StyleSánchez-González, J. L., Sánchez-Rodríguez, J. L., Juárez-Vela, R., Ruiz de Viñaspre-Hernandez, R., González-Sarmiento, R., & Martin-Vallejo, F. J. (2022). Analysis of Telomere Length and Its Implication in Neurocognitive Functions in Elderly Women. Journal of Clinical Medicine, 11(6), 1728. https://doi.org/10.3390/jcm11061728







