Goals of Care Conversations in Long-Term Care during the First Wave of the COVID-19 Pandemic
Abstract
:1. Introduction
1.1. Approach to Care Planning
- Ask the decision maker to describe their understanding of medical conditions and prognosis;
- Ask how much information the decision maker wants;
- Share information on prognosis to the degree desired;
- Elicit goals, including what is most important;
- Ask the decision maker to describe their fears and worries about future health;
- Ask the decision maker about trade-offs, such as what they are willing to accept to possibly gain more time;
- Develop treatment preferences based on goals.
- Relaying information to the decision maker. Many guidelines caution against providing prognostic information [4] and instead, tpically recommend that decision makers describe medical conditions early in the care planning conversation, putting the onus on the individual or their delegate to start the conversation. Yet, many decision makers do not fully appreciate the prognosis associated with frailty, nor the medical conditions that contribute to it [5]. Studies show that receiving realistic information is “very important” to those making medical decisions [6]. Further, providing details about co-morbid conditions and the probability of surviving cardiopulmonary resuscitation (CPR) significantly reduces the number of people who choose resuscitation [7]. Finally, providing information to the decision maker demonstrates the clinician’s understanding of the person’s health story, which allows both parties to consider the full picture. As such, early in the care planning conversation, MED-LTC clinicians described each medical condition, the expected progression of each illness, and the risk/benefit of treatments under consideration.
- Making recommendations. Care planning guidance proposes that physician recommendations “flow only from patient goals” [4]. Yet, when dealing with serious illness, individuals value physician recommendations [6,8]. In support of this evidence, the MED-LTC team made recommendations—especially when treatment had minimal benefit—while still encouraging decision makers to communicate and consider their preferences.
- Skill of counselor and time required. The Respecting Choices® model for care planning advocates a shift from physician counseling to care planning by trained facilitators and community volunteers, such as the clergy [9,10]. This objective does not consider the importance of clinician review of prognostic information, nor the expertise required for care planning conversations. Additionally, care planning recommendations do not address the time and compensation needed for an assessment and discussion related to frailty. MED-LTC assessment and communication typically took several hours.
- Values and goals. Care planning guidance suggests eliciting wishes, values, and preferences early in the conversation (#4–6 above). Instead, MED-LTC clinicians discussed preferences of the decision maker only after they understood the overall clinical prognosis and the risks and benefits of treatment.
- Capacity to make medical decisions. In a study of older patients who required medical decisions, up to 70% did not demonstrate a decision-making capacity [6]. Understanding the decision-making capacity of LTC residents is especially critical, as a significant proportion have dementia [11]. As such, MED-LTC consultations included routine assessment of decisional capacity as part of the care planning process.
- Documenting decisions. Care planning culminates in documented decisions on advance directive forms, such as physician orders for life-sustaining treatment (POLST), which generally do not describe the process used to make decisions. In addition, recorded decisions can be ambiguous, such as whether a ‘Do Not Resuscitate’ (DNR) code status implies a decision about intubation [12]. The lack of detailed, process-oriented documentation presents challenges for the follow-up and implementation of care plans. Thus, MED-LTC consultations included details of the care planning discussion, drivers of the decisions, and comprehensive information about care decisions.
- Revisiting decisions. Care planning typically consists of a single conversation, which result in decisions that remain in effect in perpetuity. The MED-LTC consultation process provided continuous and ongoing support to decision makers to help contextualize decisions when there was a change in health status.
1.2. Understanding Evidence
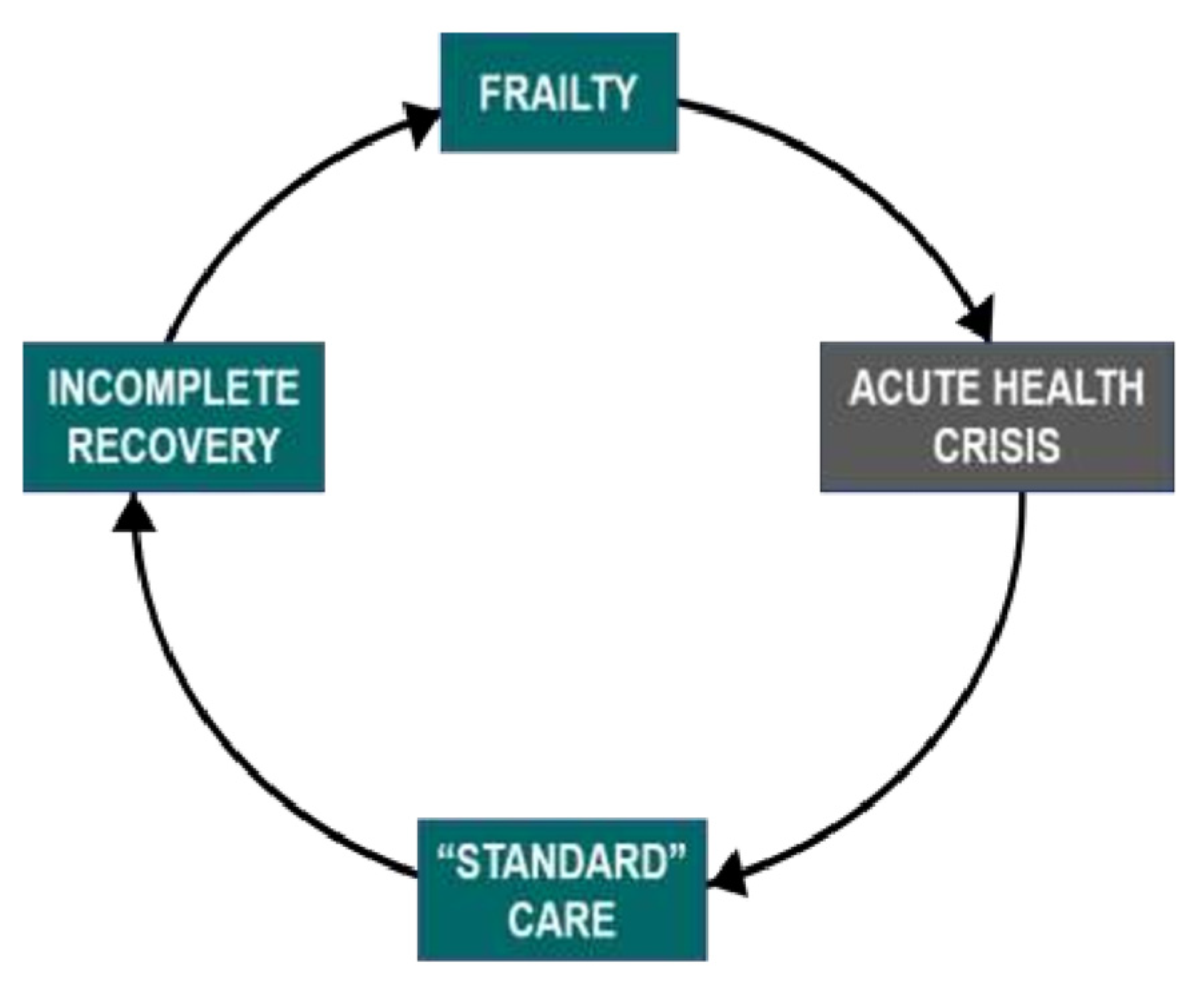
- Frailty. Frailty strongly correlates with the risk of morbidity and mortality [13]. It is typically associated with linear, gradual decline [14] in addition to stepwise deterioration following acute illness, after which there may be incomplete recovery and, thus, further frailty [13]. The sequence of decremental decline in health after acute illness can be understood as a frailty cycle (Figure 1) [15].
- 2.
- 3.
- Life expectancy for long-term care residents. In studies, median survival after admission to a nursing home ranged from 13.7 months to 2.7 years [17], while 1-year mortality ranged between 25% to 35% [18,19]. As such, many LTC residents are nearing the end of life. An important exception to this observation is for individuals with non-progressive disabilities (congenital or acquired).
- 4.
- CPR in long-term care facilities. In 10 studies of LTC residents, survival to hospital discharge after CPR ranged from 0% to 2.9% [20,21,22,23,24,25,26,27]. In three other studies, survival to hospital discharge was between 5% to 13% [27,28]. In a study of individuals with moderate or greater frailty based on the Clinical Frailty Scale, survival to hospital discharge following in-hospital cardiac arrest was 1.8% [29].
- 5.
- Prognosis related to SARS-CoV-2 infection during the first wave. The case fatality rate associated with SARS-CoV-2 infection varies by location and changes over time. Nonetheless, in all situations, mortality increases with age and poor health. During the first pandemic wave, individuals over age 80 with confirmed COVID-19 had a “death rate” of 21.9%, compared to 0.4% for younger individuals [30]. Both frailty [31] and dementia [32] appear to increase mortality.
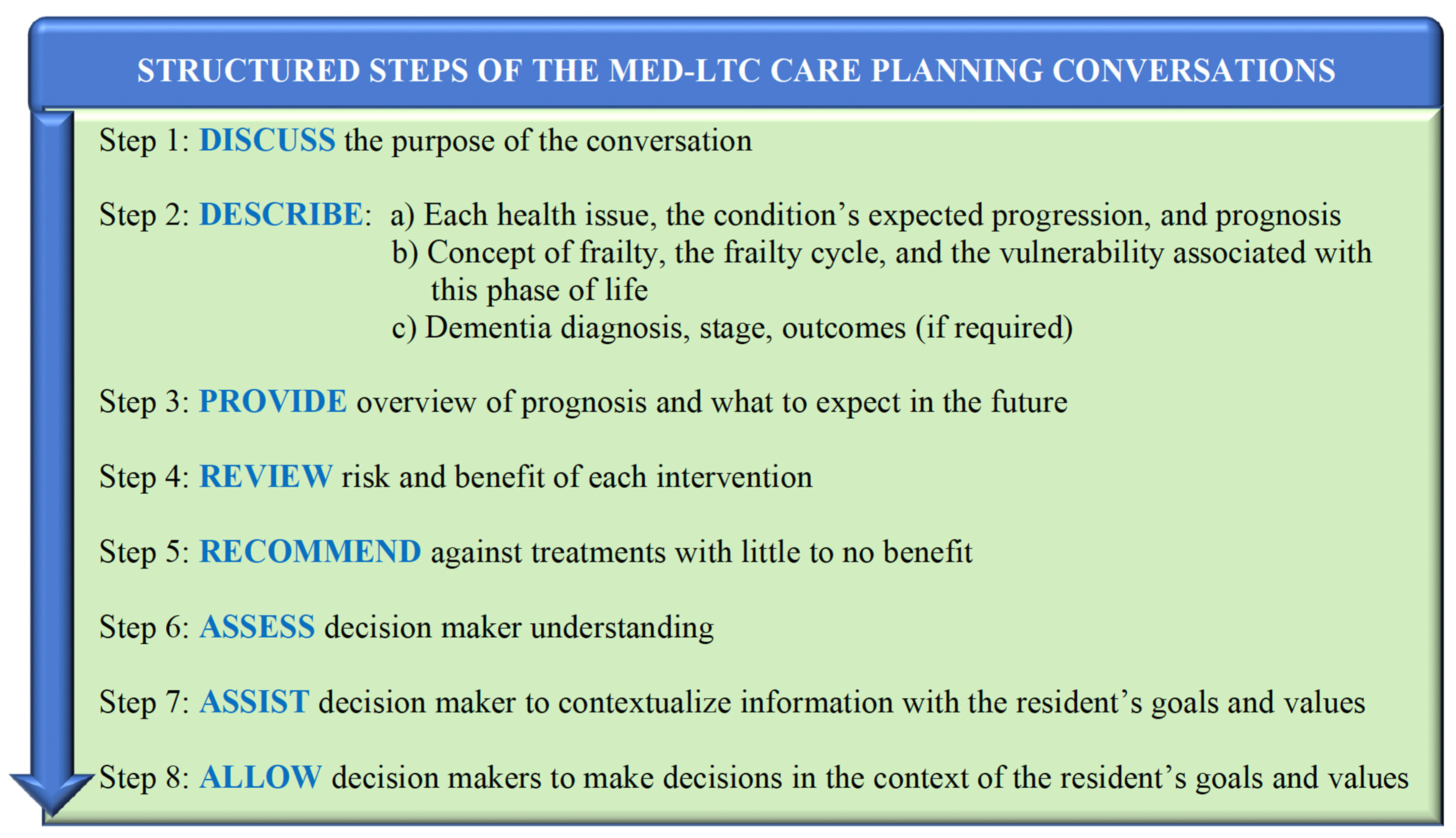
1.3. Integrating Prognosis into Care Planning
2. Materials and Methods
2.1. The MED-LTC Team
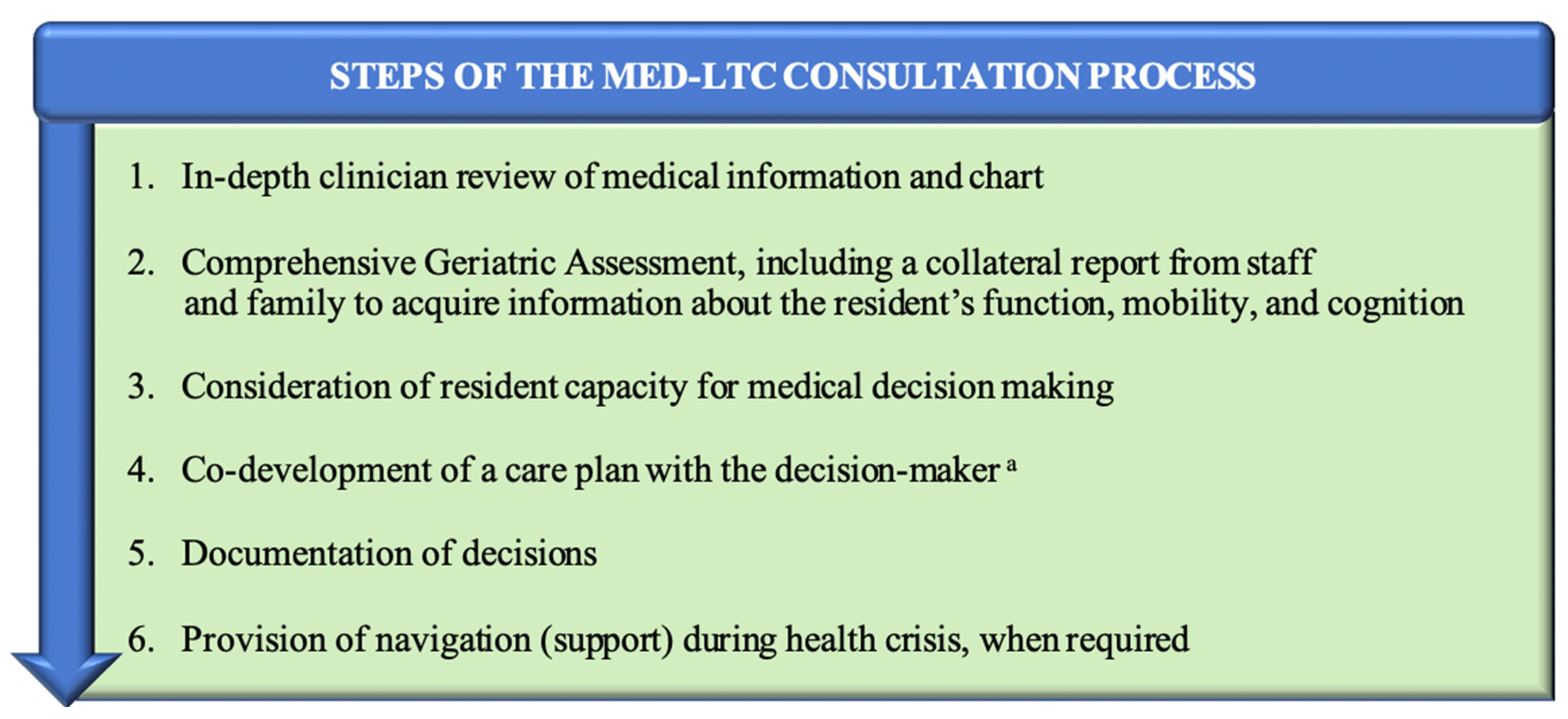
2.2. Goals of Care Consultation Process
2.3. Data Sources, Methods, and Variables
2.4. Goals of Care Outcomes
- Full code;
- NO CPR, allow intubation (or did not specify intubation status);
- NO CPR OR INTUBATION, but allow care in ICU/IMCU;
- NO CARE IN ICU/IMCU, but allow transfer to hospital;
- DO NOT HOSPITALIZE; provide full care in LTC;
- COMFORT CARE ONLY IN LTC.
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Goals of Care Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Institute for Health Information. Pandemic Experience in the Long-Term Care Sector: How Does Canada Compare with Other Countries? CIHI: Ottawa, ON, Canada, 2020; Available online: https://www.cihi.ca/sites/default/files/document/covid-19-rapid-response-long-term-care-snapshot-en.pdf (accessed on 13 September 2021).
- Sudore, R.L.; Lum, H.D.; You, J.J.; Hanson, L.C.; Meier, D.E.; Pantilat, S.Z.; Matlock, D.D.; Rietjens, J.A.; Korfage, I.J.; Ritchie, C.S.; et al. Defining Advance Care Planning for Adults: A Consensus Definition from a Multidisciplinary Delphi Panel. J. Pain Symptom Manag. 2017, 53, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.; Cosby, R.; Gzik, D.; Harle, I.; Harrold, D.; Incardona, N.; Walton, T. Provider Tools for Advance Care Planning and Goals of Care Discussions: A Systematic Review. Am. J. Hosp. Palliat. 2018, 35, 1123–1132. [Google Scholar] [CrossRef]
- Block, S. (Ed.) Discussing Goals of Care. In UpToDate; Available online: https://www.uptodate.com/contents/discussion-goals-of-care (accessed on 7 July 2021).
- Zapka, J.G.; Carter, R.; Carter, C.L.; Hennessy, W.; Kurent, J.E.; Desharnais, S. Care at the end of life: Focus on communication and race. J. Aging Health 2006, 18, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Bernacki, R.E.; Block, S.D.; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: A review and synthesis of best practices. JAMA Intern. Med. 2014, 174, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Mallery, L.; Hubbard, R.; Moorhouse, P.; Koller, K.; Eeles, E.M. Specialist physician approaches to discussing cardiopulmonary resuscitation for frail older adults: A qualitative study. J. Palliat. Care 2011, 27, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Apatira, L.; Boyd, E.A.; Malvar, G.; Evans, L.R.; Luce, J.M.; Lo, B.; White, D.B. Hope, truth, and preparing for death: Perspectives of surrogate decision makers. Ann. Intern. Med. 2008, 149, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKenzie, M.A.; Smith-Howell, E.; Bomba, P.A.; Meghani, S. Respecting Choices and Related Models of Advance Care Planning: A Systematic Review of Published Evidence. Am. J. Hosp. Palliat. Med. 2018, 35, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Person-Centered Care. Respecting Choices. Available online: https://respectingchoices.org/ (accessed on 23 March 2021).
- Dementia in Canada: Summary. Available online: https://www.cihi.ca/en/dementia-in-canada/dementia-in-canada-summary (accessed on 21 March 2021).
- Rubins, J.B. Use of Combined Do-Not-Resuscitate/Do-Not Intubate Orders Without Documentation of Intubation Preferences: A Retrospective Observational Study at an Academic Level 1 Trauma Center Code Status and Intubation Preferences. Chest 2020, 158, 292–297. [Google Scholar] [CrossRef]
- Cunha, A.I.L.; Veronese, N.; de Melo Borges, S.; Ricci, N.A. Frailty as a predictor of adverse outcomes in hospitalized older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 56, 100960. [Google Scholar] [CrossRef]
- Welstead, M.; Jenkins, N.D.; Russ, T.C.; Luciano, M.; Muniz-Terrera, G. A Systematic Review of Frailty Trajectories: Their Shape and Influencing Factors. Gerontologist 2020, 61, e463–e475. [Google Scholar] [CrossRef]
- The PATH Clinic. Available online: www.pathclinic.ca (accessed on 17 October 2021).
- Mitchell, S.L.; Teno, J.M.; Kiely, D.K.; Shaffer, M.L.; Jones, R.; Prigerson, H.G.; Volicer, L.; Givens, J.L.; Hamel, M.B. The clinical course of advanced dementia. N. Engl. J. Med. 2009, 361, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Conell-Price, J.; Covinsky, K.; Cenzer, I.S.; Chang, A.; Boscardin, W.J.; Smith, A.K. Length of stay for older adults residing in nursing homes at the end of life. J. Am. Geriatr. Soc. 2010, 58, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Braggion, M.; Pellizzari, M.; Basso, C.; Girardi, P.; Zabeo, V.; LaMattina, M.R.; Corti, M.C.; Fedeli, U. Overall mortality and causes of death in newly admitted nursing home residents. Aging Clin. Exp. Res. 2020, 32, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Middleton, A.; Ottenbacher, K.J.; Goodwin, J.S. Trajectories Over the First Year of Long-Term Care Nursing Home Residence. J. Am. Med Dir. Assoc. 2018, 19, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Benkendorf, R.; Swor, R.A.; Jackson, R.; Rivera-Rivera, E.J.; Demrick, A. Outcomes of cardiac arrest in the nursing home: Destiny or futility? Prehospital Emerg. Care 1997, 1, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.L.; Leung, L.P. Outcomes of Cardiac Arrest in Residential Care Homes for the Elderly in Hong Kong. Prehospital Emerg. Care 2017, 21, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Cheung, M. Poor Outcome of On-site CPR in a Multi-Level Geriatric Facility: Three and a Half Years Experience at the Baycrest Centre for Geriatric Care. J. Am. Geriatr. Soc. 1993, 41, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.F.; Lee, K.Y.; Lin, X.; Hao, Y.; Shahidah, N.; Ng, Y.Y.; Leong, B.S.; Sia, C.-H.; Tan, B.Y.; Tay, A.M.; et al. Nation-Wide Observational Study of Cardiac Arrests Occurring in Nursing Homes and Nursing Facilities in Singapore. Ann. Acad. Med. Singap. 2020, 49, 285–293. [Google Scholar] [CrossRef]
- Pape, M.; Rajan, S.; Hansen, S.M.; Mortensen, R.N.; Riddersholm, S.; Folke, F.; Karlsson, L.; Lippert, F.; Køber, L.; Gislason, G.; et al. Survival after out-of-hospital cardiac arrest in nursing homes—A nationwide study. Resuscitation 2018, 125, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.N.; Fairbanks, R.J.; Lerner, E.B. Cardiac Arrests in Skilled Nursing Facilities: Continuing Room for Improvement? J. Am. Med Dir. Assoc. 2006, 7, 350–354. [Google Scholar] [CrossRef]
- Vaux, J.; Lecarpentier, E.; Heidet, M.; Oubaya, N.; Hubert, H.; Baert, V.; Segal, N.; Mansouri, N.; Gueugniaud, P.-Y.; Bertrand, C.; et al. Management and outcomes of cardiac arrests at nursing homes: A French nationwide cohort study. Resuscitation 2019, 140, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Van De Glind, E.M.; Van Munster, B.C.; Van De Wetering, F.T.; Van Delden, J.J.; Scholten, R.J.; Hooft, L. Pre-arrest predictors of survival after resuscitation from out-of-hospital cardiac arrest in the elderly a systematic review. BMC Geriatr. 2013, 13, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbo, E.D.; Yuen, T.C.; Buhrmester, L.; Geocadin, R.; Volandes, A.E.; Siddique, J.; Edelson, D.P. Cardiopulmonary Resuscitation Outcomes in Hospitalized Community-Dwelling Individuals and Nursing Home Residents Based on Activities of Daily Living. J. Am. Geriatr. Soc. 2013, 61, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wharton, C.; King, E.; MacDuff, A. Frailty is associated with adverse outcome from in-hospital cardiopulmonary resuscitation. Resuscitation 2019, 143, 208–211. [Google Scholar] [CrossRef]
- Morbidity and Mortality Weekly Report. Available online: https://www.cdc.gov/mmwr/index.html (accessed on 21 April 2021).
- Pranata, R.; Henrina, J.; Lim, M.A.; Lawrensia, S.; Yonas, E.; Vania, R.; Huang, I.; Lukito, A.A.; Suastika, K.; Kuswardhani, R.T.; et al. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2020, 93, 104324. [Google Scholar] [CrossRef]
- Panagiotou, O.A.; Kosar, C.M.; White, E.M.; Bantis, L.E.; Yang, X.; Santostefano, C.M.; Feifer, R.A.; Blackman, C.; Rudolph, J.L.; Gravenstein, S.; et al. Risk Factors Associated with All-Cause 30-Day Mortality in Nursing Home Residents with COVID-19. JAMA Intern. Med. 2021, 181, 439. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. COVID-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Fried, M.W.; Crawford, J.M.; Mospan, A.R.; Watkins, S.E.; Munoz, B.; Zink, R.C.; Elliott, S.; Burleson, K.; Landis, C.; Reddy, K.R.; et al. Patient Characteristics and Outcomes of 11 721 Patients With Coronavirus Disease 2019 (COVID-19) Hospitalized Across the United States. Clin. Infect. Dis. 2020, 72, e558–e565. [Google Scholar] [CrossRef]
- Hyman, J.B.; Leibner, E.S.; Tandon, P.; Egorova, N.N.; Bassily-Marcus, A.; Kohli-Seth, R.; Arvind, V.; Chang, H.L.; Lin, H.-M.; Levin, M.A. Timing of Intubation and In-Hospital Mortality in Patients With Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0254. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062, Erratum in Lancet 2020, 395, 10229. [Google Scholar] [CrossRef]
- Twigg, H.L., 3rd; Khan, S.H.; Perkins, A.J.; Roberts, S.; Sears, C.; Rahman, O.; Smith, J.P.; Kapoor, R.; Farber, M.O.; Ellender, T.; et al. Mortality Rates in a Diverse Cohort of Mechanically Ventilated Patients with Novel Coronavirus in the Urban Midwest. Crit. Care Explor. 2020, 2, e0187. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.; Catalisano, G.; Marino, C.; Fucà, R.; Giarratano, A.; Baldi, E.; Einav, S.; Cortegiani, A. Mortality after in-hospital cardiac arrest in patients with COVID-19: A systematic review and meta-analysis. Resuscitation 2021, 164, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, P.; Mallery, L.H. Palliative and Therapeutic Harmonization: A Model for Appropriate Decision-Making in Frail Older Adults. J. Am. Geriatr. Soc. 2012, 60, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Searle, S.D.; Rockwood, K. Does determining the degree of frailty help pandemic decision-making when resources are scarce? Lancet Heal. Longev. 2021, 2, e119–e120. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, S.; Pandya, N.; Dumyati, G.; Nace, D.A.; Pandya, K.; Jump, R.L. A Structured Tool for Communication and Care Planning in the Era of the COVID-19 Pandemic. J. Am. Med Dir. Assoc. 2020, 21, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Roeland, E.; Cain, J.; Onderdonk, C.; Kerr, K.; Mitchell, W.; Thornberry, K. When Open-Ended Questions Don’t Work: The Role of Palliative Paternalism in Difficult Medical Decisions. J. Palliat. Med. 2014, 17, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.; Winch, S.; Barnett, A.; Parker, M.; Gallois, C.; Willmott, L.; White, B.; Patton, M.A.; Burridge, L.; Salkield, G.; et al. Incidence, duration and cost of futile treatment in end-of-life hospital admissions to three Australian public-sector tertiary hospitals: A retrospective multicentre cohort study. BMJ Open 2017, 7, e017661. [Google Scholar] [CrossRef]
| Characteristic | Age Groups (years) | |||
|---|---|---|---|---|
| All Combined (n = 63) | Under 65 (n = 12) | 65 and Over (n = 51) | ||
| Positive for COVID-19 at Consult—No. (%) | 28 (44.4%) | 6 (50%) | 22 (43) | |
| Age—year | Mean (SD) | 75.3 (14.2) | 51.8 (7.8) | 80.9 (8) |
| Median (range) | 77.0 (37–96) | 52.0 (37, 64) | 81.0 (65, 96) | |
| Female Sex—No. (%) | 41 (65) | 8 (66.7%) | 33 (65) | |
| Clinical Frailty Score | Mild/moderate | N/A a | N/A a | 14 (28) |
| Severe/very severe | N/A a | N/A a | 36 (72) | |
| Cognitive status—No. (%) | Dementia | 36 (57) | 2 (17) | 34 (67) |
| Abnormal cognition b | 16 (25) | 9 (75) | 7 (14) | |
| Intact cognition | 11 (18) | 1 (8) | 10 (20) | |
| Mobility—No. (%) | No aid | 8 (13) | 2 (17) | 6 (12) |
| Gait aid/needs assistance | 21 (34) | 2 (17) | 19 (38) | |
| Cannot walk | 33 (53) | 8 (67) | 25 (50) | |
|
Basic Activities of Daily Living —No. (%) | Independent | 3 (5) | 0 (0) | 3 (6) |
| Dependent for 1–2 activities | 21 (33) | 2 (16) | 19 (37) | |
| Dependent for 3 or more activities | 39 (61) | 10 (83) | 29 (57) | |
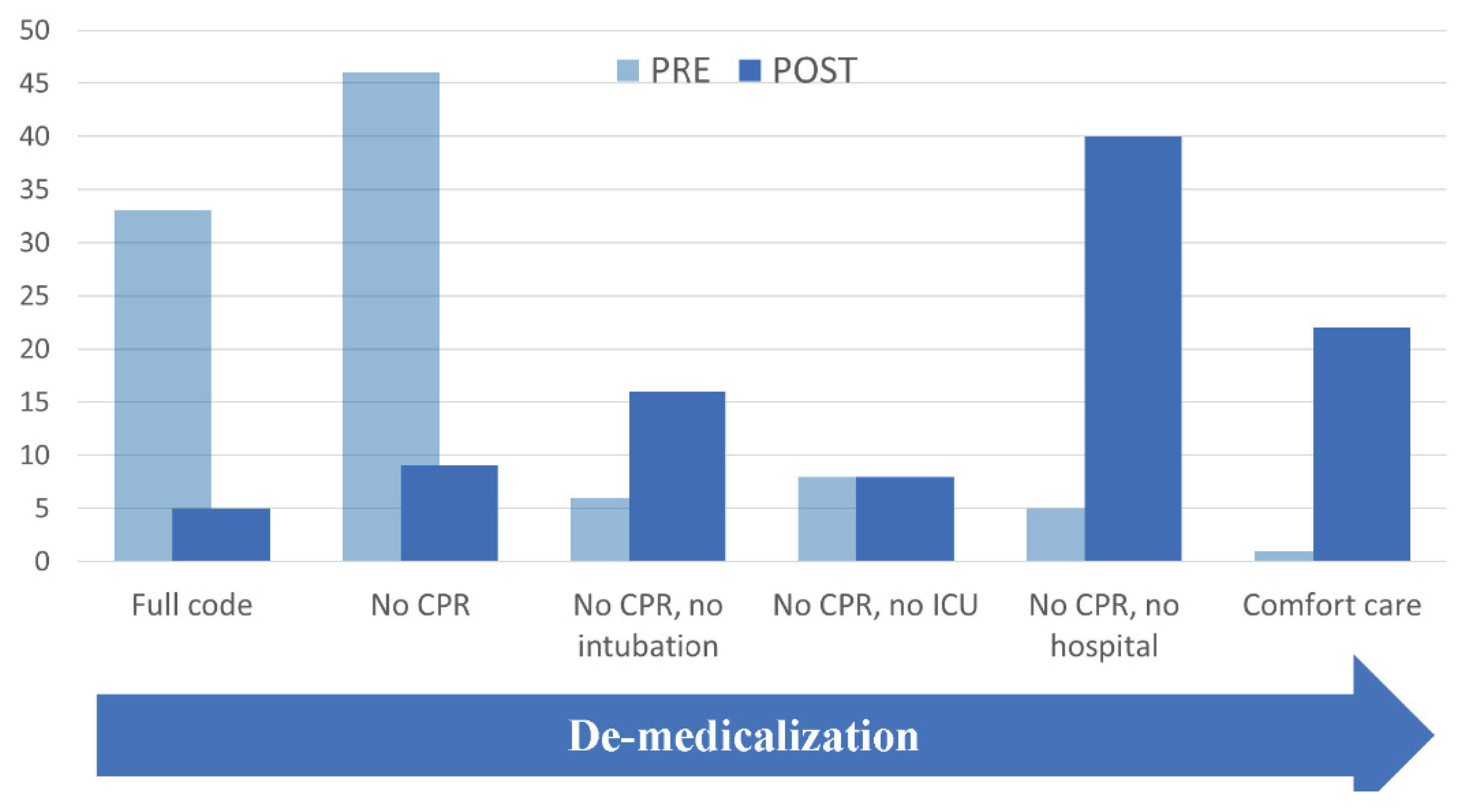
| LEVEL | Pre-Consult Level No. (%) | Post-Consult Level No. (%) |
|---|---|---|
| 1. Full code | 24 (33.4) a | 3 (4.8) |
| 2. NO CPR, allow intubation (or did not specify intubation status) | 29 (46.0) | 6 (9.5) |
| 3. NO CPR OR INTUBATION, but allow care in ICU/IMCU | 4 (6) | 10 (16) |
| 4. NO CARE IN ICU/IMCU, but allow transfer to hospital | 5 (8) | 5 (8) |
| 5. DO NOT HOSPITALIZE; provide full care in LTC | 3 (5) | 25 (40) |
| 6. COMFORT CARE ONLY IN LTC | 1 (2) | 14 (22) |
| Variable | OR (95% CI) | |
|---|---|---|
| Age group, (years) | <65 | 1, ref. |
| 65 and older | 1.82 (0.55, 6.02) | |
| Cognitive status | No dementia | 1, ref. |
| Dementia | 4.63 (1.03, 20.92) | |
| Abnormal cognition a | 0.80 (0.17, 2.64) | |
| Frailty stage according to CFS | Moderate or lower | 1, ref. |
| Severe or higher | 3.49 (1.01, 12.09) | |
| Positive for COVID-19 | No | 1, ref. |
| Yes | 4.52 (1.64, 12.42) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallery, L.; Shetty, N.; Moorhouse, P.; Miller, A.P.; von Maltzahn, M.; Buckler, M.; MacLeod, T.; Stewart, S.A.; Krueger-Naug, A.M. Goals of Care Conversations in Long-Term Care during the First Wave of the COVID-19 Pandemic. J. Clin. Med. 2022, 11, 1710. https://doi.org/10.3390/jcm11061710
Mallery L, Shetty N, Moorhouse P, Miller AP, von Maltzahn M, Buckler M, MacLeod T, Stewart SA, Krueger-Naug AM. Goals of Care Conversations in Long-Term Care during the First Wave of the COVID-19 Pandemic. Journal of Clinical Medicine. 2022; 11(6):1710. https://doi.org/10.3390/jcm11061710
Chicago/Turabian StyleMallery, Laurie, Nabha Shetty, Paige Moorhouse, Ashley Paige Miller, Maia von Maltzahn, Melissa Buckler, Tanya MacLeod, Samuel A. Stewart, and Anne Marie Krueger-Naug. 2022. "Goals of Care Conversations in Long-Term Care during the First Wave of the COVID-19 Pandemic" Journal of Clinical Medicine 11, no. 6: 1710. https://doi.org/10.3390/jcm11061710
APA StyleMallery, L., Shetty, N., Moorhouse, P., Miller, A. P., von Maltzahn, M., Buckler, M., MacLeod, T., Stewart, S. A., & Krueger-Naug, A. M. (2022). Goals of Care Conversations in Long-Term Care during the First Wave of the COVID-19 Pandemic. Journal of Clinical Medicine, 11(6), 1710. https://doi.org/10.3390/jcm11061710





