Long-Term Risk of Colectomy in Patients with Severe Ulcerative Colitis Responding to Intravenous Corticosteroids or Infliximab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Study Population and Characteristics of Patients
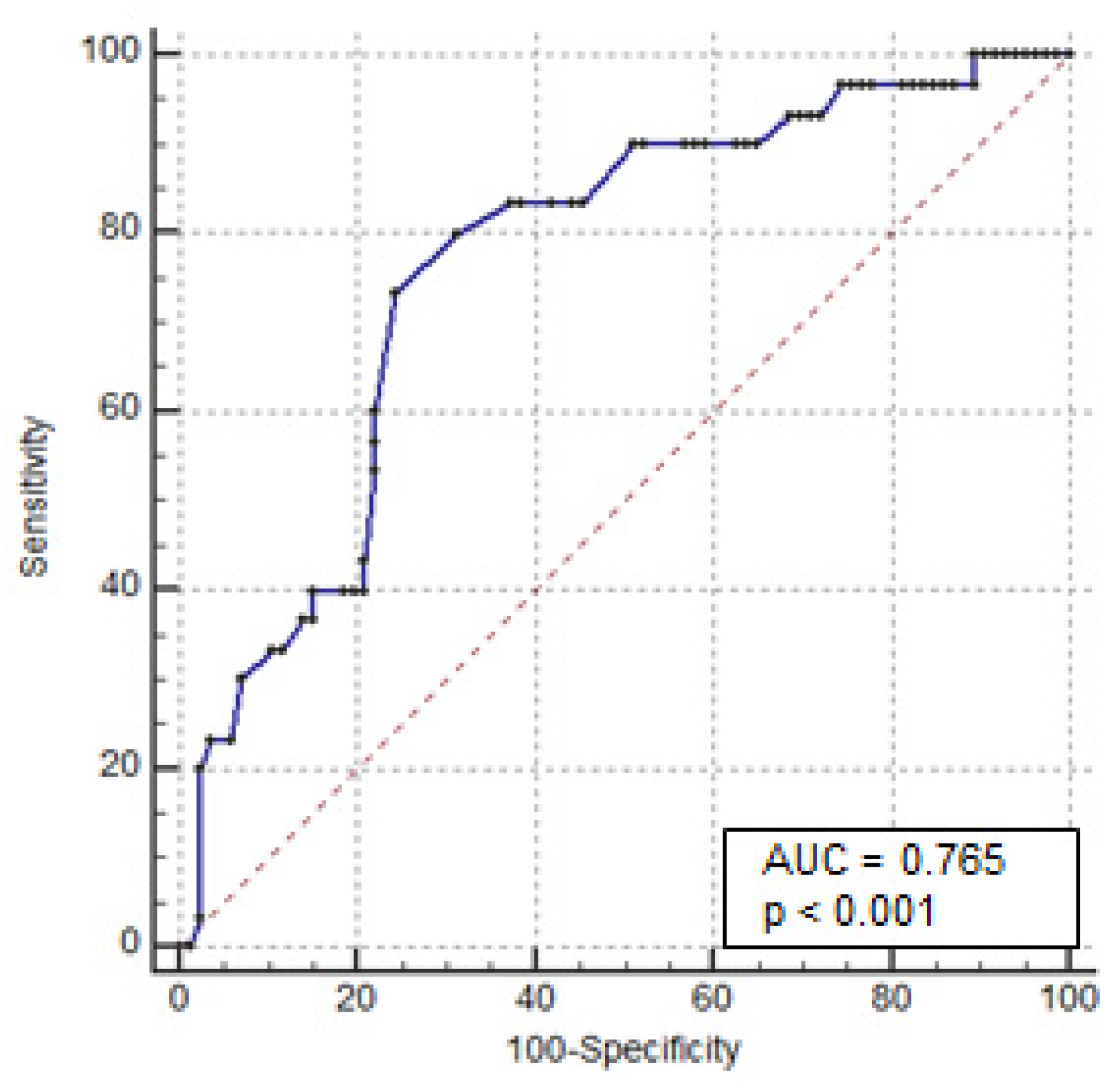
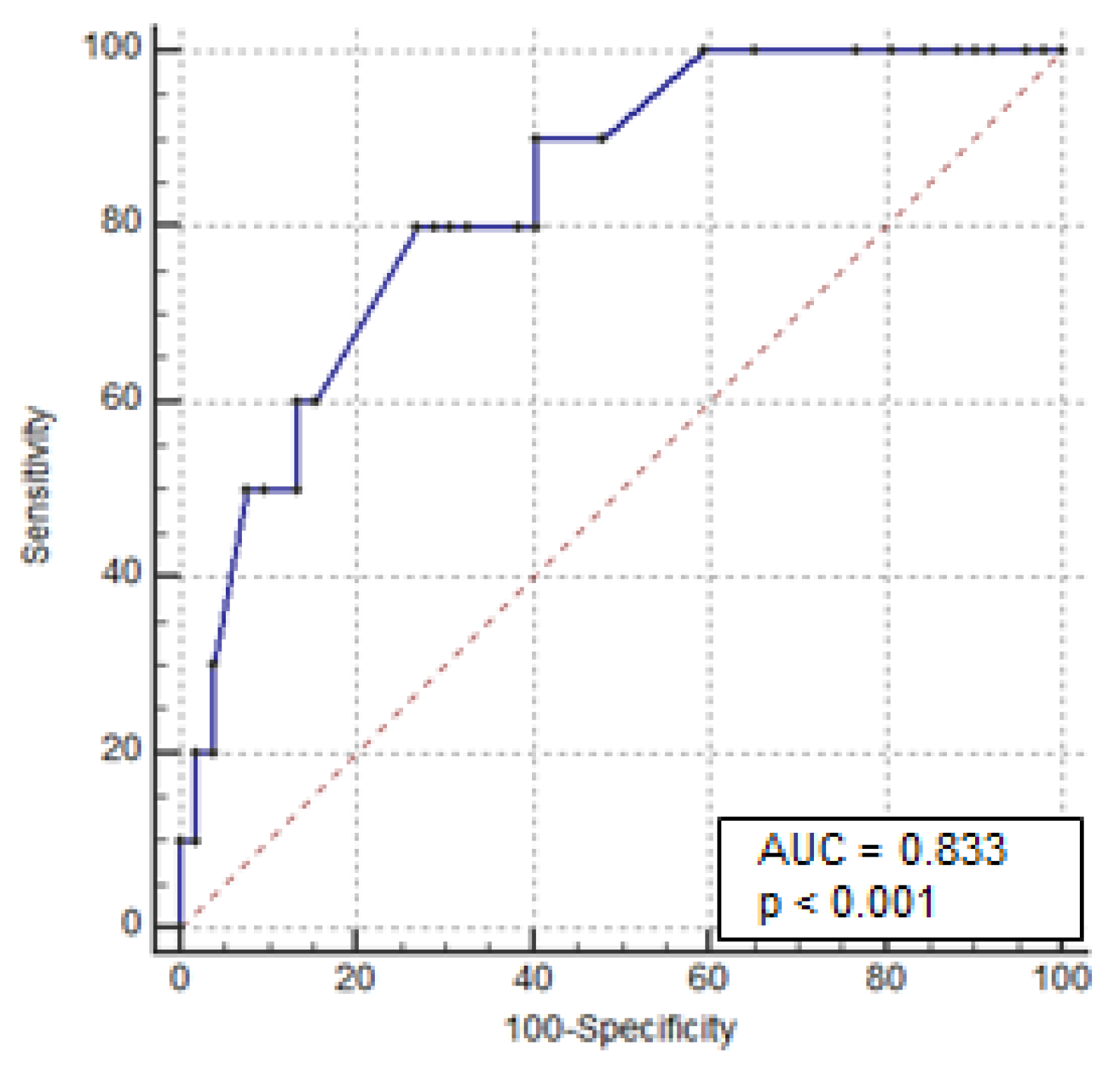
3.2. Predictors of Colectomy
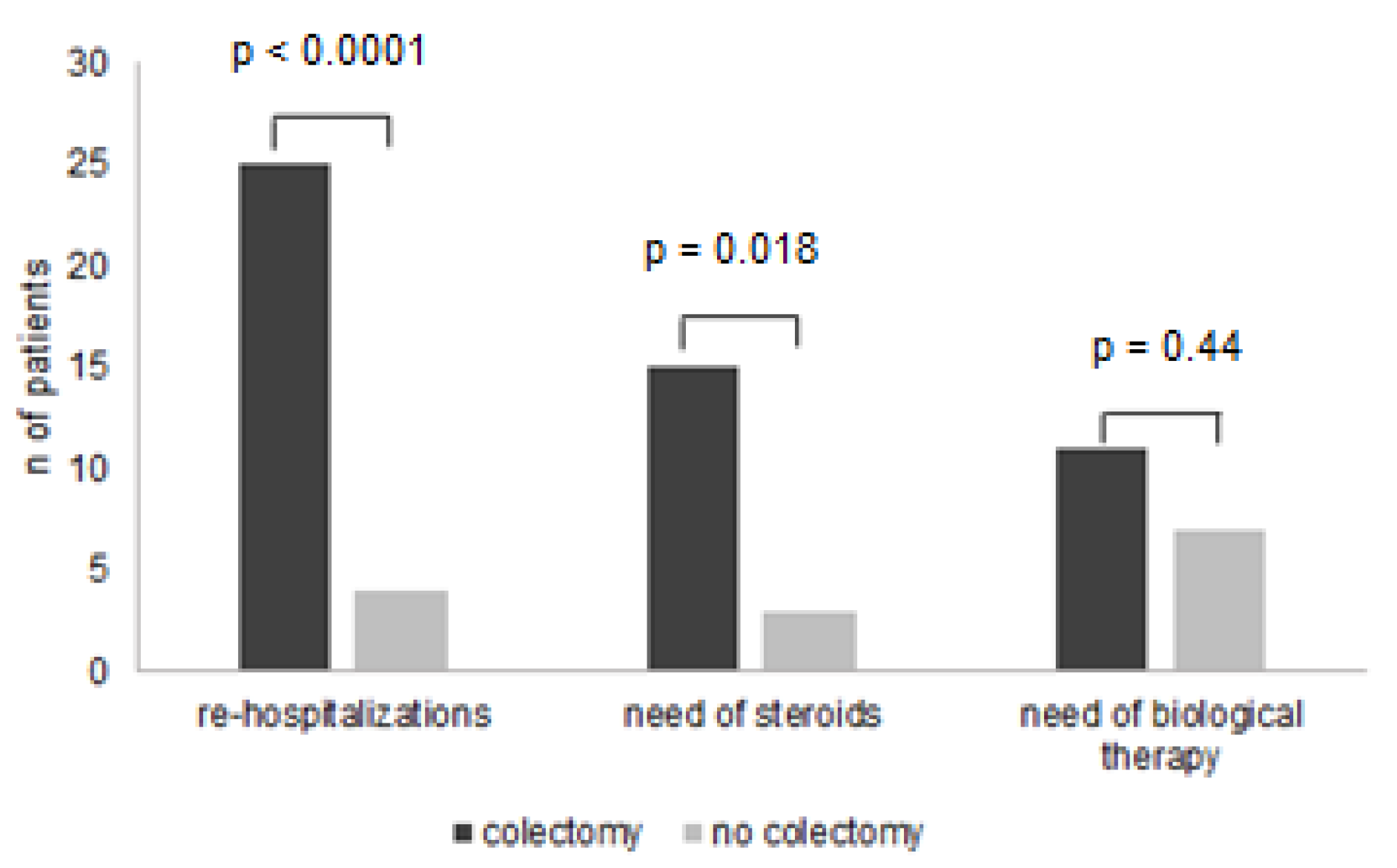
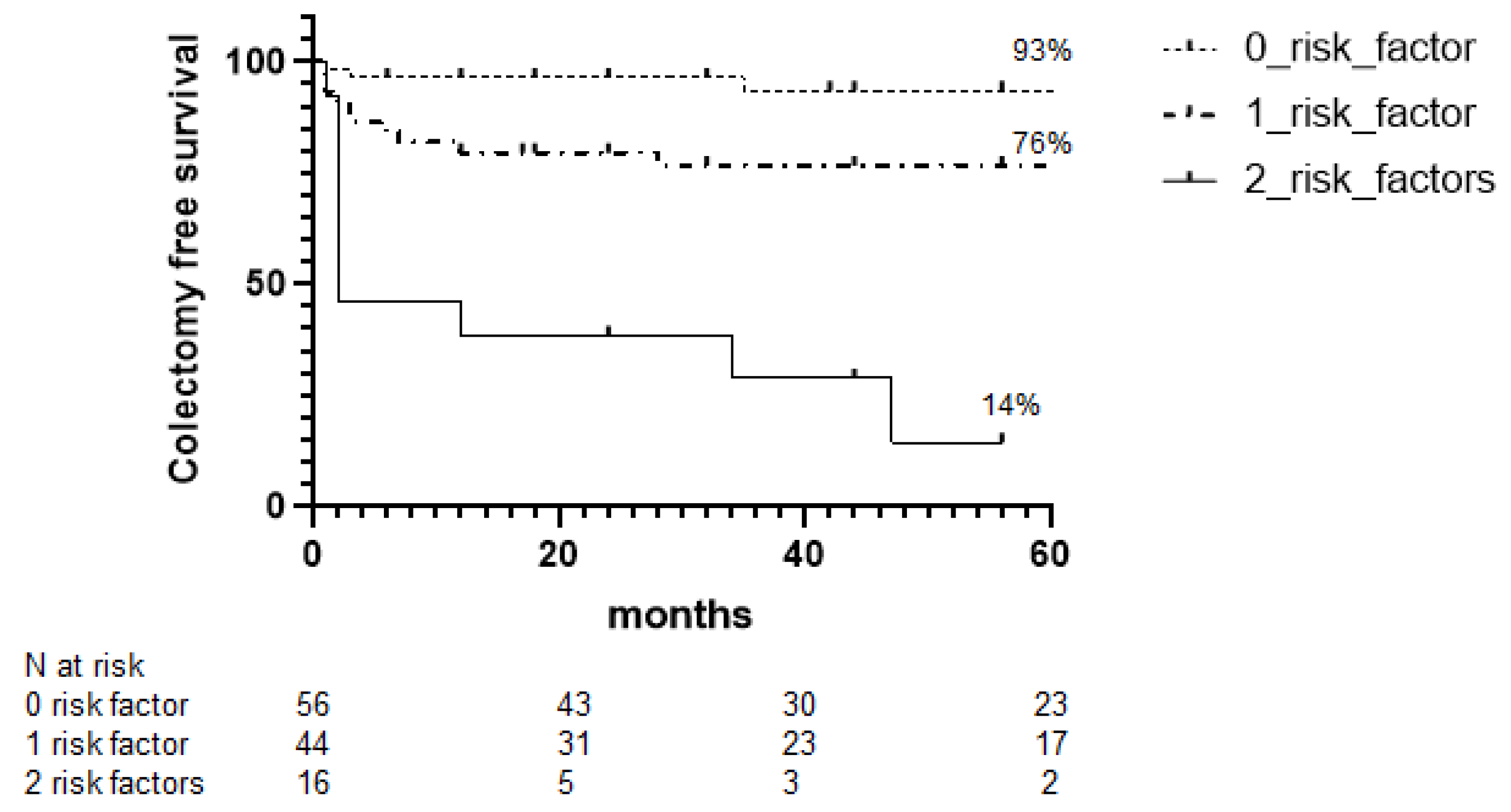
3.3. Colectomy-Free Survival during Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis: Final report on a therapeutic trial. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Walsh, C.M.; Steinhart, A.H.; Griffiths, A.M. Response to Corticosteroids in Severe Ulcerative Colitis: A Systematic Review of the Literature and a Meta-Regression. Clin. Gastroenterol. Hepatol. 2007, 5, 103–110. [Google Scholar] [CrossRef]
- Turner, D.; Walsh, C.M.; Benchimol, E.; Mann, E.H.; Thomas, K.E.; Chow, C.; McLernon, R.A.; Walters, T.D.; Swales, J.; Steinhart, A.H.; et al. Severe paediatric ulcerative colitis: Incidence, outcomes and optimal timing for second-line therapy. Gut 2008, 57, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Marshall, J.; Colombel, J.-F.; Leontiadis, G.I.; Williams, J.G.; Muqtadir, Z.; Reinisch, W. Systematic review and meta-analysis: Infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am. J. Gastroenterol. 2016, 111, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Järnerot, G.; Hertervig, E.; Friis-Liby, I.; Blomquist, L.; Karlén, P.; Grännö, C.; Vilien, M.; Ström, M.; Danielsson, A.; Verbaan, H.; et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology 2005, 128, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Ventham, N.T.; Kalla, R.; Kennedy, N.A.; Satsangi, J.; Arnott, I.D. Predicting outcomes in acute severe ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.W.; Churchhouse, A.M.D.; Protheroe, A.; Arnott, I.D.R.; The UK IBD Audit Steering Group. Predicting outcome in acute severe ulcerative colitis: Comparison of the Travis and Ho scores using UK IBD audit data. Aliment. Pharmacol. Ther. 2016, 43, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Colombel, J.-F.; Sands, B.E.; Narula, N. Mucosal Healing Is Associated with Improved Long-term Outcomes of Patients with Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Law CC, Y.; Tariq, R.; Khanna, S.; Murthy, S.; McCurdy, J.D. Systematic review with meta-analysis: The impact of Clostridium difficile infection on the short- and long-term risks of colectomy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut 2008, 57, 205–210. [Google Scholar] [CrossRef]
- Dinesen, L.C.; Walsh, A.J.; Protic, M.N.; Heap, G.; Cummings, F.; Warren, B.F.; George, B.; Mortensen, N.J.; Travis, S.P. The pattern and outcome of acute severe colitis. J. Crohn’s Colitis 2010, 4, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojic, D.; Radojicic, Z.; Nedeljkovic-Protic, M.; Al-Ali, M.; Jewell, D.P.; Travis, S.P. Long-term outcome after admission for acute severe ulcerative colitis in Oxford: The 1992–1993 cohort. Inflamm. Bowel Dis. 2009, 15, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Le Baut, G.; Kirchgesner, J.; Amiot, A.; Lefevre, J.H.; Chafai, N.; Landman, C.; Nion, I.; Bourrier, A.; Delattre, C.; Martineau, C.; et al. A Scoring System to Determine Patients’ Risk of Colectomy within 1 Year after Hospital Admission for Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1602–1610.e1. [Google Scholar] [CrossRef]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.-F.; Vermeire, S.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions and diagnosis. J. Crohn’s Colitis 2012, 6, 965–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, R.S.; Fonseca, I.; Sousa, A.S.; Jesus, A.; Pichel, F.; Amaral, T.F. Original article ESPEN diagnostic criteria for malnutrition e A validation study in hospitalized patients. Clin. Nutr. 2017, 36, 1326–1332. [Google Scholar] [CrossRef]
- Ho, G.T.; Mowat, C.; Goddard, C.J.R.; Fennell, J.M.; Shah, N.B.; Prescott, R.J.; Satsangi, J. Predicting the outcome of severe ulcerative colitis: Development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment. Pharmacol. Ther. 2004, 19, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Llaó, J.; Naves, J.E.; Ruiz-Cerulla, A.; Gordillo, J.; Mañosa, M.; Maisterra, S.; Cabré, E.; Garcia-Planella, E.; Guardiola, J.; Domènech, E. Improved outcome of acute severe ulcerative colitis while using early predictors of corticosteroid failure and rescue therapies. Dig. Liver Dis. 2016, 48, 608–612. [Google Scholar] [CrossRef]
- Tanaka, M.; Takagi, T.; Naito, Y.; Uchiyama, K.; Hotta, Y.; Toyokawa, Y.; Kashiwagi, S.; Kamada, K.; Ishikawa, T.; Yasuda, H.; et al. Low serum albumin at admission is a predictor of early colectomy in patients with moderate to severe ulcerative colitis. JGH Open 2021, 5, 377–381. [Google Scholar] [CrossRef]
- Lindgren, S.C.; Flood, L.M.; Kilander, A.F.; Löfberg, R.; Persson, T.B.; Sjödahl, R.I. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 1998, 10, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Katsanos, K.H.; Christodoulou, D.K.; Barros, L.; Magro, F.; Pedersen, N.; Kjeldsen, J.; Vegh, Z.; Lakatos, P.; Eriksson, C.; et al. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort-an Epi-IBD study. J. Crohn’s Colitis 2018, 13, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Solberg, I.C.; Høivik, M.L.; Cvancarova, M.; Moum, B. Risk matrix model for prediction of colectomy in a population-based study of ulcerative colitis patients (the IBSEN study). Scand. J. Gastroenterol. 2015, 50, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- De Cristofaro, E.; Salvatori, S.; Marafini, I.; Zorzi, F.; Alfieri, N.; Musumeci, M.; Biancone, L.; Calabrese, E.; Monteleone, G. Long-Term Outcomes and Predictive Factors of Hospitalized Patients with Severe Ulcerative Colitis Treated with Intravenous Corticosteroids. J. Clin. Med. 2021, 10, 5413. [Google Scholar] [CrossRef] [PubMed]
- Salameh, R.; Kirchgesner, J.; Allez, M.; Carbonnel, F.; Meyer, A.; Gornet, J.-M.; Beaugerie, L.; Amiot, A. Long-term outcome of patients with acute severe ulcerative colitis responding to intravenous steroids. Aliment. Pharmacol. Ther. 2020, 51, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Festa, S.; Scribano, M.L.; Pugliese, D.; Bezzio, C.; Principi, M.; Ribaldone, D.G.; Allocca, M.; Mocci, G.; Bodini, G.; Spagnuolo, R.; et al. Long-term outcomes of acute severe ulcerative colitis in therescue therapy era: A multicentre cohort study. UEG J. 2021, 9, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.P.; Farrant, J.M.; Ricketts, C.; Nolan, D.J.; Mortensen, N.M.; Kettlewell, M.G.; Jewell, D.P. Predicting outcome in severe ulcerative colitis. Gut 1996, 38, 905–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennard-Jones, J.E.; Ritchie, J.K.; Hilder, W.; Spicer, C.C. Assessment of severity in colitis: A preliminary study. Gut 1975, 16, 579–584. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Non-Colectomy (87 Patients) | Colectomy (29 Patients) | p Value |
|---|---|---|---|
| Female gender, n (%) | 36 (41%) | 21 (72%) | 0.005 |
| Age, y (median, range) | 43 (16–86) | 55 (24–85) | 0.09 |
| Disease extent, n (%) | |||
| E2: left-sided colitis | 29 (33%) | 9 (31%) | 0.9 |
| E3: extensive colitis | 58 (67%) | 20 (69%) | |
| Duration of disease | |||
| Months (median, range) | 95 (2–504) | 168 (2–516) | 0.29 |
| Previous anti-TNF therapies, n (%) | 13 (15%) | 10 (34%) | 0.029 |
| Smoking habits | |||
| Former, n (%) | 33 (38%) | 9 (31%) | 0.65 |
| Current, n (%) | 12 (14%) | 3 (10%) | 0.76 |
| Steroid dependence | |||
| Yes, n (%) | 30 (34%) | 19 (65%) | 0.04 |
| Partial Mayo Clinic score (mean ± SD) | 6.7 ± 1.03 | 7.3 ± 1.11 | 0.02 |
| Body mass index (BMI) (median, range) | 22.9 (13–32.7) | 22.6 (14.3–28.9) | 0.75 |
| C-reactive protein (CRP), mg/dL, n (%) | 38 (0.1–242) | 84 (9.5–258) | 0.02 |
| Albumin, g/dL (median, range) | 3.2 (1.6–4.5) | 2.8 (1.7–4.3) | 0.04 |
| Hemoglobin, g/dL (median, range) | 11.7 (6.2–16) | 11.2 (6.9–13.9) | 0.17 |
| Colonic dilation at X-ray, cm (median, range) | 3.1 (2.1–6) | 4.6 (3.2–6.9) | 0.02 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Risk Factors | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Female gender | 2.914 (1.21–6.97) | 0.01 | 5.511 (0.69–43.58) | 0.11 |
| Disease duration | 1.002 (0.99–1.01) | 0.26 | - | |
| Extensive colitis (E3) | 1.187 (0.48–2.92) | 0.71 | - | |
| Age at admission | 1.021 (0.99–1.05) | 0.08 | - | |
| Previous anti-TNF exposure | 2.808 (1.07–7.34) | 0.04 | 1.265 (0.09–18.4) | 0.86 |
| Steroid dependence | 3.224 (1.36–7.66) | 0.007 | 2.724 (0.03–24.52) | 0.37 |
| Stool frequency at admission | 1.019 (0.93–1.12) | 0.7 | - | |
| Hemoglobin < 10.5 g/dL | 2.102 (0.85–5.22) | 0.11 | - | |
| C-reactive protein > 30 mg/dL | 2.919 (1.01 to 8.43) | 0.034 | 1.009 (0.99–1.03) | 0.33 |
| Albumin < 3 g/dL | 8.741 (3.20–23.8) | <0.0001 | 6.887 (2.08–22.8) | 0.002 |
| Colonic dilation at X-ray > 5.5 cm | 7.581 (2.06–27.87) | 0.0016 | 8.468 (1.23–58.3) | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cristofaro, E.; Salvatori, S.; Marafini, I.; Zorzi, F.; Alfieri, N.; Musumeci, M.; Calabrese, E.; Monteleone, G. Long-Term Risk of Colectomy in Patients with Severe Ulcerative Colitis Responding to Intravenous Corticosteroids or Infliximab. J. Clin. Med. 2022, 11, 1679. https://doi.org/10.3390/jcm11061679
De Cristofaro E, Salvatori S, Marafini I, Zorzi F, Alfieri N, Musumeci M, Calabrese E, Monteleone G. Long-Term Risk of Colectomy in Patients with Severe Ulcerative Colitis Responding to Intravenous Corticosteroids or Infliximab. Journal of Clinical Medicine. 2022; 11(6):1679. https://doi.org/10.3390/jcm11061679
Chicago/Turabian StyleDe Cristofaro, Elena, Silvia Salvatori, Irene Marafini, Francesca Zorzi, Norma Alfieri, Martina Musumeci, Emma Calabrese, and Giovanni Monteleone. 2022. "Long-Term Risk of Colectomy in Patients with Severe Ulcerative Colitis Responding to Intravenous Corticosteroids or Infliximab" Journal of Clinical Medicine 11, no. 6: 1679. https://doi.org/10.3390/jcm11061679
APA StyleDe Cristofaro, E., Salvatori, S., Marafini, I., Zorzi, F., Alfieri, N., Musumeci, M., Calabrese, E., & Monteleone, G. (2022). Long-Term Risk of Colectomy in Patients with Severe Ulcerative Colitis Responding to Intravenous Corticosteroids or Infliximab. Journal of Clinical Medicine, 11(6), 1679. https://doi.org/10.3390/jcm11061679






