The Deployment of a Newly Developed Proximal Release-Type Colonic Stent Is Feasible for Malignant Colorectal Obstruction near the Anal Verge: A Single-Center Preliminary Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Participants
2.2. Inclusion and Exclusion Criteria
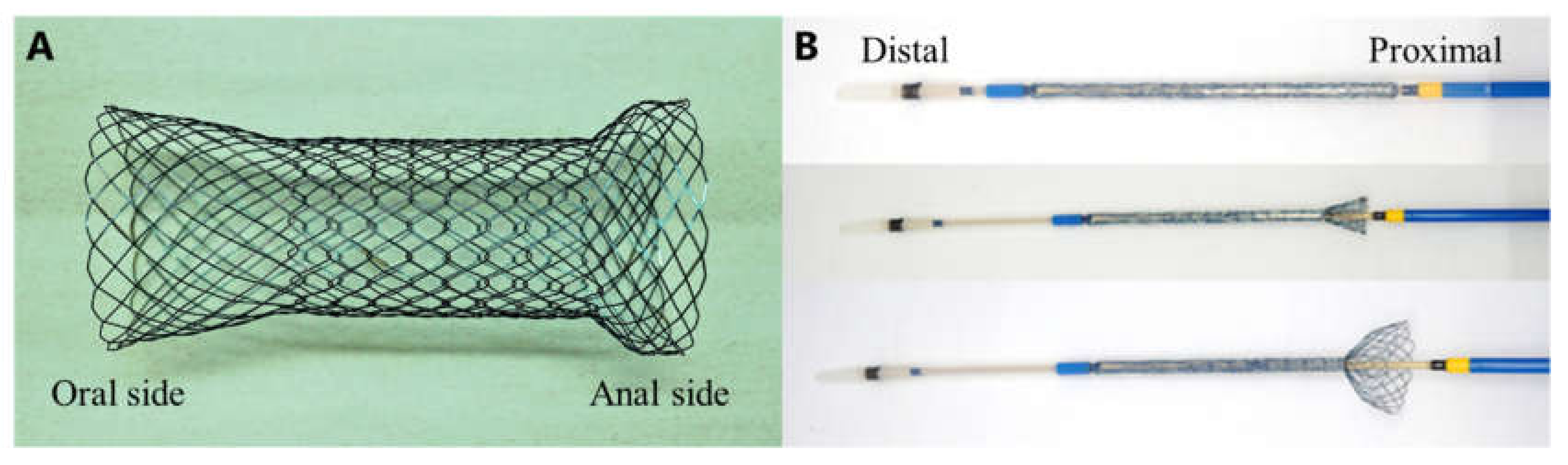
2.3. SEMS Placement Procedures
2.4. Outcomes and Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Cohort
3.2. Short-Term Outcome of SEMS Placement
3.3. Surgical and Long-Term Outcomes in BTS Cases
3.4. Long-Term Outcomes in PAL Cases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France; Available online: https://gco.iarc.fr/today (accessed on 14 March 2022).
- Cheynel, N.; Cortet, M.; Lepage, C.; Benoit, L.; Faivre, J.; Bouvier, A.-M. Trends in frequency and management of obstructing colorectal cancers in a well-defined population. Dis. Colon Rectum 2007, 50, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Jullumstrø, E.; Wibe, A.; Lydersen, S.; Edna, T.-H. Colon cancer incidence, presentation, treatment and outcomes over 25 years. Color. Dis. 2011, 13, 512–518. [Google Scholar] [CrossRef]
- Winner, M.; Mooney, S.J.; Hershman, D.L.; Feingold, D.L.; Allendorf, J.D.; Wright, J.D.; Neugut, A.I. Incidence and predictors of bowel obstruction in elderly patients with stage IV colon cancer: A population-based cohort study. JAMA Surg. 2013, 148, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Ishida, H.; Yoshida, S.; Isayama, H.; Kuwai, T.; Maetani, I.; Shimada, M.; Yamada, T.; Saito, S.; Tomita, M.; et al. A Japanese prospective multicenter study of self-expandable metal stent placement for malignant colorectal obstruction: Short-term safety and efficacy within 7 days of stent procedure in 513 cases. Gastrointest. Endosc. 2015, 82, 697–707.e1. [Google Scholar] [CrossRef] [PubMed]
- Kuwai, T.; Yamaguchi, T.; Imagawa, H.; Yoshida, S.; Isayama, H.; Matsuzawa, T.; Yamada, T.; Saito, S.; Shimada, M.; Hirata, N.; et al. Factors related to difficult self-expandable metallic stent placement for malignant colonic obstruction: A post-hoc analysis of a multicenter study across Japan. Dig. Endosc. 2019, 31, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Miyasako, Y.; Kuwai, T.; Ishaq, S.; Tao, K.; Konishi, H.; Miura, R.; Sumida, Y.; Kuroki, K.; Tamaru, Y.; Kusunoki, R.; et al. Newly developed self-expandable Niti-S MD colonic metal stent for malignant colonic obstruction. World J. Gastrointest. Surg. 2020, 12, 138–148. [Google Scholar] [CrossRef]
- Trompetas, V. Emergency management of malignant acute left-sided colonic obstruction. Ann. R. Coll. Surg. Engl. 2008, 90, 181–186. [Google Scholar] [CrossRef][Green Version]
- Tomita, M.; Saito, S.; Makimoto, S.; Yoshida, S.; Isayama, H.; Yamada, T.; Matsuzawa, T.; Enomoto, T.; Kyo, R.; Kuwai, T.; et al. Self-expandable metallic stenting as a bridge to surgery for malignant colorectal obstruction: Pooled analysis of 426 patients from two prospective multicenter series. Surg. Endosc. 2019, 33, 499–509. [Google Scholar] [CrossRef]
- Song, H.-Y.; Kim, J.H.; Kim, K.R.; Shin, J.H.; Kim, H.C.; Yu, C.-S. Malignant rectal obstruction within 5 cm of the anal verge: Is there a role for expandable metallic stent placement? Gastrointest. Endosc. 2008, 68, 713–720. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Jung, Y.S.; Hong, S.P.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Clinical outcomes and risk factors for technical and clinical failures of self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest. Endosc. 2011, 74, 858–868. [Google Scholar] [CrossRef]
- Yoshida, S.; Isayama, H.; Koike, K. Palliative self-expandable metallic stent placement for colorectal obstruction caused by an extracolonic malignancy. Gastrointest. Interv. 2014, 3, 75–79. [Google Scholar] [CrossRef][Green Version]
- Ptok, H.; Meyer, F.; Marusch, F.; Steinert, R.; Gastinger, I.; Lippert, H.; Meyer, L. Palliative stent implantation in the treatment of malignant colorectal obstruction. Surg. Endosc. 2006, 20, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Mergener, K.; Kozarek, R.A. Stenting of the gastrointestinal tract. Dig. Dis. 2002, 20, 173–181. [Google Scholar] [CrossRef] [PubMed]
- van Hooft, J.E.; Van Halsema, E.E.; Vanbiervliet, G.; Beets-Tan, R.G.; DeWitt, J.M.; Donnellan, F.; Repici, A. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2014, 46, 990–1053. [Google Scholar] [PubMed]
- Tao, K.; Kuwai, T.; Ishaq, S.; Enomoto, T.; Saida, Y. Newly developed proximal release–type colonic stent placement for malignant lower rectal obstruction. VideoGIE 2020, 5, 250–251. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, H.-Y.; Li, Y.-D.; Shin, J.H.; Park, J.-H.; Yu, C.-S. Dual-design expandable colorectal stent for malignant colorectal obstruction: Comparison of flared ends and bent ends. Am. J. Roentgenol. 2009, 193, 248–254. [Google Scholar] [CrossRef]
- Köneş, O.; Kartal, A.; Akarsu, M.; Akarsu, C.; Güneş, M.E.; Alış, H. Colonic stent use in patients with malignant flexure tumors presenting with obstruction. JSLS J. Soc. Laparoendosc. Surg. 2019, 23, 1–6. [Google Scholar] [CrossRef]
- Saida, Y. A review of colonic stents. Gastroenterol. Endosc. 2013, 55, 3–11. [Google Scholar]
- Lee, H.J.; Hong, S.P.; Cheon, J.H.; Kim, T.I.; Kim, W.H.; Park, S.J. Clinical outcomes of self-expandable metal stents for malignant rectal obstruction. Dis. Colon Rectum 2018, 61, 43–50. [Google Scholar] [CrossRef]
- Khot, U.P.; Lang, A.W.; Murali, K.; Parker, M.C. Systematic review of the efficacy and safety of colorectal stents. Br. J. Surg. 2002, 89, 1096–1102. [Google Scholar] [CrossRef]
- Repici, A.; Fregonese, D.; Costamagna, G.; Dumas, R.; Kähler, G.; Meisner, S.; Giovannini, M.; Freeman, J.; Petruziello, L.; Hervoso, C.; et al. Ultraflex precision colonic stent placement for palliation of malignant colonic obstruction: A prospective multicenter study. Gastrointest. Endosc. 2007, 66, 920–927. [Google Scholar] [CrossRef] [PubMed]

| Age, years, median (range) | 73.5 (64–80) |
| Sex, male % (n) | 37.5 (3/8) |
| ECOG performance status score % (n) | |
| 0–1 | 87.5 (7/8) |
| 2–4 | 12.5 (1/8) |
| Etiology of colorectal obstruction % (n) | |
| Primary colorectal cancer | 50.0 (4/8) |
| Anastomotic recurrence | 12.5 (1/8) |
| Gastric cancer | 25.0 (2/8) |
| Pancreatic cancer | 12.5 (1/8) |
| CROSS score before SEMS placement % (n) | |
| 0 | 12.5 (1/8) |
| 1 | 50.0 (4/8) |
| 2 | 12.5 (1/8) |
| 3 | 25.0 (2/8) |
| 4 | 0 (0/8) |
| Location of obstruction % (n) | |
| Rectosigmoid colon | 50.0 (4/8) |
| Anastomosis of the sigmoid colon | 12.5 (1/8) |
| Ra | 12.5 (1/8) |
| Rb | 25.0 (2/8) |
| Stricture length, cm, median (range) | 4 (3–10) |
| Distance from the dentate line, cm, median (range) | 5.5 (2–9) |
| Treatment intent % (n) | |
| Bridge to surgery | 50.0 (4/8) |
| Palliation | 50.0 (4/8) |
| Chemotherapy before SEMS placement % (n) | 50.0 (4/8) |
| Total | Rectal Tumors | Rectosigmoid Colon Tumors | |
|---|---|---|---|
| Technical success rate % (n) | 87.5 (7/8) | 100 (4/4) | 75.0 (3/4) |
| Procedure time, min, mean ± SD | 25.5 ± 22.0 | 12.3 ± 4.3 | 38.8 ± 25.4 * |
| Clinical success rate % (n) | 87.5 (7/8) | 100 (4/4) | 75.0 (3/4) |
| BTS success rate % (n) | 100 (4/4) | - | 100 (4/4) |
| Early adverse events rate % (n) | 12.5 (1/8) | 0 (0/4) | 25.0 (1/4) |
| Stent migration | 12.5 (1/8) | 0 (0/4) | 25.0 (1/4) |
| Perforation | 0 (0/8) | 0 (0/4) | 0 (0/4) |
| Re-obstruction | 0 (0/8) | 0 (0/4) | 0 (0/4) |
| Bleeding | 0 (0/8) | 0 (0/4) | 0 (0/4) |
| Surgical Approach % (n) | |
| Open | 0 (0/0) |
| Laparoscopy | 100 (4/4) |
| Surgical procedures % (n) | |
| Low anterior resection | 100 (4/4) |
| Without diverting stoma | 100 (4/4) |
| Postoperative complications % (n) | |
| Anastomotic leakage | 25.0 (1/4) * |
| Wound infection | 0 (0/4) |
| Intraperitoneal abscess | 0 (0/4) |
| Bowel obstruction | 25.0 (1/4) |
| Overall stoma creation rate % (n) | 25.0 (1/4) * |
| Duration from SEMS to surgery, days, median (range) | 27 (11–144) |
| Postoperative mortality rate % (n) | 0 (0/4) |
| Length of hospital stay, days, median (range) | 16.5 (8–25) |
| Survival period, days, median (range) | 249 (49–344) |
| Late Adverse Events (Including Minor) % (n) | |
| Perforation | 0 (0/4) |
| Stent migration | 0 (0/4) |
| Bleeding | 0 (0/4) |
| Duration of stent patency, days, median (range) | 113.5 (62–420) |
| Survival period, days, median (range) | 113.5 (62–420) |
| Mortality % (n) | 50.0 (2/4) |
| Chemotherapy after SEMS placement % (n) | 100 (4/4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, K.; Kuwai, T.; Sugata, S.; Hamada, T.; Moriuchi, R.; Tamaru, Y.; Kusunoki, R.; Yamaguchi, A.; Kouno, H.; Ishaq, S.; et al. The Deployment of a Newly Developed Proximal Release-Type Colonic Stent Is Feasible for Malignant Colorectal Obstruction near the Anal Verge: A Single-Center Preliminary Study. J. Clin. Med. 2022, 11, 1675. https://doi.org/10.3390/jcm11061675
Wada K, Kuwai T, Sugata S, Hamada T, Moriuchi R, Tamaru Y, Kusunoki R, Yamaguchi A, Kouno H, Ishaq S, et al. The Deployment of a Newly Developed Proximal Release-Type Colonic Stent Is Feasible for Malignant Colorectal Obstruction near the Anal Verge: A Single-Center Preliminary Study. Journal of Clinical Medicine. 2022; 11(6):1675. https://doi.org/10.3390/jcm11061675
Chicago/Turabian StyleWada, Kaoru, Toshio Kuwai, Syuhei Sugata, Takuro Hamada, Riho Moriuchi, Yuzuru Tamaru, Ryusaku Kusunoki, Atsushi Yamaguchi, Hirotaka Kouno, Sauid Ishaq, and et al. 2022. "The Deployment of a Newly Developed Proximal Release-Type Colonic Stent Is Feasible for Malignant Colorectal Obstruction near the Anal Verge: A Single-Center Preliminary Study" Journal of Clinical Medicine 11, no. 6: 1675. https://doi.org/10.3390/jcm11061675
APA StyleWada, K., Kuwai, T., Sugata, S., Hamada, T., Moriuchi, R., Tamaru, Y., Kusunoki, R., Yamaguchi, A., Kouno, H., Ishaq, S., & Kohno, H. (2022). The Deployment of a Newly Developed Proximal Release-Type Colonic Stent Is Feasible for Malignant Colorectal Obstruction near the Anal Verge: A Single-Center Preliminary Study. Journal of Clinical Medicine, 11(6), 1675. https://doi.org/10.3390/jcm11061675






