Does Additional Dietary Supplementation Improve Physiotherapeutic Treatment Outcome in Tendinopathy? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.1.1. Participants
2.1.2. Interventions
2.1.3. Comparisons
2.1.4. Outcomes
2.1.5. Study Design
2.2. Search Strategy and Study Selection
2.3. Data Extraction
2.4. Quality Assessment and Risk of Bias
2.5. Statistical Analysis
3. Results
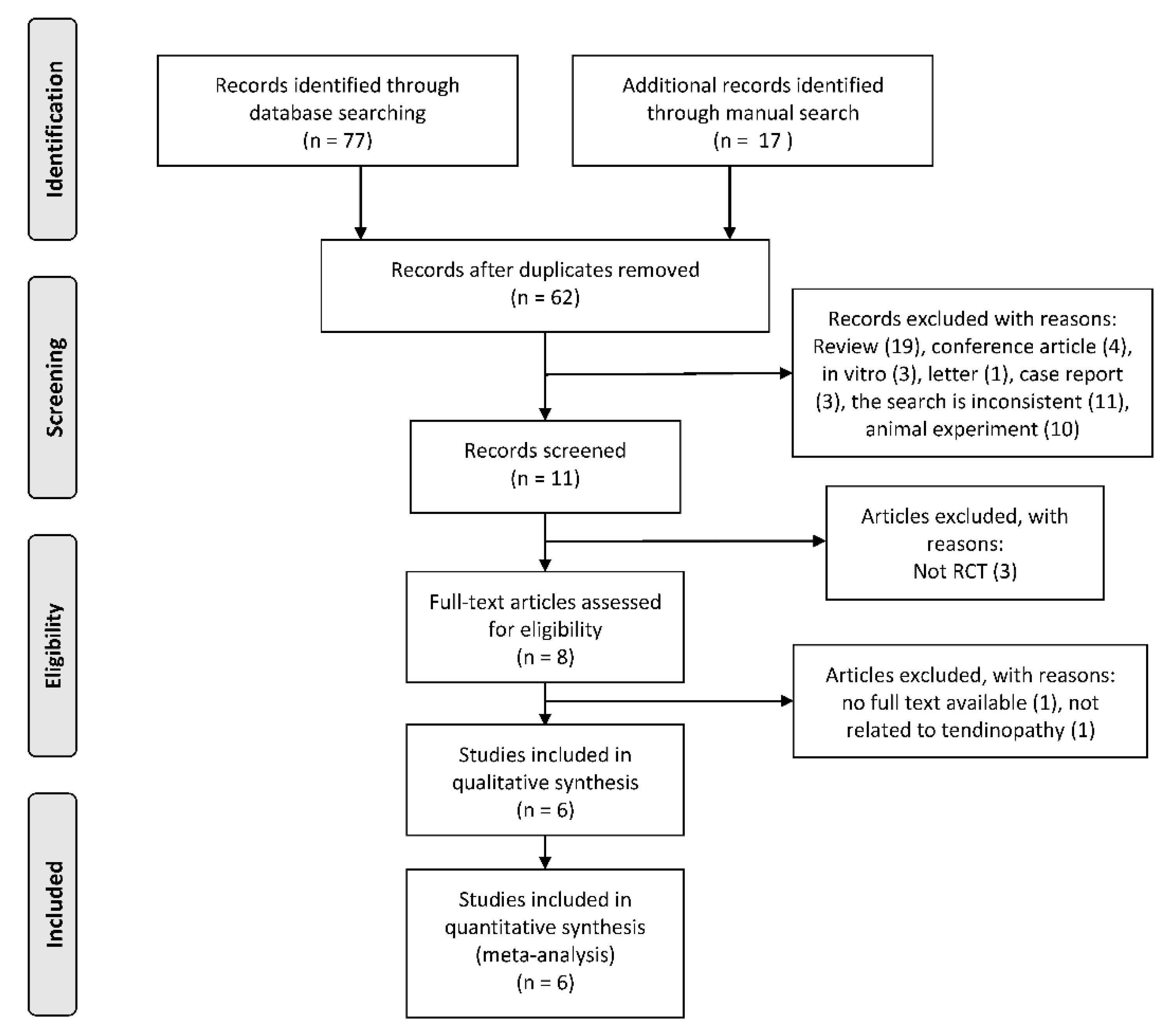
3.1. Search Yield
3.2. Characteristics of Included Studies
3.3. Assessment of Risk of Bias
3.4. Meta-Analysis
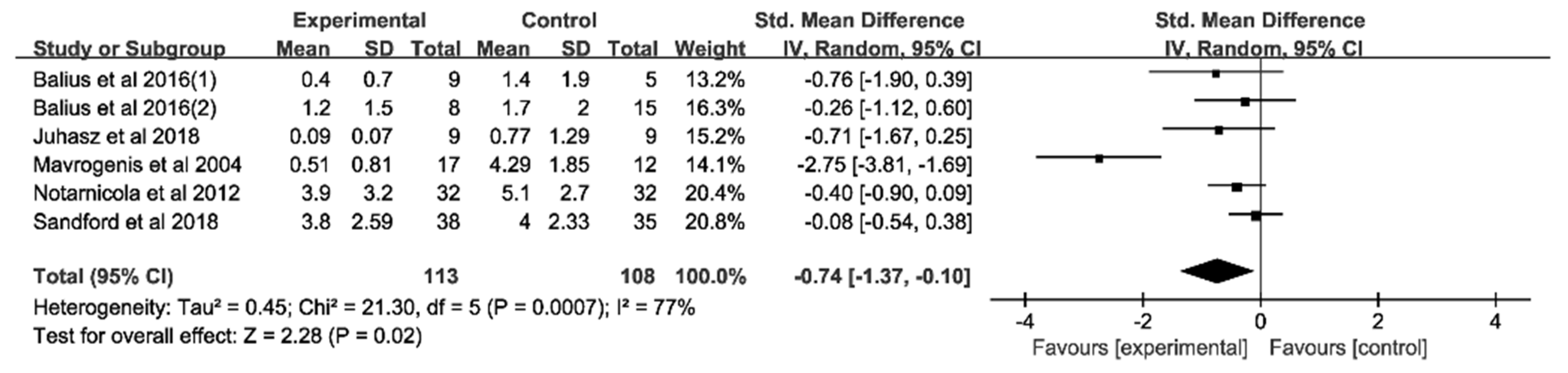
3.4.1. Analysis of Pain Score at Rest
3.4.2. Analysis of Functional Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fusini, F.; Bisicchia, S.; Bottegoni, C.; Gigante, A.; Zanchini, F.; Busilacchi, A. Nutraceutical supplement in the management of tendinopathies: A systematic review. Muscles Ligaments Tendons J. 2016, 6, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Murrell, G.A.C. The basic science of tendinopathy. Clin. Orthop. Relat. Res. 2008, 466, 1528–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skovgaard, D.; Siersma, V.D.; Klausen, S.B.; Visnes, H.; Haukenes, I.; Bang, C.W.; Bager, P.; Gravare Silbernagel, K.; Gaida, J.; Magnusson, S.P.; et al. Chronic hyperglycemia, hypercholesterolemia, and metabolic syndrome are associated with risk of tendon injury. Scand. J. Med. Sci. Sports 2021, 31, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Squier, K.; Scott, A.; Hunt, M.A.; Brunham, L.R.; Wilson, D.R.; Screen, H.; Waugh, C.M. The effects of cholesterol accumulation on Achilles tendon biomechanics: A cross-sectional study. PLoS ONE 2021, 16, e0257269. [Google Scholar] [CrossRef] [PubMed]
- Ranger, T.A.; Wong, A.M.Y.; Cook, J.L.; Gaida, J.E. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br. J. Sports Med. 2015, 50, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Knapik, J.J.; Pope, R. Achilles Tendinopathy: Pathophysiology, Epidemiology, Diagnosis, Treatment, Prevention, and Screening. J. Spec. Oper. Med. 2020, 20, 125–140. [Google Scholar] [PubMed]
- Sobhani, S.; Dekker, R.; Postema, K.; Dijkstra, P.U. Epidemiology of ankle and foot overuse injuries in sports: A systematic review. Scand. J. Med. Sci. Sports 2012, 23, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Lodise, T.P. Fluoroquinolone-Associated Tendinopathy: Does Levofloxacin Pose the Greatest Risk? Pharmacotherapy 2016, 36, 679–693. [Google Scholar] [CrossRef]
- Mani-Babu, S.; Morrissey, D.; Waugh, C.; Screen, H.; Barton, C. The Effectiveness of Extracorporeal Shock Wave Therapy in Lower Limb Tendinopathy: A Systematic Review. Am. J. Sports Med. 2014, 43, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Desjardins-Charbonneau, A.; Roy, J.-S.; Dionne, C.E.; Fremont, P.; MacDermid, J.C.; Desmeules, F. The Efficacy of Manual Therapy for Rotator Cuff Tendinopathy: A Systematic Review and Meta-analysis. J. Orthop. Sports Phys. Ther. 2015, 45, 330–350. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.; Kongsgaard, M.; Hougs Kjær, B.; Øhlenschlæger, T.; Kjær, M.; Magnusson, S.P. Heavy Slow Resistance Versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, G.; Wolfarth, B.; Legerlotz, K. Interleukin-6 levels drop after a 12 week long physiotherapeutic intervention in patients with Achilles tendinopathy—A pilot study. Transl. Sports Med. 2019, 2, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Woodley, B.L.; Newsham-West, R.J.; Baxter, G.D. Chronic tendinopathy: Effectiveness of eccentric exercise. Br. J. Sports Med. 2007, 41, 188–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Vlist, A.C.; Winters, M.; Weir, A.; Ardern, C.L.; Welton, N.J.; Caldwell, D.M.; Verhaar, J.A.N.; de Vos, R.J. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br. J. Sports Med. 2021, 55, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Lee-Barthel, A.; Ross, M.L.; Wang, B.; Baar, K. Vitamin C–enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.L.; Sebastianelli, W.; Flechsenhar, K.R.; Aukermann, D.F.; Meza, F.; Millard, R.L.; Deitch, J.R.; Sherbondy, P.S.; Albert, A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008, 24, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Zdzieblik, D.; Oesser, S.; Gollhofer, A.; König, D. Improvement of activity-related knee joint discomfort following supplementation of specific collagen peptides. Appl. Physiol. Nutr. Metab. 2017, 42, 588–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiyegbusi, A.I.; Duru, F.I.; Awelimobor, D.; Noronha, C.C.; Okanlawon, A.O. The role of aqueous extract of pineapple fruit parts on the healing of acute crush tendon injury. Niger. Q. J. Hosp. Med. 2010, 20, 223–227. [Google Scholar]
- Kao, W.W.-Y.; Flaks, J.G.; Prockop, D.J. Primary and secondary effects of ascorbate on procollagen synthesis and protein synthesis by primary cultures of tendon fibroblasts. Arch. Biochem. Biophys. 1976, 173, 638–648. [Google Scholar] [CrossRef]
- Balius, R.; Álvarez, G.; Baró, F.; Jiménez, F.; Pedret, C.; Costa, E.; Martínez-Puig, D. A 3-Arm Randomized Trial for Achilles Tendinopathy: Eccentric Training, Eccentric Training Plus a Dietary Supplement Containing Mucopolysaccharides, or Passive Stretching Plus a Dietary Supplement Containing Mucopolysaccharides. Curr. Ther. Res. Clin. Exp. 2016, 78, 1–7. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Anti-inflammatory and anti-catabolic effects of TENDOACTIVE® on human tenocytes in vitro. Histol. Histopathol. 2011, 26, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Curi, R.; Costa Rosa, L.F. Creatine supplementation reduces plasma levels of pro-inflammatory cytokines and PGE2 after a half-ironman competition. Amino Acids 2008, 35, 425–431. [Google Scholar] [CrossRef] [PubMed]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; Laprade, R.F. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef] [PubMed]
- Ömeroğlu, S.; Peker, T.; Türközkan, N.; Ömeroğlu, H. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Arch. Orthop. Trauma Surg. 2009, 129, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Puig, D.M.; Arquer, A.; García, M.; Laucirica, J.A.; Rius, M.; Blàvia, M.; Fontserè, J.; Hernández, C.; Boluda, J.; Kranjcec, T. The efficacy and safety of oral mucopolysaccharide, type I collagen and vitamin C treatment in tendinopathy patients. Apunt. Med. De L’esport 2014, 49, 31–36. [Google Scholar]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon healing in presence of chronic low-level inflammation: A systematic review. Br. Med. Bull. 2019, 132, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.C.; Cheng, W.-H.; Cheuk, Y.-C.; Mok, T.-Y.; Rolf, C.; Yung, S.-H.; Chan, K.-M. Development of vitamin C irrigation saline to promote graft healing in anterior cruciate ligament reconstruction. J. Orthop. Transl. 2013, 1, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Juhasz, I.; Kopkane, J.P.; Hajdu, P.; Szalay, G.; Kopper, B.; Tihanyi, J. Creatine Supplementation Supports the Rehabilitation of Adolescent Fin Swimmers in Tendon Overuse Injury Cases. J. Sports Sci. Med. 2018, 17, 279–288. [Google Scholar] [PubMed]
- Mavrogenis, S.; Johannessen, E.; Jensen, P.; Sindberg, C. The effect of essential fatty acids and antioxidants combined with physiotherapy treatment in recreational athletes with chronic tendon disorders: A randomised, double-blind, placebo-controlled study. Phys. Ther. Sport 2004, 5, 194–199. [Google Scholar] [CrossRef]
- Merolla, G.; Dellabiancia, F.; Ingardia, A.; Paladini, P.; Porcellini, G. Co-analgesic therapy for arthroscopic supraspinatus tendon repair pain using a dietary supplement containing Boswellia serrata and Curcuma longa: A prospective randomized placebo-controlled study. Musculoskelet. Surg. 2015, 99 (Suppl. 1), S43–S52. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Pesce, V.; Vicenti, G.; Tafuri, S.; Forcignanò, M.; Moretti, B. SWAAT Study: Extracorporeal Shock Wave Therapy and Arginine Supplementation and Other Nutraceuticals for Insertional Achilles Tendinopathy. Adv. Ther. 2012, 29, 799–814. [Google Scholar] [CrossRef]
- Praet, S.F.E.; Purdam, C.R.; Welvaert, M.; Vlahovich, N.; Lovell, G.; Burke, L.M.; Gaida, J.E.; Manzanero, S.; Hughes, D.; Waddington, G. Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients. Nutrients 2019, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Sandford, F.M.; Sanders, T.; Wilson, H.; Lewis, J.S. A randomised controlled trial of long-chain omega-3 polyunsaturated fatty acids in the management of rotator cuff related shoulder pain. BMJ Open Sport Exerc. Med. 2018, 4, e000414. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S.; European Palliative Care Research Collaborative (EPCRC). Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Dawson, J.; Hill, G.; Fitzpatrick, R.; Carr, A. The benefits of using patient-based methods of assessment. Medium-term results of an observational study of shoulder surgery. J. Bone Jt. Surg. Br. Vol. 2001, 83, 877–882. [Google Scholar] [CrossRef]
- Angst, F.; Schwyzer, H.-K.; Aeschlimann, A.; Simmen, B.R.; Goldhahn, J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short ver-sion (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI). Arthritis Care Res. 2011, 63 (Suppl. 11), S174–S188. [Google Scholar] [CrossRef]
- Bot, S.D.; Terwee, C.B.; van der Windt, D.A.; Bouter, L.M.; Dekker, J.; de Vet, H.C. Clinimetric evaluation of shoulder disability questionnaires: A systematic review of the literature. Ann. Rheum. Dis. 2004, 63, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, J.D.; McAuley, J.H. Shoulder Pain and Disability Index (SPADI). J. Physiother. 2011, 57, 197. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.; Rio, E.; Debenham, J.; Docking, S.; Travers, M.; Gibson, W. Evaluating the Progress of Mid-Portion Achilles Tendinopathy during Rehabilitation: A Review of Outcome Measures for Self- Reported Pain and Function. Int. J. Sports Phys. Ther. 2018, 13, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostuj, T.; Stief, F.; Hartmann, K.A.; Schaper, K.; Arabmotlagh, M.; Baums, M.H.; Meurer, A.; Krummenauer, F.; Lieske, S. Using the Oxford Foot Model to determine the association between objective measures of foot function and results of the AOFAS Ankle-Hindfoot Scale and the Foot Function Index: A prospective gait analysis study in Germany. BMJ Open 2018, 8, e019872. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Borenstein, M. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Bowden, J.; Tierney, J.F.; Copas, A.J.; Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Alfredson, H.; Cook, J. A treatment algorithm for managing Achilles tendinopathy: New treatment options. Br. J. Sports Med. 2007, 41, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.B.; Pizzari, T.; Kinsella, R.; Hope, D.; Cook, J.L. Current trends in tendinopathy management. Best. Pract. Res. Clin. Rheumatol. 2019, 33, 122–140. [Google Scholar] [CrossRef]
- Murphy, M.; Travers, M.; Gibson, W.; Chivers, P.; Debenham, J.; Docking, S.; Rio, E. Rate of Improvement of Pain and Function in Mid-Portion Achilles Tendinopathy with Loading Protocols: A Systematic Review and Longitudinal Meta-Analysis. Sports Med. 2018, 48, 1875–1891. [Google Scholar] [CrossRef] [PubMed]
- Vander Doelen, T.; Jelley, W. Non-surgical treatment of patellar tendinopathy: A systematic review of randomized controlled trials. J. Sci. Med. Sport 2020, 23, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigdellivol, J.; Comellas Berenger, C.; Perez Fernandez, M.A.; Cowalinsky Millan, J.M.; Carreras Vidal, C.; Gil Gil, I.; Martinez Pagan, J.; Ruiz Nieto, B.; Jimenez Gomez, F.; Comas Figuerola, F.X.; et al. Effectiveness of a Dietary Supplement Containing Hydrolyzed Collagen, Chondroitin Sulfate, and Glucosamine in Pain Reduction and Functional Capacity in Osteoarthritis Patients. J. Diet. Suppl. 2019, 16, 379–389. [Google Scholar] [CrossRef]
- Dragan, S.; Serban, M.C.; Damian, G.; Buleu, F.; Valcovici, M.; Christodorescu, R. Dietary Patterns and Interventions to Alleviate Chronic Pain. Nutrients 2020, 12, 2510. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, A.-S.; Svensson, R.B.; Malmgaard-Clausen, N.M.; Couppé, C.; Hjortshoej, M.H.; Doessing, S.; Kjaer, M.; Magnusson, S.P. Clinical Outcomes, Structure, and Function Improve with Both Heavy and Moderate Loads in the Treatment of Patellar Tendinopathy: A Randomized Clinical Trial. Am. J. Sports Med. 2021, 49, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of inflammatory mediators with pain perception. Biomed. Pharm. 2017, 96, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Legerlotz, K.; Jones, E.R.; Screen, H.R.; Riley, G.P. Increased expression of IL-6 family members in tendon pathology. Rheumatology 2012, 51, 1161–1165. [Google Scholar] [CrossRef] [Green Version]
- Vitali, M.; Naim Rodriguez, N.; Pironti, P.; Drossinos, A.; Di Carlo, G.; Chawla, A.; Gianfranco, F. ESWT and nutraceutical supplementation (Tendisulfur Forte) vs ESWT-only in the treatment of lateral epicondylitis, Achilles tendinopathy, and rotator cuff tendinopathy: A comparative study. J. Drug Assess. 2019, 8, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Dierckxsens, Y.; Delisse, G.; Seidel, L.; Albert, A. Curcuminoids and Boswellia serrata extracts combination decreases tendinopathy symptoms: Findings from an open-label post-observational study. Curr. Med. Res. Opin. 2021, 37, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Gumina, S.; Passaretti, D.; Gurzi, M.D.; Candela, V. Arginine L-alpha-ketoglutarate, methylsulfonylmethane, hydrolyzed type I collagen and bromelain in rotator cuff tear repair: A prospective randomized study. Curr. Med. Res. Opin. 2012, 28, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Sahu, S.; Vasudeva, A.; Sheikh, N.A.; Venkataraman, S.; Handa, G.; Wadhwa, S.; Singh, U.; Gamanagati, S.; Yadav, S.L. Comparing Effectiveness of Combination of Collagen Peptide Type-1, Low Molecular Weight Chondroitin Sulphate, Sodium Hyaluronate, and Vitamin-C Versus Oral Diclofenac Sodium in Achilles Tendinopathy: A Prospective Randomized Control Trial. Cureus 2021, 13, e19737. [Google Scholar] [CrossRef] [PubMed]
- Morina, D.; Fernandez-Castillejo, S.; Valls, R.M.; Pedret, A.; Taltavull, N.; Romeu, M.; Giralt, M.; Montero, M.; Bernal, G.; Faba, J.; et al. Effectiveness of a low-fat yoghurt supplemented with rooster comb extract on muscle strength in adults with mild knee pain and mechanisms of action on muscle regeneration. Food Funct. 2018, 9, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Pipis, H.; Freitag, M.S.C.; Izquierdo, M.D.C.; Freitag, K.; Theoharides, T.C. Effects of an Extract of Salmon Milt on Symptoms and Serum TNF and Substance P in Patients with Fibromyalgia Syndrome. Clin. Ther. 2019, 41, 1564–1574.e1562. [Google Scholar] [CrossRef] [PubMed]
- Baar, K. Stress Relaxation and Targeted Nutrition to Treat Patellar Tendinopathy. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemeier, K.M.; Øhlenschlæger, T.F.; Mikkelsen, U.R.; Sønder, F.; Schjerling, P.; Svensson, R.B.; Kjaer, M. Effects of anti-inflammatory (NSAID) treatment on human tendinopathic tissue. J. Appl. Physiol. 2017, 123, 1397–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cömert Kılıç, S. Does glucosamine, chondroitin sulfate, and methylsulfonylmethane supplementation improve the outcome of temporomandibular joint osteoarthritis management with arthrocentesis plus intraarticular hyaluronic acid injection. A randomized clinical trial. J. Cranio-Maxillofac. Surg. 2021, 49, 711–718. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, B.J.; Theocharidis, A.; Adebero, T.; Kurgan, N.; Fajardo, V.A.; Roy, B.D.; Josse, A.R.; Logan-Sprenger, H.M.; Falk, B.; Klentrou, P. Effects of Post-Exercise Whey Protein Consumption on Recovery Indices in Adolescent Swimmers. Int. J. Environ. Res. Public Health 2020, 17, 7761. [Google Scholar] [CrossRef]
- Shahnazi, M.; Mohammadi, M.; Mohaddes, G.; Latifi, Z.; Ghasemnejad, T.; Nouri, M.; Fattahi, A. Dietary omega-3 and -6 fatty acids affect the expression of prostaglandin E2 synthesis enzymes and receptors in mice uteri during the window of pre-implantation. Biochem. Biophys. Res. Commun. 2018, 503, 1754–1760. [Google Scholar] [CrossRef]
- Baugé, C.; Leclercq, S.; Conrozier, T.; Boumediene, K. TOL19-001 reduces inflammation and MMP expression in monolayer cultures of tendon cells. BMC Complement. Altern. Med. 2015, 15, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.-M.; Fu, S.-C. Anti-inflammatory management for tendon injuries-friends or foes? BMC Sports Sci. Med. Rehabil. 2009, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerschnig, M.; Stolberg-Stolberg, J.; Schmidt, C.; Wienerroither, V.; Plecko, M.; Schlichting, K.; Perka, C.; Dynybil, C. Effect of COX-2 inhibition on tendon-to-bone healing and PGE2 concentration after anterior cruciate ligament reconstruction. Eur. J. Med. Res. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, B.; Dandanell, S.; Kjaer, M.; Langberg, H. Effect of anti-inflammatory medication on the running-induced rise in patella tendon collagen synthesis in humans. J. Appl. Physiol. 2010, 110, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åström, M.; Westlin, N. No effect of piroxicam on Achilles tendinopathy: A randomized study of 70 patients. Acta Orthop. Scand. 1992, 63, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Pattanittum, P.; Turner, T.; Green, S.; Buchbinder, R. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst. Rev. 2013, 5, CD003686. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, P.; Lin, H.; Geng, H. Curcumin improves tendon healing in rats: A histological, biochemical, and functional evaluation. Connect. Tissue Res. 2016, 57, 20–27. [Google Scholar] [CrossRef] [PubMed]

| First Author (Year) | Tendon Investigated | Study Groups | Sample Size (n) | Type of Population | Mean Age (Years; Mean ± SD) | Intervention Duration (Weeks) | Symptom Duration (Months) | Time Points of Measurement |
|---|---|---|---|---|---|---|---|---|
| Balius et al. (2016) | Achilles tendon (mid-portion) | MCVC + EC | 17 | non-athletic | 43.5 ± 14.5 | 12 | >3 | baseline, 6 and 12 w |
| EC | 20 | 38.9 ± 6.6 | ||||||
| Juhasz et al. (2018) | Musculus flexor hallucis longus | Creatine | 9 | athletic | 15.5 ± 1.4 | 6 | 1–1.5 | 2, 4 and 6 w |
| Placebo | 9 | 14.8 ± 1.6 | ||||||
| Mavrogenis et al. (2004) | Patellar & several upper body tendons * | EFA, AO and US | 17 | athletic | 31 | 5 | >3 | 8, 16, 24 and 32 d |
| Placebo and US | 14 | 32 | ||||||
| Notarnicola et al. (2012) | Achilles tendon (insertional) | ESWT and tenosan | 32 | non-athletic | 55.8 ± 13.2 | 8 | >6 | 2 and 6 m |
| ESWT and placebo | 32 | |||||||
| Praet et al. (2019) | Achilles tendon (mid-portion) | TENDOFORTE + EccEx | 10 | non-athletic | 45.3 ± 6.4 | 12 | 18 | 3 and 6 m |
| Placebo + EccEx | 10 | 42.0 ± 9.4 | ||||||
| Sandford et al. (2018) | Rotator cuff | PUFAs | 38 | non-athletic | 52.2 ± 12.0 | 8 | >3 | 8 w, 3, 6 and 12 m |
| Placebo | 35 | 52.0 ± 16.2 |
| First Author (Year) | Dietary Supplements (Company) | Ingredients of Dietary Supplement | Assumed Effect of Supplement |
|---|---|---|---|
| Balius et al. (2016) | TendoActive (Bioiberica SA, Palafolls, Spain) | mucopolysaccharides, collagen type I, vitamin C | suppression of NF-κB mediated IL-1ß catabolic signalling pathways in tenocytes |
| Juhasz et al. (2018) | Micronized Cr monohydrate (BioTech, Inc., Ft. Lauderdale, FL, USA) | Cr monohydrate, dextrose, and vitamin C | reduction of inflammatory markers |
| Mavrogenis et al. (2004) | Bio-Sport (Pharma Nord ApS, Vejle, Denmark) | EPA, DHA and GLA. selenium, zinc, vitamin A, vitamin B6, vitamin C and vitamin E | reduction of inflammation caused by essential fatty acids and antioxidants |
| Notarnicola et al. (2012) | Tenosan (Agave s.r.l., Prato, Italy) | arginine-L-alpha-ketoglutarate, MSM, hydrolysed collagen type I, Vinitrox, bromelain, and vitamin C | stimulation of metabolism and proliferation; reduction of inflammation and neoangiogenesis |
| Praet et al. (2019) | TENDOFORTE® (GELITA AG, Eberbach, Germany) | Hydrolysed specific collagen peptides | stimulation of collagen type I and III, proteoglycans and elastin content synthesis by sCPs; reduction of TNF-alpha, matrix metalloproteases and stimulation of tissue inhibitors of metalloproteinases by Glycine |
| Sandford et al. (2018) | MaxEPA (Seven Seas Ltd., Hull, UK) | EPA, DHA and vitamin E acetate | reduction of inflammation |
| First Author (year) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Balius et al. (2016) | + | - | - | - | ? | - | - |
| Juhasz et al. (2018) | ? | ? | ? | ? | + | + | + |
| Mavrogenis et al. (2004) | + | + | + | + | - | + | ? |
| Notarnicola et al. (2012) | - | ? | + | ? | - | + | - |
| Praet et al. (2019) | + | + | + | + | + | + | + |
| Sandford et al. (2018) | + | + | + | + | + | + | + |
| Study Characteristics | Studies | Pain at Rest | p Value | Functional Outcomes | ||
|---|---|---|---|---|---|---|
| Effect Size (95%CI) | Studies | Effect Size (95%CI) | p Value | |||
| Type of Tendinopathy | ||||||
| Achilles Tendon | 2 | −0.41 (−0.83, 0.00) | >0.05 | 3 | 0.53 (0.16, 0.90) | 0.005 |
| Other Type | 2 | −1.72 (−3.72, 0.28) | >0.05 | - | - | - |
| Intervention Duration | ||||||
| ≤8 weeks | 4 | −0.88 (−1.78, 0.08) | >0.05 | 2 | 0.28 (−0.06, 0.62) | >0.05 |
| >8 weeks | 2 | 0.32 (−0.22, 0.86) | >0.05 | |||
| Type of physiotherapy | ||||||
| Exercise therapy | 3 | −0.26 (−0.62, 0.09) | >0.05 | 3 | 0.09 (−0.26, 0.44) | > 0.05 |
| ESWT/US | 2 | −1.53 (−3.83, 0.78) | >0.05 | - | - | - |
| Type of population | ||||||
| Athletic | 2 | −1.72 (−3.72, 0.28) | >0.05 | - | - | - |
| Non-athletic | 3 | −0.26 (−0.57, 0.05) | >0.05 | 4 | 0.29 (0.00, 0.58) | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Li, J.; Legerlotz, K. Does Additional Dietary Supplementation Improve Physiotherapeutic Treatment Outcome in Tendinopathy? A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1666. https://doi.org/10.3390/jcm11061666
Qiu F, Li J, Legerlotz K. Does Additional Dietary Supplementation Improve Physiotherapeutic Treatment Outcome in Tendinopathy? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(6):1666. https://doi.org/10.3390/jcm11061666
Chicago/Turabian StyleQiu, Fanji, Jinfeng Li, and Kirsten Legerlotz. 2022. "Does Additional Dietary Supplementation Improve Physiotherapeutic Treatment Outcome in Tendinopathy? A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 6: 1666. https://doi.org/10.3390/jcm11061666
APA StyleQiu, F., Li, J., & Legerlotz, K. (2022). Does Additional Dietary Supplementation Improve Physiotherapeutic Treatment Outcome in Tendinopathy? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(6), 1666. https://doi.org/10.3390/jcm11061666






