Characteristic Assessment of Angiographies at Different Depths with AS-OCTA: Implication for Functions of Post-Trabeculectomy Filtering Bleb
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Technique
2.3. Outcome Measures
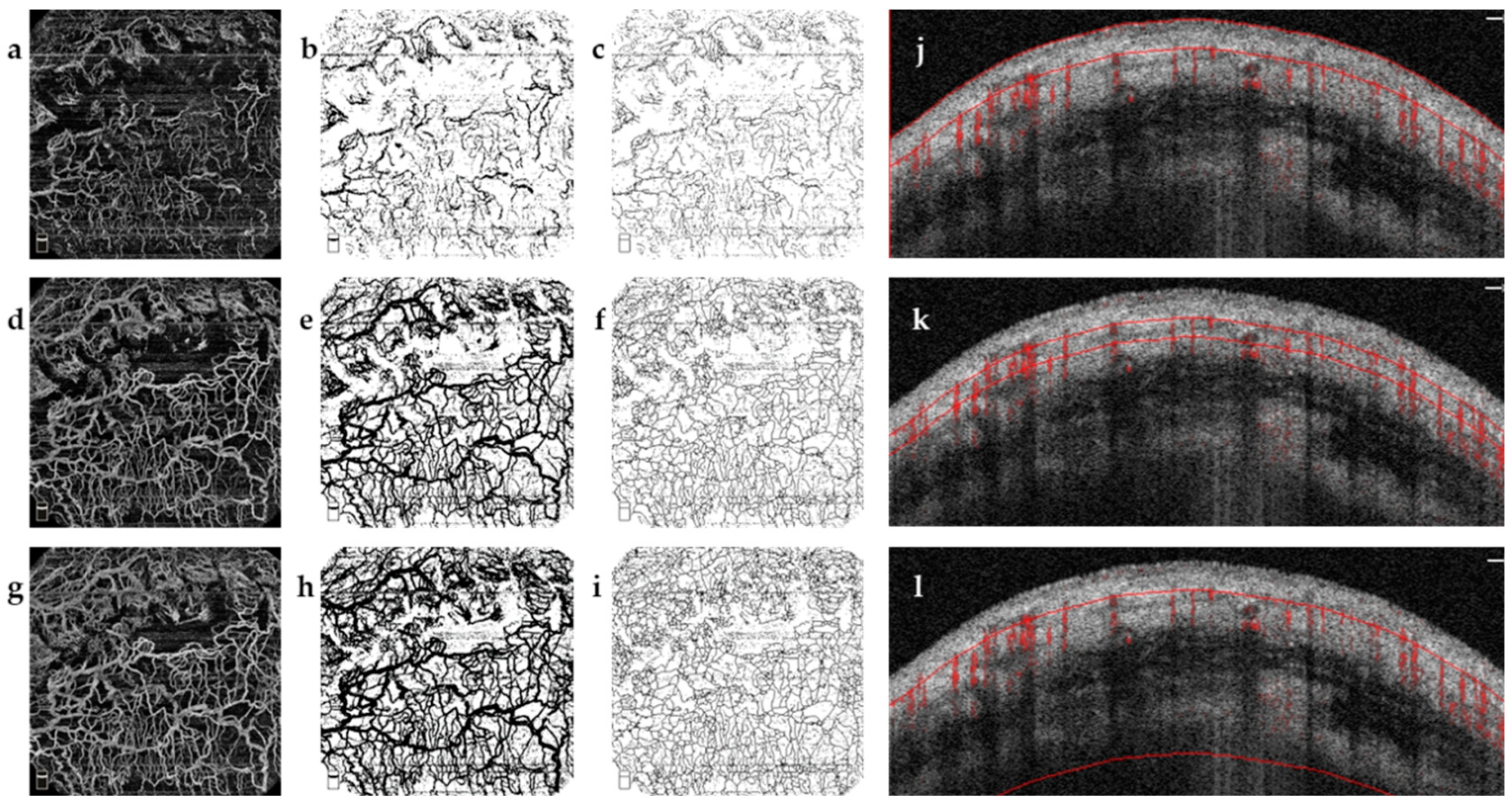
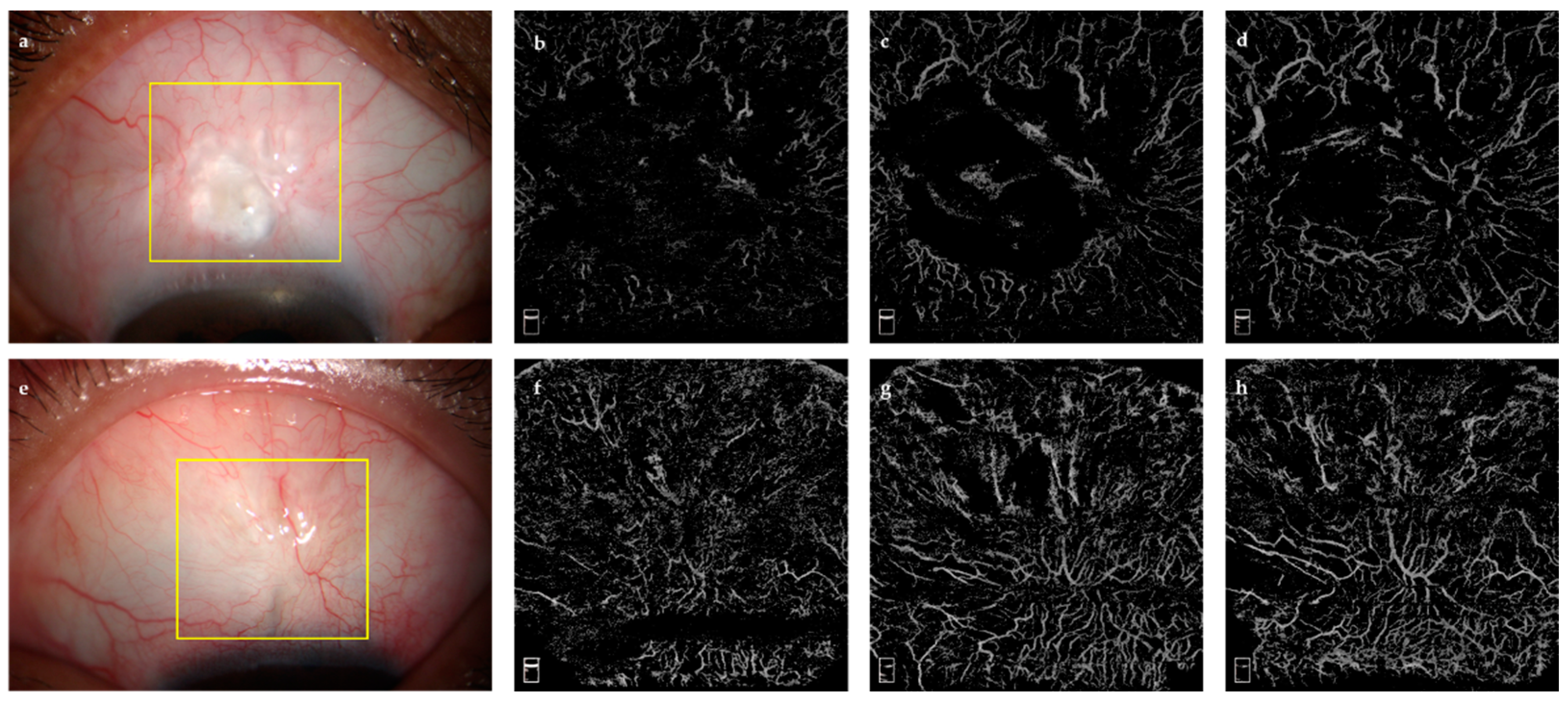
2.3.1. Bleb Evaluation Using OCTA
- AS-OCTA Image Acquisition and Processing
- 2.
- Quantitative Analysis
2.3.2. Bleb Evaluation Using OCT
2.3.3. Grading Using Conventional Bleb Grading Systems
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. VD and VDI in Bleb Area
3.3. Predictive Factors for Surgical Outcomes
3.3.1. Setting the IOP as the Dependent Variable
3.3.2. Setting an IOP of ≤21 mmHg as the Criteria for Surgical Success
3.4. Diagnostic Accuracy and Cut-Off Value
3.5. Influence of PG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Wang, N.L.; Wong, T.Y.; Congdon, N.; He, M.G.; Wang, Y.X.; Braithwaite, T.; Casson, R.J.; Cicinelli, M.V.; Das, A.; et al. Prevalence and causes of vision loss in East Asia in 2015: Magnitude, temporal trends and projections. Br. J. Ophthalmol. 2020, 104, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef]
- Cairns, J.E. Trabeculectomy. Preliminary report of a new method. Am. J. Ophthalmol. 1968, 66, 673–679. [Google Scholar] [CrossRef]
- Joseph, J.P.; Miller, M.H.; Hitchings, R.A. Wound healing as a barrier to successful filtration surgery. Eye 1988, 2, S113–S123. [Google Scholar] [CrossRef]
- Richter, C.U.; Shingleton, B.J.; Bellows, A.R.; Hutchinson, B.T.; O’Connor, T.; Brill, I. The development of encapsulated filtering blebs. Ophthalmology 1988, 95, 1163–1168. [Google Scholar] [CrossRef]
- Beer, T.W.; Baldwin, H.C.; Goddard, J.R.; Gallagher, P.J.; Wright, D.H. Angiogenesis in pathological and surgical scars. Hum. Pathol. 1998, 29, 1273–1278. [Google Scholar] [CrossRef]
- Shaunak, S.; Thomas, S.; Gianasi, E.; Godwin, A.; Jones, E.; Teo, I.; Mireskandari, K.; Luthert, P.; Duncan, R.; Patterson, S.; et al. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat. Biotechnol. 2004, 22, 977–984. [Google Scholar] [CrossRef]
- Brown, N.J.; Smyth, E.A.E.; Cross, S.S.; Reed, M.W.R. Angiogenesis induction and regression in human surgical wounds. Wound Repair Regen. 2002, 10, 245–251. [Google Scholar] [CrossRef]
- Schlunck, G.; Meyer-ter-Vehn, T.; Klink, T.; Grehn, F. Conjunctival fibrosis following filtering glaucoma surgery. Exp. Eye Res. 2016, 142, 76–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Nie, F.F.; Chen, X.L.; Qin, Z.L.; Kang, C.F.; Chen, B.; Ma, J.X.; Pan, B.L.; Ma, Y.G. Upregulated periostin promotes angiogenesis in keloids through activation of the ERK 1/2 and focal adhesion kinase pathways, as well as the upregulated expression of VEGF and angiopoietin-1. Mol. Med. Rep. 2015, 11, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Sacu, S.; Rainer, G.; Findl, O.; Georgopoulos, M.; Vass, C. Correlation between the early morphological appearance of filtering blebs and outcome of trabeculectomy with mitomycin C. J. Glaucoma 2003, 12, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.R.; Clarke, J.C.K.; Peto, T.; Minassian, D.; Khaw, P.T. Postoperative increased bleb vascularity persists for over one year and has implications for intraocular pressure control. Investig. Ophthalmol. Vis. Sci. 2004, 45, U377. [Google Scholar]
- Palanca-Capistrano, A.M.; Hall, J.; Cantor, L.B.; Morgan, L.; Hoop, J.; WuDunn, D. Long-term outcomes of intraoperative 5-fluorouracil versus intraoperative mitomycin C in primary trabeculectomy surgery. Ophthalmology 2009, 116, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Cantor, L.B.; Mantravadi, A.; WuDunn, D.; Swamynathan, K.; Cortes, A. Morphologic classification of filtering blebs after glaucoma filtration surgery: The Indiana Bleb Appearance Grading Scale. J. Glaucoma 2003, 12, 266–271. [Google Scholar] [CrossRef]
- Wells, A.P.; Crowston, J.G.; Marks, J.; Kirwan, J.F.; Smith, G.; Clarke, J.C.; Shah, R.; Vieira, J.; Bunce, C.; Murdoch, I.; et al. A pilot study of a system for grading of drainage blebs after glaucoma surgery. J. Glaucoma 2004, 13, 454–460. [Google Scholar] [CrossRef]
- Picht, G.; Grehn, F. Classification of filtering blebs in trabeculectomy: Biomicroscopy and functionality. Curr. Opin. Ophthalmol. 1998, 9, 2–8. [Google Scholar] [CrossRef]
- Wells, A.P.; Ashraff, N.N.; Hall, R.C.; Purdie, G. Comparison of two clinical Bleb grading systems. Ophthalmology 2006, 113, 77–83. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakuma, T.; Kitazawa, Y. An ultrasound biomicroscopic study of filtering blebs after mitomycin C trabeculectomy. Ophthalmology 1995, 102, 1770–1776. [Google Scholar] [CrossRef]
- McWhae, J.A.; Crichton, A.C. The use of ultrasound biomicroscopy following trabeculectomy. Can. J. Ophthalmol. 1996, 31, 187–191. [Google Scholar]
- Leung, C.K.; Yick, D.W.; Kwong, Y.Y.; Li, F.C.; Leung, D.Y.; Mohamed, S.; Tham, C.C.; Chung-chai, C.; Lam, D.S. Analysis of bleb morphology after trabeculectomy with Visante anterior segment optical coherence tomography. Br. J. Ophthalmol. 2007, 91, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Labbe, A.; Hamard, P.; Iordanidou, V.; Dupont-Monod, S.; Baudouin, C. Utility of the Visante OCT in the follow-up of glaucoma surgery. J. Fr. Ophtalmol. 2007, 30, 225–231. [Google Scholar] [PubMed]
- Singh, M.; Aung, T.; Friedman, D.S.; Zheng, C.; Foster, P.J.; Nolan, W.P.; See, J.L.; Smith, S.D.; Chew, P.T.K. Anterior segment optical coherence tomography imaging of trabeculectomy blebs before and after laser suture lysis. Am. J. Ophthalmol. 2007, 143, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chew, P.T.K.; Friedman, D.S.; Nolan, W.P.; See, J.L.; Smith, S.D.; Zheng, C.; Foster, P.J.; Aung, T. Imaging of trabeculectomy blebs using anterior segment optical coherence tomography. Ophthalmology 2007, 114, 47–53. [Google Scholar] [CrossRef]
- Kawana, K.; Kiuchi, T.; Yasuno, Y.; Oshika, T. Evaluation of Trabeculectomy Blebs Using 3-Dimensional Cornea and Anterior Segment Optical Coherence Tomography. Ophthalmology 2009, 116, 848–855. [Google Scholar] [CrossRef]
- Singh, M.; Aung, T.; Aquino, M.C.; Chew, P.T.K. Utility of Bleb Imaging With Anterior Segment Optical Coherence Tomography in Clinical Decision-making After Trabeculectomy. J. Glaucoma 2009, 18, 492–495. [Google Scholar] [CrossRef]
- Messmer, E.M.; Zapp, D.M.; Mackert, M.J.; Thiel, M.; Kampik, A. In vivo confocal microscopy of filtering blebs after trabeculectomy. Arch. Ophthalmol. Chic. 2006, 124, 1095–1103. [Google Scholar] [CrossRef][Green Version]
- Caglar, C.; Karpuzoglu, N.; Batur, M.; Yasar, T. In Vivo Confocal Microscopy and Biomicroscopy of Filtering Blebs After Trabeculectomy. J. Glaucoma 2016, 25, e377–e383. [Google Scholar] [CrossRef]
- Alsagoff, Z.; Chew, P.T.; Chee, C.K.; Wong, J.S.; Aung, T. Indocyanine green anterior segment angiography for studying conjunctival vascular changes after trabeculectomy. Clin. Exp. Ophthalmol. 2001, 29, 22–26. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wei, E.; Wang, X.G.; Zhang, X.B.; Morrison, J.C.; Parikh, M.; Lombardi, L.H.; Gattey, D.M.; Armour, R.L.; Edmunds, B.; et al. Optical Coherence Tomography Angiography of Optic Disc Perfusion in Glaucoma. Ophthalmology 2014, 121, 1322–1332. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Fasanella, V.; Agnifili, L.; Curcio, C.; Ciancaglini, M.; Mastropasqua, L. Anterior Segment Optical Coherence Tomography Imaging of Conjunctival Filtering Blebs after Glaucoma Surgery. BioMed Res. Int. 2014, 2014, 610623. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Romano, A. The impact of new methods of investigation and treatment on the understanding of the pathology of scleral inflammation. Eye 2014, 28, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Pepple, K.L.; Zhi, Z.W.; Wang, R.K. Optical coherence tomography based microangiography for quantitative monitoring of structural and vascular changes in a rat model of acute uveitis in vivo: A preliminary study. J. Biomed. Opt. 2015, 20, 016015. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Uji, A.; Huang, A.S.; Weinreb, R.N.; Yamada, T.; Miyata, M.; Kameda, T.; Ikeda, H.O.; Tsujikawa, A. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. Am. J. Ophthalmol. 2018, 196, 1–9. [Google Scholar] [CrossRef]
- Yin, X.; Cai, Q.H.; Song, R.; He, X.F.; Lu, P.R. Relationship between filtering bleb vascularization and surgical outcomes after trabeculectomy: An optical coherence tomography angiography study. Graefe’s Arch. Clin. Exp. 2018, 256, 2399–2405. [Google Scholar] [CrossRef]
- Hayek, S.; Labbe, A.; Brasnu, E.; Hamard, P.; Baudouin, C. Optical Coherence Tomography Angiography Evaluation of Conjunctival Vessels during Filtering Surgery. Transl. Vis. Sci. Technol. 2019, 8, 4. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Brescia, L.; Di Antonio, L.; Guarini, D.; Giattini, D.; Zuppardi, E.; Agnifili, L. Angiographic biomarkers of filtering bleb function after XEN gel implantation for glaucoma: An optical coherence tomography-angiography study. Acta Ophthalmol. 2020, 98, e761–e767. [Google Scholar] [CrossRef]
- Uji, A.; Murakami, T.; Suzuma, K.; Yoshitake, S.; Arichika, S.; Ghashut, R.; Fujimoto, M.; Yoshimura, N. Influence of Vitrectomy Surgery on the Integrity of Outer Retinal Layers in Diabetic Macular Edema. Retin. J. Retin. Vitr. Dis. 2018, 38, 163–172. [Google Scholar] [CrossRef]
- Chu, Z.D.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.Q.; Chen, C.L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K.K. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Opt. 2016, 21, 66008. [Google Scholar] [CrossRef]
- Su, L.; Ji, Y.O.; Tong, N.; Sarraf, D.; He, X.G.; Sun, X.D.; Xu, X.; Sadda, S.R. Quantitative assessment of the retinal microvasculature and choriocapillaris in myopic patients using swept-source optical coherence tomography angiography. Graefe’s Arch. Clin. Exp. 2020, 258, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Seven, E.; Batur, M.; Ozer, M.D.; Yasar, T. Evaluation of Successful and Failed Filtering Blebs after Trabeculectomy Using Anterior Segment Optical Coherence Tomography. J. Curr. Ophthalmol. 2021, 33, 1–5. [Google Scholar] [PubMed]
- Singh, M.; See, J.L.; Aquino, M.C.; Thean, L.S.; Chew, P.T. High-definition imaging of trabeculectomy blebs using spectral domain optical coherence tomography adapted for the anterior segment. Clin. Exp. Ophthalmol. 2009, 37, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kudsieh, B.; Fernandez-Vigo, J.I.; Canut Jordana, M.I.; Vila-Arteaga, J.; Urcola, J.A.; Ruiz Moreno, J.M.; Garcia-Feijoo, J.; Fernandez-Vigo, J.A. Updates on the utility of anterior segment optical coherence tomography in the assessment of filtration blebs after glaucoma surgery. Acta Ophthalmol. 2021, 100, e29–e37. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Tube Versus Trabeculectomy Study Group. Three-year follow-up of the tube versus trabeculectomy study. Am. J. Ophthalmol. 2009, 148, 670–684. [Google Scholar] [CrossRef]
- Jampel, H.D.; Solus, J.F.; Tracey, P.A.; Gilbert, D.L.; Loyd, T.L.; Jefferys, J.L.; Quigley, H.A. Outcomes and bleb-related complications of trabeculectomy. Ophthalmology 2012, 119, 712–722. [Google Scholar] [CrossRef]
- Landers, J.; Martin, K.; Sarkies, N.; Bourne, R.; Watson, P. A twenty-year follow-up study of trabeculectomy: Risk factors and outcomes. Ophthalmology 2012, 119, 694–702. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, Y.; Shin, J.H.; Kim, Y.A.; Park, K.H. Comparison of conjunctival vascularity changes using optical coherence tomography angiography after trabeculectomy and phacotrabeculectomy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2239–2255. [Google Scholar] [CrossRef]
- Miller, M.H.; Grierson, I.; Unger, W.I.; Hitchings, R.A. Wound healing in an animal model of glaucoma fistulizing surgery in the rabbit. Ophthalmic Surg. 1989, 20, 350–357. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.; Payne, R.; Yue, B.Y.; Chang, J.H.; Ying, H. Angiogenesis in glaucoma filtration surgery and neovascular glaucoma: A review. Surv. Ophthalmol. 2015, 60, 524–535. [Google Scholar] [CrossRef]
- Kronfeld, P.C. Functional Characteristics of Surgically Produced Outflow Channels. Am. J. Ophthalmol. 1969, 67, 451–463. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, Y.A.; Park, K.H.; Lee, Y. Evaluation of Functional Filtering Bleb Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Uji, A.; Okamoto, Y.; Suda, K.; Kameda, T.; Nakanishi, H.; Ikeda, H.O.; Miyake, M.; Nakano, E.; Motozawa, N.; et al. Anterior Segment Optical Coherence Tomography Angiography Imaging of Conjunctiva and Intrasclera in Treated Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 2019, 208, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Okamoto, Y.; Kameda, T.; Suda, K.; Nakanishi, H.; Miyake, M.; Ikeda, H.O.; Yamada, T.; Kadomoto, S.; Uji, A.; et al. Short-Term Effects of Different Types of Anti-Glaucoma Eyedrop on the Sclero-Conjunctival Vasculature Assessed Using Anterior Segment OCTA in Normal Human Eyes: A Pilot Study. J. Clin. Med. 2020, 9, 4016. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Kolker, A.E.; Stewart, J.A.; Leech, J.; Jackson, A.L. Conjunctival hyperemia in healthy subjects after short-term dosing with latanoprost, bimatoprost, and travoprost. Am. J. Ophthalmol. 2003, 135, 314–320. [Google Scholar] [CrossRef]
- Yanagi, M.; Kiuchi, Y.; Yuasa, Y.; Yoneda, T.; Sumi, T.; Hoshikawa, Y.; Kobayashi, M.; Fukushima, A. Association between glaucoma eye drops and hyperemia. Jpn. J. Ophthalmol. 2016, 60, 72–77. [Google Scholar] [CrossRef]
- Seet, L.F.; Finger, S.N.; Chu, S.W.; Toh, L.Z.; Wong, T.T. Novel insight into the inflammatory and cellular responses following experimental glaucoma surgery: A roadmap for inhibiting fibrosis. Curr. Mol. Med. 2013, 13, 911–928. [Google Scholar] [CrossRef]



| Variables | Success Group | Failure Group | p Value |
|---|---|---|---|
| (28 Eyes) | (18 Eyes) | ||
| Age (year) | 48.50 [33.00] | 48.50 [5.50] | 0.973 |
| Sex (male/female) | 12/16 | 10/8 | 0.916 |
| Preoperative IOP (mmHg) | 25.58 (9.26) | 24.92 (11.67) | 0.857 |
| Preoperative topical ocular hypotensive drugs (n) | 1.00 [3.00] | 1.00 [3.00] | 0.215 |
| Preoperative PG (n, %) | 13.00 (46.43%) | 6.00 (33.33%) | 0.541 |
| Follow-up time (year) | 2.00 [9.00] | 1.00 [2.25] | 0.008 |
| Postoperative topical ocular hypotensive drugs (n) | 0.00 [1.00] | 0.50 [3.00] | 0.190 |
| Postoperative PG (n, %) | 7 (25.00%) | 6 (33.33%) | 0.540 |
| Variables | Success Group (28 Eyes) | Failure Group (18 Eyes) | p Value |
|---|---|---|---|
| SL | |||
| VD (%) 1 | 4.93 (3.36) | 19.90 (5.82) | <0.001 |
| VDI (pixel−1) 2 | 7.94 [2.08] | 15.40 [1.48] | <0.001 |
| TL | |||
| VD (%) 1 | 10.94 (6.13) | 27.83 (6.24) | <0.001 |
| VDI (pixel−1) 2 | 8.65 [3.00] | 16.97 [1.66] | <0.001 |
| DL | |||
| VD (%) 1 | 14.37 (7.70) | 31.40 (6.25) | <0.001 |
| VDI (pixel−1) 2 | 9.52 [3.29] | 17.39 [2.16] | <0.001 |
| Factors | Success Group (28 Eyes, 28 Subjects) | Failure Group (18 Eyes, 18 Subjects) | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| SL | ||||
| VD (%) 1 | 0.016 | 0.936 | −0.089 | 0.726 |
| VDI (pixel−1) 2 | 0.462 | 0.013 | 0.273 | 0.274 |
| TL | ||||
| VD (%) 1 | −0.052 | 0.791 | 0.580 | 0.012 |
| VDI (pixel−1) 2 | 0.450 | 0.016 | 0.517 | 0.028 |
| DL | ||||
| VD (%) 1 | 0.092 | 0.642 | 0.597 | 0.009 |
| VDI (pixel−1) 2 | 0.408 | 0.031 | 0.329 | 0.183 |
| Variable | Univariable Model | Multivariable Model 1 3 | Multivariable Model 2 4 | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p Value | Coefficient (95% CI) | p Value | Coefficient (95% CI) | p Value | |
| Age (y) | 0.083 (−0.107–0.268) | 0.392 | 0.070 (−0.027–0.167) | 0.151 | 0.057 (−0.060–0.173) | 0.331 |
| TVD 1 | ||||||
| <15% | 0.332 (−0.319–0.982) | 0.310 | 0.150 (−0.415–0.715) | 0.595 | 0.114 (−0.593–0.820) | 0.746 |
| ≥15% | 1.115 (0.768–1.462) | <0.001 | 0.630 (0.125–0.908) | 0.011 | 0.667 (0.178–1.155) | 0.009 |
| TVDI | 1.659 (1.250–2.069) | <0.001 | 0.788 (0.197–1.379) | 0.016 | 0.720 (0.024–1.415) | 0.043 |
| Bleb height | −0.745 (−5.853–4.386) | 0.771 | 1.084 (−1.861–4.028) | 0.460 | 2.503 (−1.586–6.591) | 0.223 |
| IBAGS 2 | ||||||
| 2 | 6.348 (1.858–10.838) | 0.007 | 1.266 (−2.279–4.811) | 0.474 | ||
| 3 | 13.403 (7.981–18.826) | <0.001 | 7.540 (3.353–11.725) | 0.001 | ||
| 4 | 23.691 (13.990–33.391) | <0.001 | 3.797 (3.549–18.948) | 0.005 | ||
| KGS 2 | ||||||
| 2 | −0.196 (−5.539–5.148) | 0.941 | 0.864 (−3.597–5.325) | 0.223 | ||
| 3 | 12.342 (6.832–17.851) | <0.001 | 4.074 (−1.893–10.041) | 0.806 | ||
| 4 | 11.865 (5.261–18.469) | 0.001 | 1.990 (−4.301–8.282) | 0.698 | ||
| Variable | Univariable Model | Multivariable Model 1 3 | Multivariable Model 2 4 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age (y) | 1.000 (0.961–1.043) | 0.946 | ||||
| TVD | 1.448 (1.165–1.799) | 0.001 | 1.470 (1.037–2.085) | 0.031 | 1.470 (1.037–2.085) | 0.031 |
| TVDI | 1.862 (1.370–2.530) | <0.001 | 2.295 (1.008–5.224) | 0.048 | 2.295 (1.008–5.224) | 0.048 |
| Bleb height | 0.895 (0.289–2.774) | 0.848 | ||||
| IBAGS 1 | ||||||
| 2 | 3.37 (0.76–16.62) | 0.115 | 0.012 (0.000–6.533) | 0.169 | ||
| 3 | 13.5 (2.23–120.78) | 0.008 | 20.485 (0.258–1626.1) | 0.176 | ||
| 4 | n/a 2 | 0.999 | n/a 2 | n/a 2 | ||
| KGS 1 | ||||||
| 2 | 0.37 (0.02–3.02) | 0.410 | 0.864 (−3.597–5.325) | 0.387 | ||
| 3 | 8.75 (1.67–58.61) | 0.015 | 4.074 (−1.893–10.041) | 0.257 | ||
| 4 | n/a 2 | 0.994 | n/a 2 | n/a 2 | ||
| Variables | Group A (29 Eyes, 29 Subjects) | Group B (13 Eyes, 13 Subjects) | Group C (4 Eyes, 4 Subjects) | p Value |
|---|---|---|---|---|
| VD (%) 1 | ||||
| SL | 9.27 (7.94) | 14.11 (9.45) | 11.00 (9.84) | 0.248 |
| TL | 15.37 (10.00) | 22.09 (9.44) | 18.57 (13.08) | 0.146 |
| DL | 18.13 (10.44) | 27.29 (9.06) | 21.76 (14.74) | 0.041 |
| VDI 2 | ||||
| SL | 9.54 [6.93] | 11.32 [6.46] | 9.55 [4.30] | 0.264 |
| TL | 10.60 [8.06] | 15.36 [6.11] | 9.95 [5.54] | 0.191 |
| DL | 11.59 [8.08] | 16.61 [7.83] | 10.52 [6.15] | 0.206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Zhu, Y.; Xiao, H.; Huang, J.; Ling, J.; Huang, H.; Li, Y.; Zhuo, Y. Characteristic Assessment of Angiographies at Different Depths with AS-OCTA: Implication for Functions of Post-Trabeculectomy Filtering Bleb. J. Clin. Med. 2022, 11, 1661. https://doi.org/10.3390/jcm11061661
Luo M, Zhu Y, Xiao H, Huang J, Ling J, Huang H, Li Y, Zhuo Y. Characteristic Assessment of Angiographies at Different Depths with AS-OCTA: Implication for Functions of Post-Trabeculectomy Filtering Bleb. Journal of Clinical Medicine. 2022; 11(6):1661. https://doi.org/10.3390/jcm11061661
Chicago/Turabian StyleLuo, Man, Yingting Zhu, Hui Xiao, Jingjing Huang, Jin Ling, Haishun Huang, Yiqing Li, and Yehong Zhuo. 2022. "Characteristic Assessment of Angiographies at Different Depths with AS-OCTA: Implication for Functions of Post-Trabeculectomy Filtering Bleb" Journal of Clinical Medicine 11, no. 6: 1661. https://doi.org/10.3390/jcm11061661
APA StyleLuo, M., Zhu, Y., Xiao, H., Huang, J., Ling, J., Huang, H., Li, Y., & Zhuo, Y. (2022). Characteristic Assessment of Angiographies at Different Depths with AS-OCTA: Implication for Functions of Post-Trabeculectomy Filtering Bleb. Journal of Clinical Medicine, 11(6), 1661. https://doi.org/10.3390/jcm11061661





