Triple-Negative Apocrine Breast Carcinoma Has Better Prognosis despite Poor Response to Neoadjuvant Chemotherapy
Abstract
1. Introduction
2. Methods
2.1. Study Population
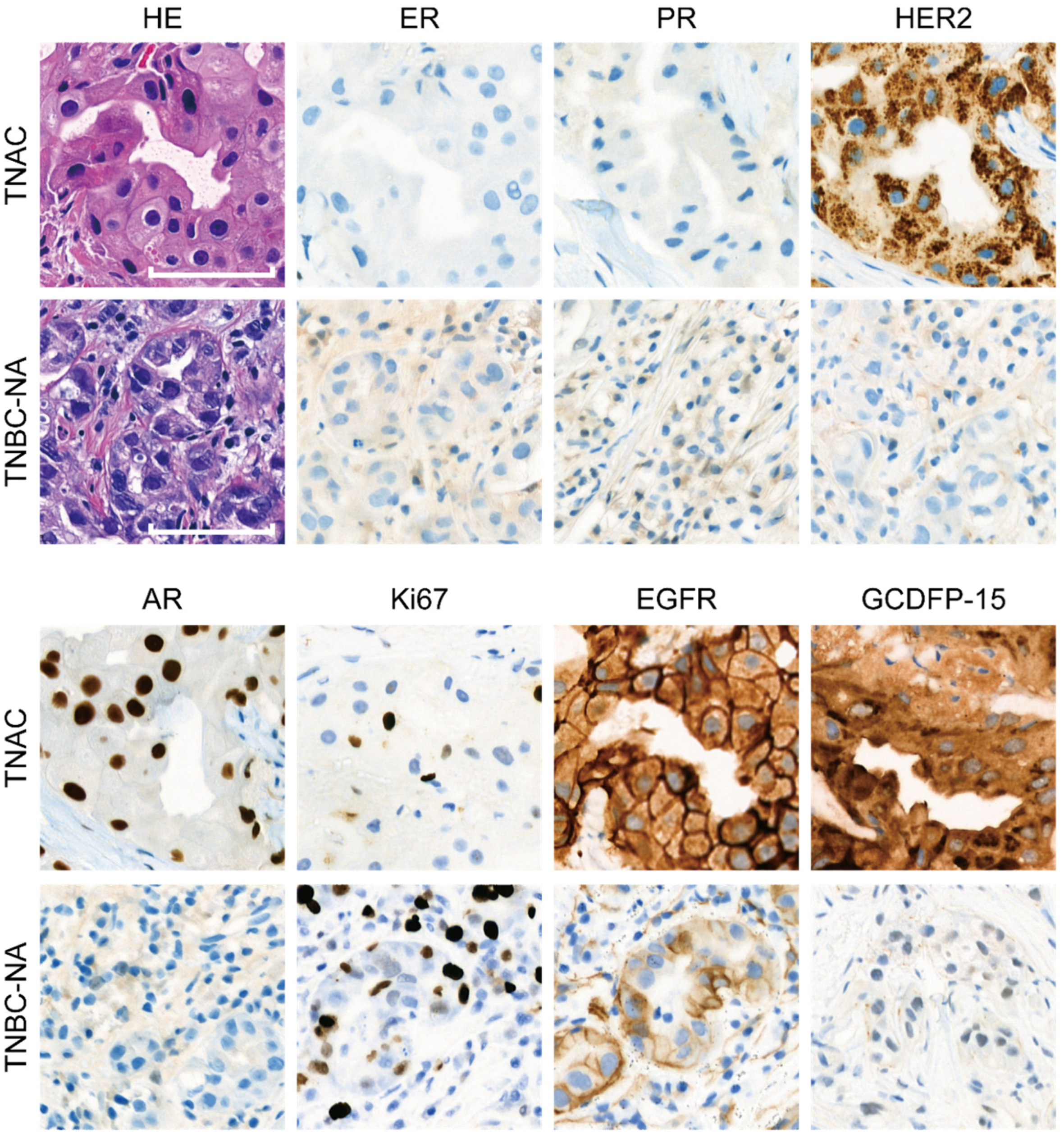
2.2. Immunohistochemical Staining
2.3. SEER Analysis
3. Results
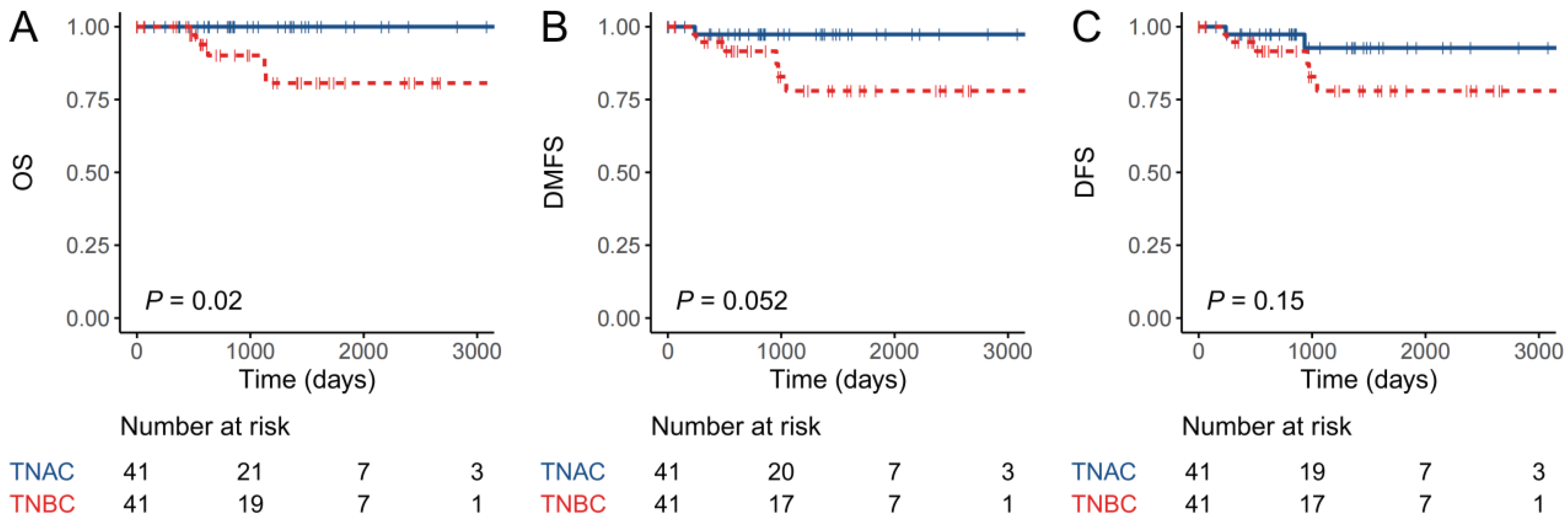
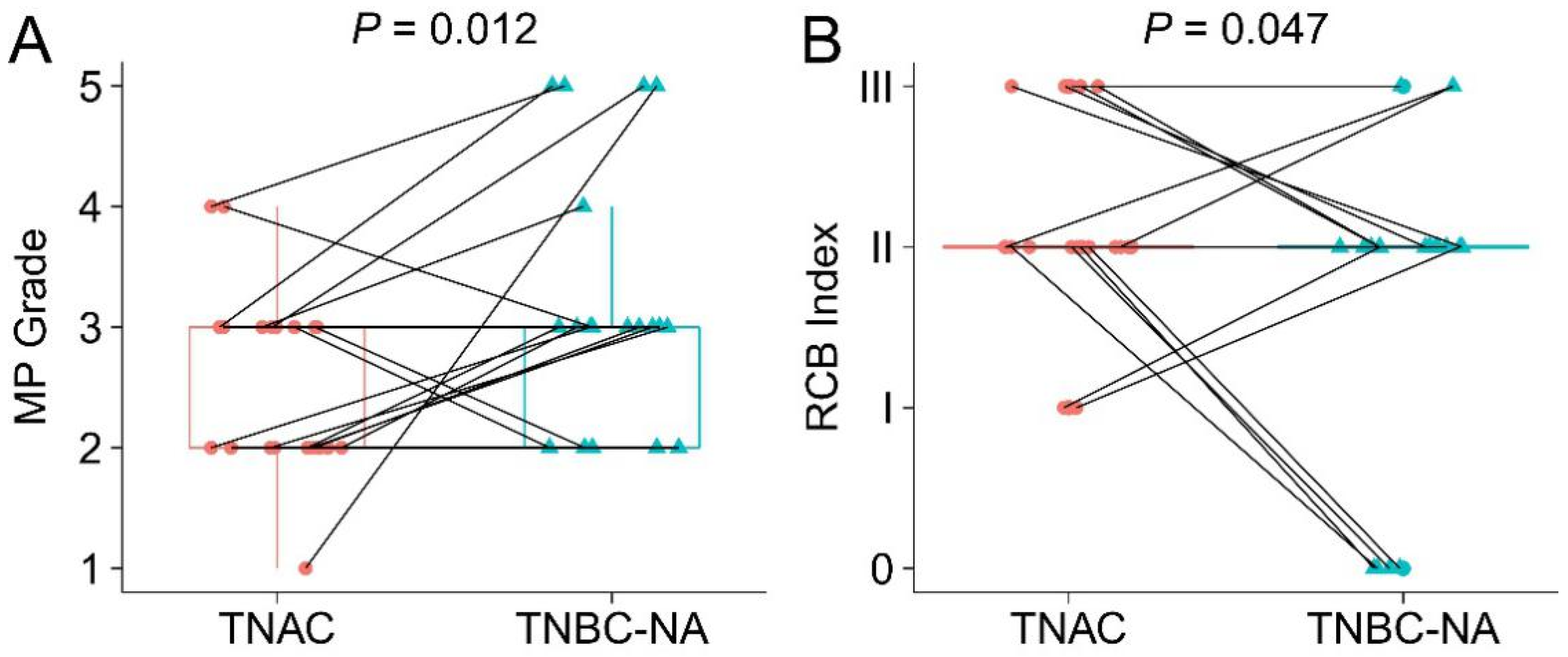
3.1. Patients with TNAC Have Better Short-Term Prognosis Than Those with TNBC-NA Despite a Poorer Response to Neoadjuvant Chemotherapy
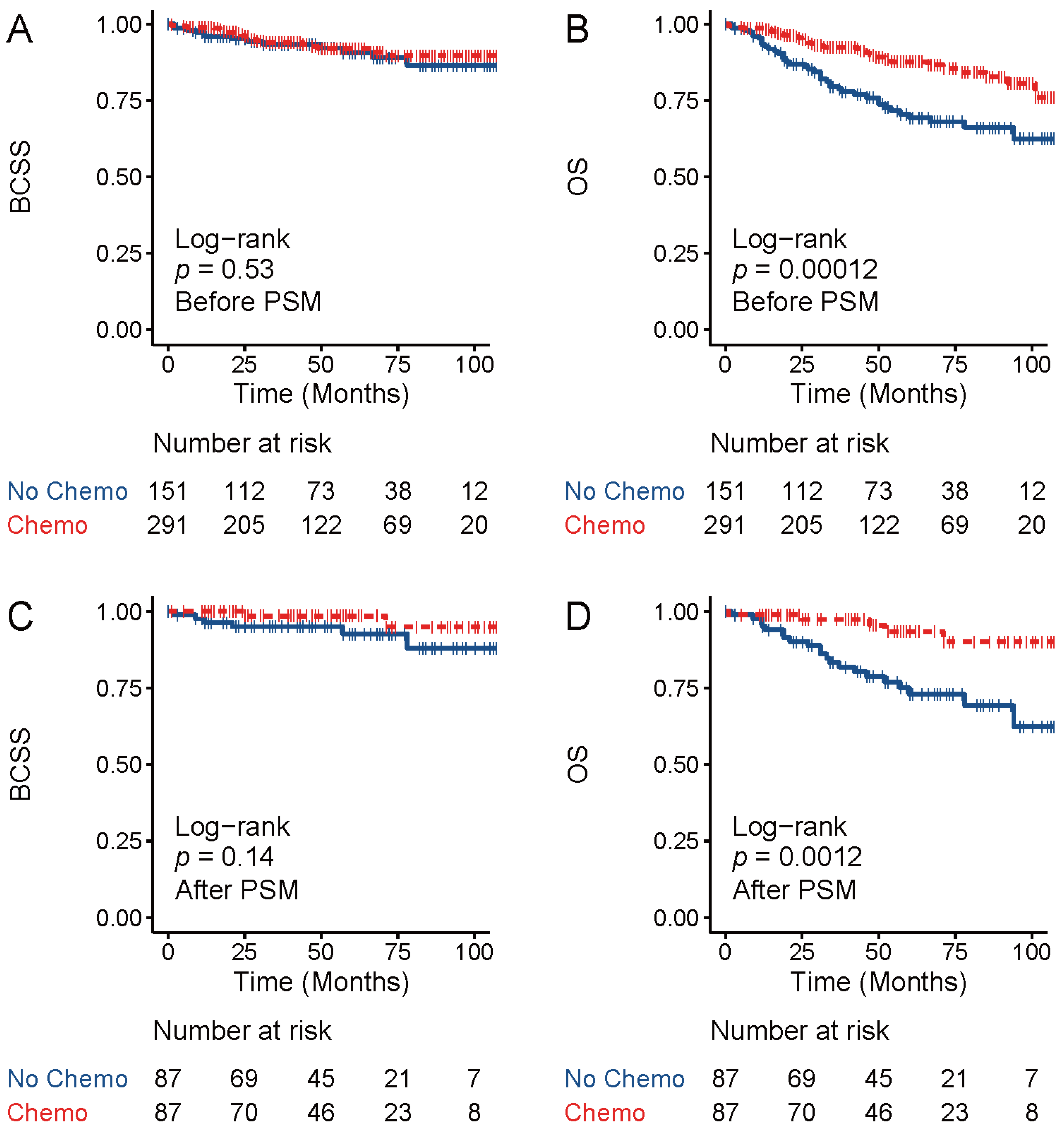
3.2. Chemotherapy did Not Improve Breast-Cancer-Specific Survival for TNAC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dellapasqua, S.; Maisonneuve, P.; Viale, G.; Pruneri, G.; Mazzarol, G.; Ghisini, R.; Mazza, M.; Iorfida, M.; Rotmensz, N.; Veronesi, P.; et al. Immunohistochemically defined subtypes and outcome of apocrine breast cancer. Clin. Breast Cancer 2013, 13, 95–102. [Google Scholar] [CrossRef]
- Vranic, S.; Marchio, C.; Castellano, I.; Botta, C.; Scalzo, M.S.; Bender, R.P.; Payan-Gomez, C.; di Cantogno, L.V.; Gugliotta, P.; Tondat, F.; et al. Immunohistochemical and molecular profiling of histologically defined apocrine carcinomas of the breast. Hum. Pathol. 2015, 46, 1350–1359. [Google Scholar] [CrossRef]
- Astvatsaturyan, K.; Yue, Y.; Walts, A.E.; Bose, S. Androgen receptor positive triple negative breast cancer: Clinicopathologic, prognostic, and predictive features. PLoS ONE 2018, 13, e0197827. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, L.A.; Dabbs, D.J.; Beriwal, S.; Striebel, J.M.; Bhargava, R. Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 2010, 23, 205–212. [Google Scholar] [CrossRef]
- Vera-Badillo, F.E.; Templeton, A.J.; de Gouveia, P.; Diaz-Padilla, I.; Bedard, P.L.; Al-Mubarak, M.; Seruga, B.; Tannock, I.F.; Ocana, A.; Amir, E. Androgen receptor expression and outcomes in early breast cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, djt319. [Google Scholar] [CrossRef]
- Bozovic-Spasojevic, I.; Zardavas, D.; Brohee, S.; Ameye, L.; Fumagalli, D.; Ades, F.; de Azambuja, E.; Bareche, Y.; Piccart, M.; Paesmans, M.; et al. The Prognostic Role of Androgen Receptor in Patients with Early-Stage Breast Cancer: A Meta-analysis of Clinical and Gene Expression Data. Clin. Cancer Res. 2017, 23, 2702–2712. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.D.; Liu, Y.R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440.e425. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanovic, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Baggerly, K.A.; Wang, Y.; Zhang, Y.; Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Valero, V.; Lehmann, B.D.; Pietenpol, J.A.; Hortobagyi, G.N.; et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin. Cancer Res. 2013, 19, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Moon, B.I.; Lim, W.; Park, S.; Cho, M.S.; Sung, S.H. Feasibility of Classification of Triple Negative Breast Cancer by Immunohistochemical Surrogate Markers. Clin. Breast Cancer 2018, 18, e1123–e1132. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, M.; Peng, G.; Shi, D.; Zhang, J. Prognosis in triple-negative apocrine carcinomas of the breast: A population-based study. Cancer Med. 2019, 8, 7523–7531. [Google Scholar] [CrossRef] [PubMed]
- Arciero, C.A.; Diehl, A.H., 3rd; Liu, Y.; Sun, Q.; Gillespie, T.; Li, X.; Subhedar, P. Triple-negative apocrine carcinoma: A rare pathologic subtype with a better prognosis than other triple-negative breast cancers. J. Surg. Oncol. 2020, 122, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Montagna, E.; Cancello, G.; Pagan, E.; Bagnardi, V.; Munzone, E.; Dellapasqua, S.; Viale, G.; Mazzarol, G.; Veronesi, P.; Galimberti, V.; et al. Prognosis of selected triple negative apocrine breast cancer patients who did not receive adjuvant chemotherapy. Breast 2020, 53, 138–142. [Google Scholar] [CrossRef]
- Nagao, T.; Kinoshita, T.; Hojo, T.; Tsuda, H.; Tamura, K.; Fujiwara, Y. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: The relationship between the outcome and the clinicopathological characteristics. Breast 2012, 21, 289–295. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Honma, N.; Horii, R.; Iwase, T.; Saji, S.; Younes, M.; Ito, Y.; Akiyama, F. Ki-67 evaluation at the hottest spot predicts clinical outcome of patients with hormone receptor-positive/HER2-negative breast cancer treated with adjuvant tamoxifen monotherapy. Breast Cancer 2015, 22, 71–78. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Ogston, K.N.; Miller, I.D.; Payne, S.; Hutcheon, A.W.; Sarkar, T.K.; Smith, I.; Schofield, A.; Heys, S.D. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast 2003, 12, 320–327. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Anderson, S.J.; Dignam, J.J.; Bear, H.D.; Julian, T.B.; Geyer, C.E., Jr.; Taghian, A.; Wickerham, D.L.; Wolmark, N. Predictors of locoregional recurrence after neoadjuvant chemotherapy: Results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J. Clin. Oncol. 2012, 30, 3960–3966. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, E.; Bossuyt, V.; Viale, G.; Cameron, D.; Badve, S.; Denkert, C.; MacGrogan, G.; Penault-Llorca, F.; Boughey, J.; Curigliano, G.; et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: Recommendations from an international working group. Mod. Pathol. 2015, 28, 1185–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.; Chen, T.; Yang, Q. Dose invasive apocrine adenocarcinoma has worse prognosis than invasive ductal carcinoma of breast: Evidence from SEER database. Oncotarget 2017, 8, 24579–24592. [Google Scholar] [CrossRef] [PubMed]
- Saridakis, A.; Berger, E.R.; Harigopal, M.; Park, T.; Horowitz, N.; Le Blanc, J.; Zanieski, G.; Chagpar, A.; Greenup, R.; Golshan, M.; et al. Apocrine Breast Cancer: Unique Features of a Predominantly Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2021, 28, 5610–5616. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.Y.; Zhang, W.W.; Sun, J.Y.; Li, F.Y.; He, Z.Y.; Wu, S.G. The Clinicopathological Features and Survival Outcomes of Different Histological Subtypes in Triple-negative Breast Cancer. J. Cancer 2018, 9, 296–303. [Google Scholar] [CrossRef]
- Bareche, Y.; Venet, D.; Ignatiadis, M.; Aftimos, P.; Piccart, M.; Rothe, F.; Sotiriou, C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 2018, 29, 895–902. [Google Scholar] [CrossRef]
- Sun, X.; Zuo, K.; Yao, Q.; Zhou, S.; Shui, R.; Xu, X.; Bi, R.; Yu, B.; Cheng, Y.; Tu, X.; et al. Invasive apocrine carcinoma of the breast: Clinicopathologic features and comprehensive genomic profiling of 18 pure triple-negative apocrine carcinomas. Mod. Pathol. 2020, 33, 2473–2482. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef]
- Meattini, I.; Pezzulla, D.; Saieva, C.; Bernini, M.; Orzalesi, L.; Sanchez, L.J.; Desideri, I.; Francolini, G.; Bonomo, P.; Greto, D.; et al. Triple Negative Apocrine Carcinomas as a Distinct Subtype of Triple Negative Breast Cancer: A Case-control Study. Clin. Breast Cancer 2018, 18, e773–e780. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Gatalica, Z. An Update on the Molecular and Clinical Characteristics of Apocrine Carcinoma of the Breast. Clin. Breast Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Iwase, H.; Kurebayashi, J.; Tsuda, H.; Ohta, T.; Kurosumi, M.; Miyamoto, K.; Yamamoto, Y.; Iwase, T. Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer 2010, 17, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Moran, M.S.; Li, Y.; Liang, Y.; Su, P.; Haffty, B.G.; Yang, Q. Special subtypes with favorable prognosis in breast cancer: A registry-based cohort study and network meta-analysis. Cancer Treat. Rev. 2020, 91, 102108. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Chen, C.; Di, G.; Liu, G.; Wu, J.; Shao, Z. Pathological complete response as a surrogate for relapse-free survival in patients with triple negative breast cancer after neoadjuvant chemotherapy. Oncotarget 2017, 8, 18399–18408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, Z.; Chaudhri, S.; Guo, M.; Zhang, L.; Rea, D. Neoadjuvant Chemotherapy in Triple Negative Breast Cancer: An Observational Study. Oncol. Res. 2016, 23, 291–302. [Google Scholar] [CrossRef]
- Huang, M.; O’Shaughnessy, J.; Zhao, J.; Haiderali, A.; Cortes, J.; Ramsey, S.D.; Briggs, A.; Hu, P.; Karantza, V.; Aktan, G.; et al. Association of Pathologic Complete Response with Long-Term Survival Outcomes in Triple-Negative Breast Cancer: A Meta-Analysis. Cancer Res. 2020, 80, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; O’Shaughnessy, J.; Zhao, J.; Haiderali, A.; Cortes, J.; Ramsey, S.; Briggs, A.; Karantza, V.; Aktan, G.; Qi, C.Z.; et al. Evaluation of Pathologic Complete Response as a Surrogate for Long-Term Survival Outcomes in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 1096–1104. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Sala, I.; Oriecuia, C.; De Pas, T.; Specchia, C.; Graffeo, R.; Pagan, E.; Queirolo, P.; Pennacchioli, E.; et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: Systematic review and meta-analysis. BMJ 2021, 375, e066381. [Google Scholar] [CrossRef]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, X.; Yu, Q.; Eng, C. Androgen receptor-induced tumor suppressor, KLLN, inhibits breast cancer growth and transcriptionally activates p53/p73-mediated apoptosis in breast carcinomas. Hum. Mol. Genet. 2013, 22, 2263–2272. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann. Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Santonja, A.; Sanchez-Munoz, A.; Lluch, A.; Chica-Parrado, M.R.; Albanell, J.; Chacon, J.I.; Antolin, S.; Jerez, J.M.; de la Haba, J.; de Luque, V.; et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 2018, 9, 26406–26416. [Google Scholar] [CrossRef]
- Shousha, S.; Bull, T.B.; Southall, P.J.; Mazoujian, G. Apocrine carcinoma of the breast containing foam cells. An electron microscopic and immunohistological study. Histopathology 1987, 11, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Fuchinoue, F.; Hirotani, Y.; Nakanishi, Y.; Yamaguchi, H.; Nishimaki, H.; Noda, H.; Tang, X.Y.; Iizuka, M.; Amano, S.; Sugitani, M.; et al. Overexpression of PGC1alpha and accumulation of p62 in apocrine carcinoma of the breast. Pathol. Int. 2015, 65, 19–26. [Google Scholar] [CrossRef] [PubMed]
| TNAC | TNBC-NA | p | |
|---|---|---|---|
| Age at diagnosis (y) | 1.000 | ||
| 20–49 | 6 (30.0) | 6 (30.0) | |
| 50–69 | 11 (55.0) | 11 (55.0) | |
| 70–89 | 3 (15.0) | 3 (15.0) | |
| T stage | 0.196 | ||
| T1 | 16 (80.0) | 11 (55.0) | |
| T2 | 4 (20.0) | 8 (40.0) | |
| T3 | 0 (0) | 0 (0) | |
| T4 | 0 (0) | 1 (5.0) | |
| N stage | |||
| N0 | 18 (90.0) | 14 (70.0) | 0.348 |
| N1 | 1 (5.0) | 4 (20.0) | |
| N2 | 1 (5.0) | ||
| N3 | 1 (5.0) | 1 (5.0) | |
| AJCC stage | 0.323 | ||
| IA | 14 (70.0) | 10 (50.0) | |
| IIA | 5 (25.0) | 5 (25.0) | |
| IIB | 0 (0) | 3 (15.0) | |
| IIIA | 0 (0) | 1 (5.0) | |
| IIIB | 0 (0) | 0 (0) | |
| IIIC | 1 (5.0) | 1 (5.0) | |
| Radiation | 0.500 | ||
| No | 15 (75.0) | 12 (60.0) | |
| Yes | 5 (25.0) | 8 (40.0) | |
| Surgery type | 1.000 | ||
| BCS * | 7 (35.0) | 6 (30.0) | |
| Mastectomy | 13 (65.0) | 14 (70.0) | |
| Laterality | 0.747 | ||
| Left | 7 (35.0) | 9 (45.0) | |
| Right | 13 (65.0) | 11 (55.0) | |
| Ki-67 (%) | <0.001 | ||
| 0–29 | 18 (90.0) | 2 (10.0) | |
| 30–59 | 2 (10.0) | 3 (15.0) | |
| 60–99 | 0 (0.0) | 15 (75.0) | |
| Histologic grade | 0.006 | ||
| I | 8 (40.0) | 1 (5.0) | |
| II | 5 (25.0) | 4 (20.0) | |
| III | 5 (25.0) | 15 (75.0) | |
| Missing | 2 (10.0) | ||
| TILs | 0.051 | ||
| 0–10 | 11 (61.1) | 6 (30.0) | |
| 11–40 | 6 (33.3) | 7 (35.0) | |
| 41–90 | 1 (5.6) | 7 (35.0) |
| TNAC | TNBC-NA | p | |
|---|---|---|---|
| Age at diagnosis (y) | |||
| 20–49 | 6 (28.6) | 6 (28.6) | 1.000 |
| 50–69 | 15 (71.4) | 15 (71.4) | |
| 70–89 | 0 (0.0) | 0 (0.0) | |
| T stage | |||
| T1 | 16 (76.2) | 13 (61.9) | 0.767 |
| T2 | 3 (14.3) | 5 (23.8) | |
| T3 | 1 (4.8) | 1 (4.8) | |
| T4 | 1 (4.8) | 2 (9.5) | |
| N stage | |||
| N0 | 16 (76.2) | 12 (57.1) | 0.065 |
| N1 | 0 (0) | 6 (28.6) | |
| N2 | 4 (19.0) | 2 (9.5) | |
| N3 | 1 (4.8) | 1 (4.8) | |
| AJCC stage | |||
| IA | 13 (61.9) | 8 (38.1) | 0.541 |
| IIA | 2 (9.5) | 6 (28.6) | |
| IIB | 1 (4.8) | 2 (9.5) | |
| IIIA | 3 (14.3) | 2 (9.5) | |
| IIIB | 1 (4.8) | 2 (9.5) | |
| IIIC | 1 (4.8) | 1 (4.8) | |
| Radiation | |||
| No | 12 (57.1) | 10 (47.6) | 0.757 |
| Yes | 9 (42.9) | 11 (52.4) | |
| Surgery type | |||
| BCS | 8 (38.1) | 6 (28.6) | 0.743 |
| Mastectomy | 13 (61.9) | 15 (71.4) | |
| Laterality | |||
| Left | 14 (66.7) | 13 (61.9) | 1.000 |
| Right | 7 (33.3) | 8 (38.1) | |
| Ki-67 (%) | |||
| 0–29 | 19 (90.5) | 4 (19.0) | <0.001 |
| 30–59 | 2 (9.5) | 7 (33.3) | |
| 60–99 | 0 (0.0) | 10 (47.6) | |
| Histologic grade | |||
| I | 6 (28.6) | 2 (9.5) | 0.164 |
| II | 11 (52.4) | 11 (52.4) | |
| III | 2 (9.5) | 7 (33.3) | |
| Missing | 2 (9.5) | 1 (4.8) | |
| TILs | |||
| 0–10 | 14 (73.7) | 9 (45.0) | 0.097 |
| 11–40 | 2 (10.5) | 8 (40.0) | |
| 41–90 | 3 (15.8) | 3 (15.0) |
| Neoadjuvant Therapy | Clinical Evaluation | T Stage | N Stage | MP Grade | RCB | Ki-67 (%) | Histologic Grade | TILs (%) | |
|---|---|---|---|---|---|---|---|---|---|
| TNAC-1 | ddEC/T1w | Unk/SD | 1 | 0 | 3 | II | 5 | I | 2 |
| TNAC-2 | AC-T | Unk | 4 | 2 | 2 | III | 30 | II | 15 |
| TNAC-3 | TP1w/CEF/DF | SD/SD/PR | 1 | 0 | 2 | II | 20 | I | 5 |
| TNAC-4 | TP/CEF/NP | SD/SD/PR | 1 | 2 | 2 | III | 10 | II | 40 |
| TNAC-5 | TPX | PR | 1 | 0 | 2 | II | 5 | II | 8 |
| TNAC-6 | TP1w | PR | 1 | 0 | 4 | I | 10 | II | 1 |
| TNAC-7 | ddEC/T1w | SD/PR | 1 | 0 | 3 | II | 20 | II | 1 |
| TNAC-8 | TP1w | SD | 1 | 0 | 3 | II | 10 | II | 60 |
| TNAC-9 | CEF/TP1w | Unk/PR | 1 | 0 | 3 | I | 10 | I | 3 |
| TNAC-10 | T1w/EC | Unk/PR | 2 | 0 | 4 | II | 5 | II | 5 |
| TNAC-11 | T1w/AC | Unk/SD | 2 | 2 | 2 | III | 15 | II | 10 |
| TNAC-12 | ddEC/ddT175 | Unk | 1 | 0 | 2 | II | 20 | I | 5 |
| TNAC-13 | TPX/AC | Unk/PR | 1 | 0 | 3 | II | 15 | I | 0 |
| TNAC-14 | CEF | SD | 1 | 2 | 2 | III | 20 | Unk | Unk |
| TNAC-15 | EC | SD | 1 | 0 | 2 | II | 20 | II | 3 |
| TNAC-16 | TP/NE/DCF/NP | Unk/Unk/Unk/PR | 1 | 0 | 2 | II | 5 | I | 0 |
| TNAC-17 | CEF/TP1w | Unk/PR | 1 | 3 | 2 | III | 25 | III | 3 |
| TNAC-18 | ddEC/ddT175 | Unk/PR | 1 | 0 | 3 | II | 20 | II | 45 |
| TNAC-19 | ddEC/T1w | Unk/SD | 2 | 0 | 3 | II | 5 | II | 80 |
| TNAC-20 | ddEC/T1w | PR/SD | 1 | 0 | 3 | II | 40 | Unk | Unk |
| TNAC-21 | TX | SD | 3 | 0 | 1 | II | 15 | III | 2 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| No Chemo | Chemo | p-Value | No Chemo | Chemo | p-Value | |
| Sample size | 151 | 291 | 87 | 87 | ||
| Age group | <0.001 | 1.000 | ||||
| <50 years | 5 (3.3) | 40 (13.7) | 3 (3.4) | 3 (3.4) | ||
| 50–69 years | 41 (27.2) | 191 (65.6) | 36 (41.4) | 36 (41.4) | ||
| 70+ years | 105 (69.5) | 60 (20.6) | 48 (55.2) | 48 (55.2) | ||
| Income | 0.579 | 0.456 | ||||
| USD 50,000–69,999 | 74 (49.0) | 129 (44.3) | 43 (49.4) | 35 (40.2) | ||
| USD 70,000+ | 56 (37.1) | 113 (38.8) | 30 (34.5) | 34 (39.1) | ||
| <USD 50,000 | 21 (13.9) | 49 (16.8) | 14 (16.1) | 18 (20.7) | ||
| Race | 0.542 | 0.765 | ||||
| Hispanic (All races) | 11 (7.3) | 25 (8.6) | 7 (8.0) | 6 (6.9) | ||
| Non-Hispanic Asian or Pacific Islander | 19 (12.6) | 37 (12.7) | 13 (14.9) | 16 (18.4) | ||
| Non-Hispanic Black | 26 (17.2) | 40 (13.7) | 13 (14.9) | 9 (10.3) | ||
| Non-Hispanic White | 95 (62.9) | 185 (63.6) | 54 (62.1) | 56 (64.4) | ||
| Others | 0 (0.0) | 4 (1.4) | 0 (0.0) | 0 (0.0) | ||
| Stage | <0.001 | 1.000 | ||||
| I | 81 (53.6) | 68 (23.4) | 44 (50.6) | 44 (50.6) | ||
| II | 25 (16.6) | 92 (31.6) | 18 (20.7) | 18 (20.7) | ||
| III | 5 (3.3) | 27 (9.3) | 3 (3.4) | 3 (3.4) | ||
| IV | 1 (0.7) | 5 (1.7) | 0 (0.0) | 0 (0.0) | ||
| Unk | 39 (25.8) | 99 (34.0) | 22 (25.3) | 22 (25.3) | ||
| Grade | <0.001 | 1.000 | ||||
| Well-differentiated; Grade I | 21 (13.9) | 12 (4.1) | 8 (9.2) | 8 (9.2) | ||
| Moderately differentiated; Grade II | 90 (59.6) | 159 (54.6) | 62 (71.3) | 62 (71.3) | ||
| Poorly differentiated; Grade III | 34 (22.5) | 106 (36.4) | 16 (18.4) | 16 (18.4) | ||
| Undifferentiated; anaplastic; Grade IV | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Unk | 5 (3.3) | 14 (4.8) | 1 (1.1) | 1 (1.1) | ||
| Sequence | 0.926 | 0.316 | ||||
| 1st of 2 or more primaries | 19 (12.6) | 39 (13.4) | 12 (13.8) | 18 (20.7) | ||
| One primary only | 132 (87.4) | 252 (86.6) | 75 (86.2) | 69 (79.3) | ||
| Surgery | 0.033 | 1.000 | ||||
| No | 9 (6.0) | 5 (1.7) | 0 (0.0) | 0 (0.0) | ||
| Yes | 142 (94.0) | 286 (98.3) | 87 | 87 | ||
| Radiation | 0.019 | 1.000 | ||||
| No | 77 (51.0) | 113 (38.8) | 39 (44.8) | 39 (44.8) | ||
| Yes | 74 (49.0) | 178 (61.2) | 48 (55.2) | 48 (55.2) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Liu, Y.; Wu, J.; Hu, X.L.; Zhao, G.; Liang, B.; Wang, S.; Long, M. Triple-Negative Apocrine Breast Carcinoma Has Better Prognosis despite Poor Response to Neoadjuvant Chemotherapy. J. Clin. Med. 2022, 11, 1607. https://doi.org/10.3390/jcm11061607
Hu T, Liu Y, Wu J, Hu XL, Zhao G, Liang B, Wang S, Long M. Triple-Negative Apocrine Breast Carcinoma Has Better Prognosis despite Poor Response to Neoadjuvant Chemotherapy. Journal of Clinical Medicine. 2022; 11(6):1607. https://doi.org/10.3390/jcm11061607
Chicago/Turabian StyleHu, Taobo, Yiqiang Liu, Jinbo Wu, Xuejiao Lina Hu, Guiyang Zhao, Baosheng Liang, Shu Wang, and Mengping Long. 2022. "Triple-Negative Apocrine Breast Carcinoma Has Better Prognosis despite Poor Response to Neoadjuvant Chemotherapy" Journal of Clinical Medicine 11, no. 6: 1607. https://doi.org/10.3390/jcm11061607
APA StyleHu, T., Liu, Y., Wu, J., Hu, X. L., Zhao, G., Liang, B., Wang, S., & Long, M. (2022). Triple-Negative Apocrine Breast Carcinoma Has Better Prognosis despite Poor Response to Neoadjuvant Chemotherapy. Journal of Clinical Medicine, 11(6), 1607. https://doi.org/10.3390/jcm11061607






