Abstract
Background: Interstitial lung disease (ILD) is associated with high rates of comorbidities and non-infectious lung disease mortality. Against this background, we aimed to evaluate the prognostic capacity of lung function and cardiopulmonary exercise testing (CPET) in patients with ILD. Materials and Methods: A total of 183 patients with diverse ILD entities were included in this monocentric analysis. Prediction models were determined using Cox regression models with age, sex, body mass index (BMI), and all parameters from pulmonary function testing and CPET. Kaplan–Meier curves were plotted for selected variables. Results: The median follow-up period was 3.0 ± 2.5 years. Arterial hypertension (57%) and pulmonary hypertension (38%) were the leading comorbidities. The Charlson comorbidity index score was 2 ± 2 points. The 3-year and 5-year survival rates were 68% and 50%, respectively. VO2peak (mL/kg/min or %pred.) was identified as a significant prognostic parameter in patients with ILD. The cut-off value for discriminating mortality was 61%. Conclusion: The present analyses consistently revealed the high prognostic power of VO2peak %pred. and other parameters evaluating breathing efficacy (VÉ/VCO2 @AT und VÉ/VCO2 slope) in ILD patients. VO2peak %pred., in contrast to the established prognostic values FVC %pred., DLCO/KCO %pred., and GAP, showed an even higher prognostic ability in all statistical models.
1. Introduction
The main entities of interstitial lung diseases (ILDs) are idiopathic pulmonary fibrosis (IPF), idiopathic nonspecific interstitial pneumonia (NSIP), sarcoidosis with fibrosis, and hypersensitivity pneumonitis [1]. Some revisions of ILD entities have been performed recently [1,2]. Due to the diagnostic complexity of ILDs, a dynamic integrated approach using multidisciplinary discussion (MDD) is considered the standard for classification of these diseases [3]. ILDs (sarcoidosis included) occupy the third position among non-infectious pulmonary diseases in terms of the mortality rate [4]. The German INSIGHT-IPF-Registry data indicated a 1-year and 2-year survival rate of 87% versus 46% and 62% versus 21%, respectively, for patients with versus without antifibrotic therapy [5]. In comparison to the general population, patients with ILDs more frequently show several comorbidities, especially cardiac diseases, diabetes mellitus, dyslipidaemia, obstructive sleep apnoea, cancer, and depression [6,7,8,9]. Additionally, these diseases are associated with limited cardiorespiratory fitness (CRF). The CRF, which is measured as ‘power of work’ [10] or ‘maximum oxygen uptake (VO2peak)’ [11] in addition to other parameters [12,13], has a prognostic influence in ILD subgroups. There are multiple pathophysiologic reasons for the reduced CRF, such as lung functional pathologies, disturbances in gas exchange, and haemodynamic limitations [14]. Therefore, it seems plausible that pulmonary hypertension (PH), which indicates an impairment of pulmonary perfusion, has an influence on cardiopulmonary function, affecting both exercise capacity (measured as 6-MWD or VO2peak) and prognosis [15,16,17,18,19].
Similarly to the indices used for evaluation of COPD (chronic obstructive pulmonary disease), such as the BODE (body-mass, airflow obstruction, dyspnoea, and exercise capacity index), ADO (age, dyspnoea, and airflow obstruction index), and DOSE (dyspnoea, obstruction, smoking, and exacerbation index), various indices have been established for prognosis evaluation in IPF patients [20,21,22]. These indices consider gender, age, and lung function data to be prognostically relevant. However, these multidimensional indices did not outperform the single parameter ‘diffusion capacity’, which is evaluated by measuring the diffusing capacity for carbon monoxide (DLCO). Data for IPF patients have consistently shown that DLCO is the best individual prognostic marker, even outperforming forced vital capacity (FVC), and FVC and DLCO are known to represent the progression of high-resolution computed tomography (HRCT) findings in IPF patients [23].
The six-minute walk distance (6-MWD) evaluation is a simple exercise test with prognostic relevance in patients with ILD [24,25,26,27]. The 6-MWD and the associated oxygen desaturation show good correlation with VO2peak (mL/min/kg) and breathing efficacy (measured as the VÉ/VCO2 slope) in IPF patients [24]. This is also true for patients with sarcoidosis [28] and other ILDs [29,30,31]. In sarcoidosis patients, the peak oxygen uptake, maximum respiratory rate, breathing reserve, alveolar–arterial oxygen pressure gradient at peak exercise, and delta SpO2 values show a strong correlation with the relative differences in FVC %pred. and DLCO %pred. over five years [32].
Because of the frequent cardiovascular gas exchange defects and muscular comorbidities in ILD patients, cardiopulmonary exercise testing (CPET) has proven to be an elegant and relatively easy method to determine the individual performance and compensation ability of the respiratory system (lung–heart–circulation–muscle) and to differentiate the leading disturbances causing limitations of exercise capacity [33,34,35,36]. Against this background, CPET is used to evaluate intervention efficacy in pulmonary diseases [37]. The standardisation of CPET in ILD patients is a part of current investigations [38]. In the current literature, a couple of studies have demonstrated the prognostic relevance of CPET parameters in diverse ILD subgroups [39,40]. Nevertheless, a meta-analysis of 13 retrospective studies stated that there was insufficient evidence to confirm the value of CPET in facilitating ‘real-world’ clinical decision making in patients with ILD and that additional prospective studies are required to validate the putative prognostic associations reported in previous studies in carefully phenotyped patient populations [41].
Thus, the aim of this outpatient study was to assess the prognostic value of multiple CPET-derived parameters in a defined group of patients with ILD, with detailed analyses in IPF patients.
2. Material and Methods
2.1. Patients
In this single-centre retrospective study, a total of 215 patients with ILD were included. This centre follows the IPF-guidelines on diagnosis and therapy such as multidisciplinary discussion (MDD) [2,6,42]. No CPET data were available for 32 of the 215 patients. Therefore, the final study population consisted of 183 patients.
A definite/probable UIP (usual interstitial pneumonia) pattern was found in 55 of the 183 patients, and IPF was classified on the basis of this finding. Patients with CPFE syndrome were evaluated separately as a subgroup of patients with UIP [43,44]. In line with ATS/ERS guidelines [1], patients with a non-UIP pattern were classified as showing ‘sarcoidosis with fibrosis’ [45], ‘exogen-allergic alveolitis with fibrosis’ [46], or other conditions, including fibrotic non-specific interstitial pneumonia (NSIP) [47], fibrotic ‘interstitial pneumonia with autoimmune features’ (IPAF) [48], or unclassifiable ILD [49].
For the characterisation of the patients, sex, age, body mass index (BMI), and number of comorbidities (according to the Charlson comorbidity Index [50]) were analysed. The Charlson comorbidity Index predicts 10-year survival in patients focusing on 19 comorbidities with different assessment scores. The severity grading of IPF patients followed the modified GAP index [21].
2.2. Lung Function and Diffusing Capacity
Lung function parameters were calculated according to normative values, as described previously [51,52,53]. Obstructive pulmonary disease was defined as forced expiratory volume in 1 s (FEV1)/FVC < 70%; restrictive pulmonary disease by total lung capacity (TLC) < 80%; and clinically relevant diffusion impairment by DLCO < 60%.
2.3. Cardiopulmonary Exercise Testing
CPET was performed according to the modified JONES-protocol using a bicycle ergometer as a symptom-limited test. Performance and analysis methods have been previously described in detail [54]. Briefly, the test started with a 3-min resting phase and unloaded cycling of 1 min followed by a protocol with a step-increment protocol of 16 W∙min−1.
2.4. Right Heart Catheterisation
Right heart catheterisation (RHC) was performed in accordance with the guidelines of the ESC/ERS [55] and German recommendations [56]. PH was defined as mean pulmonary artery pressure (PAPmean) >20 mmHg, and pulmonary arterial hypertension was defined as PAPmean >20 mmHg, pulmonary artery wedge pressure (PAWP) ≤15 mmHg, and pulmonary vascular resistance (PVR) ≥3 Wood units (≥240 dyn∙s∙cm−5) [57].
2.5. Echocardiography
Resting echocardiography was performed by experienced physicians according to relevant guidelines [58,59]. TR was classified according to the American College of Cardiology/European Society of Cardiology (ESC) recommendations, and PAPsys was estimated using a simplified Bernoulli equation via TR velocity (v) as RVsys (mmHg) = 4v2, with the addition of 5 mmHg if the inferior vena cava was not dilated and there was visible respiratory variability and 10 mmHg if the inferior vena cava was dilated or without respiratory variability.
2.6. Follow-Up Assessments
The patients were contacted by phone and provided written informed consent for data collection. The date of evaluation was 01.03.2020 (mean observation time, 3.0 ± 2.5 years). The study was approved by the ethics committee of the University of Greifswald (Reg.-Nr. BB 057/2017).
2.7. Methodological Limitations
The selection of patients was inherently biased, since only patients who underwent CPET were included in the analyses. As a result, despite the retrospective nature of the study, only five patients were lost to follow-up (2.7%). Moreover, due to the retrospective approach, not all clinical and functional data were available. Due to the fact that our institution is a supra-regional centre for PH-diagnosis and therapy, the proportion of PH-patients in this study is high.
In all patients, the modified JONES-protocol was used on a braked cycle ergometer, which has been only evaluated for COPD patients so far and did not significantly influence the comparability of exercise parameters to other protocols [60,61]. Studies comparing different exercise protocols are not known for patients with ILD. Moreover, the current study focused on the prognostic evaluation of CPET parameters. Therefore, data on therapy are not provided. However, the effect of available antifibrotic medication on pulmonary function and cardiopulmonary exercise capacity is undisputable [8,62,63,64].
2.8. Statistical Analyses
Continuous variables, stratified by group status, were reported as the median and interquartile range (IQR, in brackets). Categorical variables were reported as absolute numbers and percentages. Differences among groups were verified using Wilcoxon (continuous data) and χ2 tests (categorical data). Potential associations of group status and parameters from pulmonary function testing and CPET with mortality were tested using Cox regression models adjusted for age and sex. For group status, the follow-up duration was calculated based on the time of diagnosis; for the other variables, the time of first examination was defined as the starting point. Prediction models were determined using Cox regression models with age, sex, body mass index (BMI), and all parameters from pulmonary function testing and CPET as explanatory variables. For the final model, variables using a backward selection procedure with a cut-off p-value of 0.1 were eliminated. The discrimination of these models was reported using Harrell’s C-statistic. Based on logistic regression models with the outcome ‘death: yes/no’, conducted receiver operating characteristic (ROC) analyses for selected variables were conducted. Kaplan–Meier curves were plotted for selected variables; for continuous variables, cut-off values were defined as the point which maximised the Youden index for the outcome ‘death’. The Youden index was defined as sensitivity + specificity − 1. All analyses were carried out using Stata 14.1 (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Patient Characteristics
The median age of the included patients (n = 183, 68% male) was 68.1 ± 10.4 years. The median age of patients with a UIP pattern was 72.8 ± 7.9 years and that of those with CPFE syndrome was 71.1 ± 8.3 years (Table 1). In the latter group, 95% of the patients were men. Patients with EAA (64.3 ± 10.5 years) and sarcoidosis (60.6 ± 12.1 years) were significantly younger than those with IPF (p < 0.001). The percentage of male patients was the smallest in the sarcoidosis group (53%). At the time of study inclusion, the diagnosis had been established for 2.7 ± 6.1 years in ILD patients and 2.0 ± 1.7 years in CPFE syndrome patients.

Table 1.
Data of patients with different interstitial lung diseases.
The average Charlson comorbidity index of the patients was 2 ± 2 points. The mean number of comorbidities was 2.4, and more than three comorbidities were reported in 41% of the patients (Figure 1). The GAP index in the IPF subgroup was ≥3 in 88% of the cases (Figure 1a).
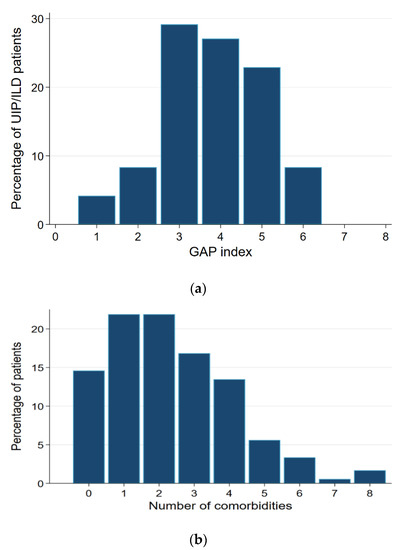
Figure 1.
(a) The GAP-Index in IPF-patients (n = 48). UIP: Usual interstitial pneumonia; ILD: interstitial lung disease. (b) Number of comorbidities (in percent).
3.2. Echocardiography
On average, 71% of all patients had normal left ventricular function, and the corresponding value in patients with the UIP pattern was 59%. Reduced left ventricular function was documented in 11% of the patients. Right ventricular function was comparable in all patients, with an approximate tricuspid annular plane systolic excursion (TAPSE) of 21 ± 5 mm (Table 1).
3.3. Right Heart Catheterisation
Haemodynamic data were available in a subgroup of 87 patients, and PH was diagnosed in 79% of these patients (68% of IPF patients and up to 100% of patients with sarcoidosis), Table 1. The RHC and non-RHC groups showed significant differences (Supplementary Table S1), especially in relation to the time between diagnosis and inclusion in the present study (1.3 ± 5.4 vs. 3.0 ± 6.8 years, p = 0.008). Patients who underwent invasive diagnostic procedures (RHC, n = 87) experienced diverse comorbidities more often (e.g., arterial hypertension, atrial fibrillation, renal insufficiency, coronary artery disease, venous thromboembolic disease). PH was known beforehand in 77% of patients who received RHC (Supplementary Table S1).
Although the RHC group and overall study group showed no significant differences in echocardiographic (except for PAPsyst: 33.4 ± 11.0 vs. 58.3 ± 19.9 mmHg for the RHC group, p < 0.001) and lung functional findings (except for DLCO and KCO% pred., p < 0.001), the RHC group showed poorer findings for CPET parameters such as performance (97 ± 32 vs. 68 ± 27 W, p < 0.001), oxygen uptake (17.1 ± 4.6 vs. 11.4 ± 3.3 mL/kg/min, p < 0.001), and breathing efficacy (VÉ/VCO2 slope: 37.9 ± 10.2 vs. 50.7 ± 15.5, p < 0.001).
3.4. Lung Function Values
The overall group of patients had FEV1 (%pred.), FVC (%pred.), TLC value (%pred.), and DLCO value (%pred.) values of 81% ± 22%, 81% ± 22%, 79% ± 20%, and 44% ± 26%, respectively. The proportion of patients with TLC (%pred.) < 80%, FVC (%pred.) < 80%, DLCO (%pred.) < 60%, and KCO (%pred.) < 60% was 54%, 29%, 82%, and 49%, respectively (Table 1).
3.5. Cardiopulmonary Exercise Testing
The determined maximum power in watts (W) was 67% ± 30% pred., peak oxygen uptake was 62% ± 21% pred., and the VÉ/MVV ratio was 68% ± 21% pred. In 36 (20%) patients of the overall group, this value was >80% and, therefore, demonstrated pulmonary exercise limitation. The respiratory efficacy (measured as the VÉ/VCO2 slope) was 44 ± 14 in 77% of the patients, with values > 34 considered pathological. The PaetCO2 max value > 6 mmHg at the end of exercise demonstrated an inhomogeneity of ratio perfusion/ventilation and was pathological in 73% of the overall group. Interestingly, 31% of all ILD patients showed dynamic hyperinflation (defined as EELVmax − EELFrest > 0); in sarcoidosis patients, it was even evident in 65% of the patients (Table 1).
3.6. Survival
Figure 2 depicts the survival rates of the entire group of ILD patients. The 3-year and 5-year survival rates were 68% and 50%, respectively. Figure 3 shows the survival rates of subgroups of ILD patients. The 3-year and 5-year survival rates were the lowest in patients with CPFE. For IPF patients, the rates were 72% and 58%, respectively.
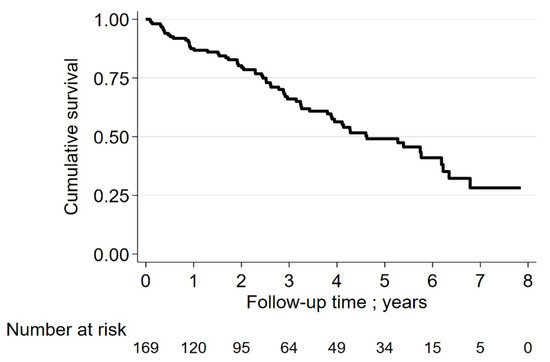
Figure 2.
Survival rate of ILD-patients.
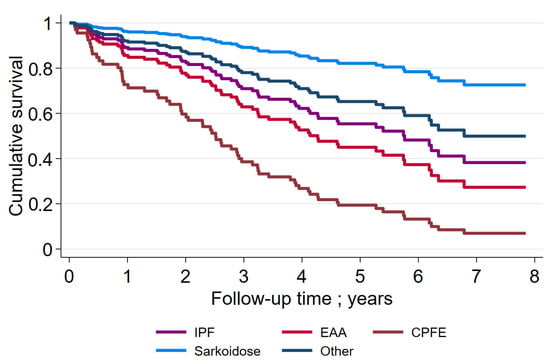
Figure 3.
Survival rate in subgroups of ILD-patients. IPF: idiopathic pulmonary fibrosis; EAA: exogen allergic alveolitis; CPFE: combined pulmonary fibrosis and emphysema.
3.7. Parameters Relevant to Prognosis
Parameters relevant to prognosis over the years were determined by Cox regression analyses of the data at study entry (adjusted for age, sex, and body mass index, Supplementary Table S2). Three models were established:
Model 1 (dyslipidaemia, PHT (medical history), TAPSE, PAPsys, FEV1 pred., and DLCO pred. (or KCO pred., n = 98). Using backward elimination, significance was observed for PAPsys (HR, 1.03; 95% CI, 1.01–1.04; p = 0.003) and TAPSE (HR, 0.88; 95% CI 0.82–0.96; p = 0.004). The C-statistic was 0.810.
Subgroup analyses in IPF patients (n = 55, mean age 72.8 ± 7.9 years, 76% male revealed a significant influence of dyslipidaemia (HR, 25.65; 95% CI 1.71–385.15; p = 0.019), PH (medical history) (HR, 31.73; 95% CI 3.15–319.91; p = 0.003), TAPSE (HR, 0.82; 95% CI 0.68–0.98; p = 0.032), and DLCO (%pred.) (HR, 1.02; 95% CI 1.00–1.04, p = 0.032). The C-statistic was 0.928.
Model 2 (CPET values, without AaDO2 and PaetCO2, n = 134) demonstrated a significant influence of max. power (%pred.) (HR, 0.98; 95% CI 0.97–0.99; p = 0.039), petCO2 at rest (HR, 1.16; 95% CI 1.04–1.29; p = 0.006), VE/VCO2 @AT (HR, 1.09; 95% CI 1.03–1.16; p = 0.002), and VO2peak (%pred.) (HR, 0.97; 95% CI 0.94–0.99; p = 0.038) upon prognosis. The C-statistic was 0.826.
Subgroup analyses in IPF patients revealed prognostic significance for petCO2 at rest (HR, 1.45; 95% CI 1.14–1.86; p = 0.003), petCO2@AT (HR, 0.59; 95% CI 0.43–0.80; p = 0.001), and VO2peak (%pred.) (HR, 0.92; 95% CI 0.87–0.97; p = 0.001). The C-statistic was 0.869.
Model 3 (comorbidities of medical history, patient characteristics (BMI, gender, and age), lung function, diffusing capacity, and CPET values without AaDO2 and PaetCO2, n = 112). This model demonstrated a significant influence of dyslipidaemia (HR, 3.37; 95% CI 1.19–9.57; p = 0.023), BMI (HR, 0.81; 95% CI 0.72–0.91; p = 0.000), RV (%pred.) (HR, 0.95; 95% CI 0.90–0.99; p = 0.023), TLC (%pred.) (HR, 1.09; 95% CI 1.00–1.20; p = 0.042), FEV1 (%pred.) (HR, 0.94; 95% CI 0.90–0.98; p = 0.008), VO2peak (mL/kg/min) (HR, 0.75; 95% CI 0.63–0.89; p = 0.001), petCO2@AT (mmHg) (HR, 0.74; 95% CI 0.63–0.87; p = 0.000), and petCO2 at rest (mmHg) (HR, 1.51; 95% CI 1.22–1.87; p = 0.000). The C-statistic was 0.869 (better than that of models 1 and 2).
The IPF patient subgroup was too small for these analyses. In two of the three models, VO2peak (as mL/kg/min or %pred.) in the entire group of patients with ILD was of significant prognostic relevance. For the entire group, the best cut-off VO2peak was 61% pred. for the discrimination of mortality (for VÉ/VCO2@AT, 39; for VÉ/VCO2 slope, 40; and for VO2peak/HRpeak, 8.6 mL) (Supplementary Table S3, Figure 4).
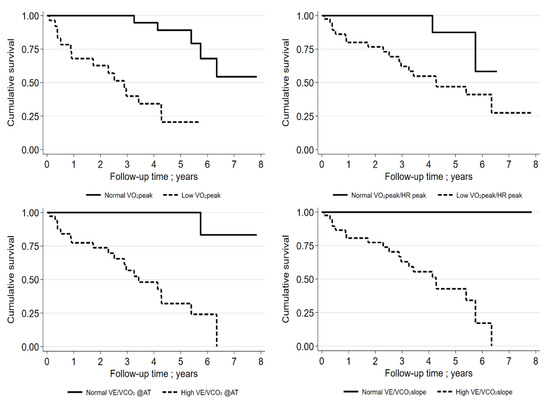
Figure 4.
Kaplan–Meier curves for IPF patients (VO2peak % pred cut-off: 61%; VO2peak/HRpeak cut-off: 8.6 mL; VE/VCO2@AT cut-off: 39; VE/VCO2 slope cut-off: 40). “Low“ means beneath cut-off, “normal“ means above cut-off.
In the subgroup of IPF patients (n = 55; mean age, 72.8 ± 7.9; 76% males), the best cut-off for VO2peak was 69% pred. for the discrimination of mortality (for VÉ/VCO2@AT, 37; for VÉ/VCO2 slope, 33; and for VO2peak/HRpeak, 13 mL). An additional analysis of the prognostic relevance of the GAP index (including the FEV values) was performed and was found to be significant for survival (HR, 2.44; 95% CI 1.5–3.96; p < 0.001). The C-statistic was 0.760. Only VO2peak %pred. remained statistically significant in the model with the GAP index, VO2peak %pred., and VÉ/VCO2 slope (HR, 0.93; 95% CI 0.88–0.98; p = 0.006). The C-statistic was found to be 0.883. Interestingly, FVC (%pred.) did not significantly influence the survival of patients with IPF (HR, 0.979; 95% CI 0.955–1.003; p = 0.087).
For the subgroup of patients who underwent RHC, the prognostic relevance of the presence of PH (defined as PAPmean > 20 mmHg) was examined (adjusted for age, sex, and BMI) but did not prove to be of significance for survival (HR, 2.3; 95% CI 0.90–5.94; p = 0.082).
4. Discussion
Our study with 183 ILD patients clearly demonstrated that the maximum oxygen uptake (VO2peak) had a highly significant prognostic influence. The best mortality predictive cut-off value for VO2peak in ILD patients was 61% pred., while in IPF patients, it was 69% pred. Only four studies included more than 100 patients. However, these studies lacked detailed information on comorbidities, and the included exercise variables were limited [41].
Dyslipidaemia, as well as the existence of PH (medical records), echocardiographically measured right ventricular function (TAPSE), and PAPsys were of prognostic significance. In addition to male sex and age > 70 years, the degree of dyspnoea; DLCO < 60% pred., 6-MWD < 250 m, and SpO2 < 88% measured during the 6-MWD; and the existence of PH and cardiovascular comorbidities were considered prognostically relevant baseline data in IPF patients [13,65,66]. Additionally, in the literature, histological data (number of fibroblastic foci), the extent of fibrotic alterations measured with HRCT, and selected biomarkers were prognostically relevant. Some of the prognostically relevant data are also reflected by our data, especially dyslipidaemia and PH. Against this background, it appears strange that comorbidities were not regularly part of investigations of ILD prognosis. With regard to dyslipidaemia, there were no differences between IPF and NSIP patients (20% and 17%) [7]. This is similar to our data. Although the prognostic significance of PH (medical history) is adequately familiar [15,67] it has not yet been described for dyslipidaemia in ILD-patients.
Focusing on lung function, only FEV1 and DLCO (as well as KCO) were reported to be prognostically relevant in our Cox analysis. In contrast to other studies [39,40,68], the prognostic significance for FVC was not observed. However, a good correlation between FVC and FEV1 is known [10]. The small number of included patients with IPF (n = 55/183) might explain the missing prognostic relevance of FVC.
The prognostic significance of diffusion parameters in ILD patients is undisputable [24,39], justifying their inclusion in prognosis scores [22]. DLCO is a sensitive marker for gas-exchange pathologies in fibrotic lung diseases. However, it is decisively influenced by the capillary blood volume [69]. Against this background, it is a good parameter for the detection of early pulmonary vascular injury [70]. It is reduced in manifest vascular disturbances and often associated with PH. In our study, 94% of the patients with PH showed pathological diffusion capacity (DLCO < 60% pred.), while the corresponding percentage in evaluations based on KCO pred. was 83%. The optimal prognostic cut-off value was 36% for DLCO pred. and 48% for KCO pred.
The prognostic relevance of CPET parameters for diverse subgroups of patients with ILD is commonly known [41]. In contrast to the straightforward and inexpensive 6-MWD, the most convincing advantage of CPET is the early detection of pathophysiological conditions and cardiopulmonary limitations. The limited lung compliance in ILD patients leads to only a small increase in breathing volume (Vt), which results in an increase in breathing frequency. Predominant dead-space ventilation leads to a pathological breathing efficacy. Hyperventilation is enhanced by the stimulation of mechanoreceptors of the lung. Additionally, the alveolo-arterial oxygen difference (AaDO2) at rest is often elevated in patients with ILD and increases during exercise due to oxygen exploitation in the muscle tissue. This eventually results in acidosis, which aggravates hyperventilation and impairs breathing efficacy. Impaired oxygen exchange in the lung eventually limits oxygen supply to the muscle tissue and exercise performance. The increased breathing effort claims a relevant proportion of oxygen uptake that is no longer available to the peripheral muscles. Depending on the extent of disease, it is comprehensible that ventilation, perfusion, and gas exchange are impaired in ILD patients [14,71]. During exercise, despite lung parenchymal disease, the heart rate is elevated and the stroke volume is reduced in ILD patients. Our data support these statements and prove the prognostic relevance of these parameters (Supplementary Tables S1 and S2).
Independent of the primary disease, which was also true for ILD patients, the existence of PH resulted in typical CPET changes: reduced VO2peak < 60% pred., increased VÉ/VCO2 slope > 45, PetCO2 < 33 mmHg at rest, and an increase of <3 mmHg during exercise [15,17,18,19,72,73]. Therefore, our PH patients showed a VO2peak of 47.4% ± 15.3% pred., a VÉ/VCO2 slope of 53.9 ± 15.9, and a PetCO2 at rest of 26.1 ± 5.5 mmHg. Interestingly, our analyses did not reveal a significant influence of invasively measured PH on survival in ILD patients. However, other ILD-studies (especially IPF studies) have described the prognostic relevance of PH in these patients [74,75,76,77].
In summary, the present data revealed that VO2peak pred., VÉ/VCO2 slope, and VÉ/VCO2@AT are of prognostic relevance in ILD patients (including those with IPF). This is consistent with other studies [41,78]. Surprisingly, in our patients, dyslipidaemia was prognostically relevant, but has not been described to show prognostic significance in ILD patients so far. None of the established prognostic parameters, including FVC %pred. and DLCO/KCO %pred., other than the VO2peak %pred. were shown to be relevant prognostic markers in the entire group of patients with ILD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11061609/s1, Table S1: Patients with/without Right heart catheter; Table S2: Associations of measurements from medical history, echocardiography, right heart catheter, lung function testing and cardiopulmonary exercise testing with mortality; Table S3: Sensitivity, specificity, and Youden index for the perfect cut-offs for mortality (selected data).
Author Contributions
Conceptualization, R.E. and B.S.; methodology, R.E. and B.S.; software, T.I.; validation, T.I., S.G. and B.S.; formal analysis, T.I.; investigation, C.W. and A.G.; resources, R.E.; data curation, A.G. and C.W.; writing—original draft preparation, R.E. and B.S.; writing—review and editing, S.G., A.G. and C.W.; visualization, R.E.; supervision, B.S.; project administration, R.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the University of Greifswald (Reg.-Nr. BB 057/2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
On request data can be made available.
Acknowledgments
We wish to thank the staff of our clinic, especially Dagmar Fimmel, Kathrin Gutsche, Jeannette Pieper, and Claudia Pohl.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ILD | interstitial lung disease |
| RHC | right heart catheter |
| CRF | cardiorespiratory fitness |
| BMI | body mass index |
| FVC | forced vital capacity |
| CPET | cardiopulmonary exercise testing |
| PH | pulmonary hypertension |
| COPD | chronic obstructive pulmonary disease |
| BODE | body-mass, airflow obstruction, dyspnea, and exercise capacity index |
| ADO | age, dyspnoea, airflow obstruction index |
| DOSE | dyspnoea, obstruction, smoking, exacerbation index |
| HRCT | high-resolution computed tomography |
| NSIP | non-specific interstitial pneumonia |
| UIP | usual interstitial pneumonia |
| IPAF | interstitial pneumonia with autoimmune features |
| DLCO | diffusion capacity |
| CPFE | combined pulmonary fibrosis and emphysema |
| VO2peak | peak oxygen uptake |
| VO2@AT | oxygen uptake at anaerobic threshold |
| VÉ/VCO2 slope | breathing efficacy |
| VÉ/VCO2@AT | breathing efficacy at anaerobic threshold |
| 6-MWD | 6-min walking distance |
| FEV1 | forced expiratory volume in 1 s |
| SD | standard deviation |
| ROC | receiver operating characteristic |
| TLC | total lung capacity |
| W | Watt |
| RR | relative risk |
| CPI | composite physiological index |
References
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Horst, C.; Gholipour, B.; Nair, A.; Jacob, J. Differential diagnoses of fibrosing lung diseases. BJR/Open 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Wirtz, H.; Koschel, D.; Pittrow, D.; Held, M.; Klotsche, J.; Andreas, S.; Claussen, M.; Grohé, C.; et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: Long-term results of the INSIGHTS-IPF registry. Eur. Respir. J. 2020, 56, 1902279. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Günther, A.; Bonella, F.; Geißler, K.; Koschel, D.; Kreuter, M.; Prasse, A.; Schönfeld, N.; Sitter, H.; Müller-Quernheim, J.; et al. German Guideline for Idiopathic Pulmonary Fibrosis—Update on Pharmacological Therapies 2017. Pneumologie 2017, 71, 474. [Google Scholar] [CrossRef] [PubMed]
- Hagmeyer, L.; Herkenrath, S.; Anduleit, N.; Treml, M.; Randerath, W. Cardiopulmonary Exercise Testing Allows Discrimination Between Idiopathic Non-specific Interstitial Pneumonia and Idiopathic Pulmonary Fibrosis in Mild to Moderate Stages of the Disease. Lung 2019, 197, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.E.; Ley, B.; Kreuter, M.; Wijsenbeek, M.; Vittinghoff, E.; Collard, H.R.; Vancheri, C. The added value of comorbidities in predicting survival in idiopathic pulmonary fibrosis: A multicentre observational study. Eur. Respir. J. 2019, 53, 1801587. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.B.; Smith, C.; Le Jeune, I.; Gribbin, J.; Fogarty, A.W. The association between idiopathic pulmonary fibrosis and vascular disease: A population-based study. Am. J. Respir. Crit. Care Med. 2008, 178, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- King, T.E.; Tooze, J.A., Jr.; Schwarz, M.I.; Brown, K.R.; Cherniack, R.M. Predicting survival in idiopathic pulmonary fibrosis: Scoring system and survival model. Am. J. Respir. Crit. Care Med. 2001, 164, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E. Review series: Aspects of interstitial lung disease: Exercise limitation in interstitial lung disease—mechanisms, significance and therapeutic options. Chronic Respir. Dis. 2010, 7, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Ferrazza, A.; Martolini, D.; Valli, G.; Palange, P. Cardiopulmonary Exercise Testing in the Functional and Prognostic Evaluation of Patients with Pulmonary Diseases. Respiration 2009, 77, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Fabrellas, E.F.; Sanchez, R.P.; Abad, C.S.; Samper, G.J. Prognosis and Follow-Up of Idiopathic Pulmonary Fibrosis. Med. Sci. 2018, 6, 51. [Google Scholar]
- Troy, L.K.; Young, I.H.; Lau, E.M.; Corte, T.J. Exercise pathophysiology and the role of oxygen therapy in idiopathic interstitial pneumonia. Respirology 2015, 21, 1005–1014. [Google Scholar] [CrossRef]
- van der Plas, M.N.; van Kan, C.; Blumenthal, J.; Jansen, H.M.; Wells, A.U.; Bresser, P. Pulmonary vascular limitation to exercise and survival in idiopathic pulmonary fibrosis. Respirology 2014, 19, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Villaquirán, C.; Valera, J.L.; Molina-Molina, M.; Xaubet, A.; Rodríguez-Roisin, R.; Barberà, J.A.; Roca, J. Peak Oxygen Uptake During the Six-minute Walk Test in Diffuse Interstitial Lung Disease and Pulmonary Hypertension. Archivos Bronconeumol. 2010, 46, 122–128. [Google Scholar] [CrossRef]
- Boutou, A.K.; Pitsiou, G.G.; Trigonis, I.; Papakosta, D.; Kontou, P.K.; Chavouzis, N.; Nakou, C.; Argyropoulou, P.; Wasserman, K.; Stanopoulos, I. Exercise capacity in idiopathic pulmonary fibrosis: The effect of pulmonary hypertension. Respirology 2011, 16, 451–458. [Google Scholar] [CrossRef]
- Gläser, S.; Obst, A.; Koch, B.; Henkel, B.; Grieger, A.; Felix, S.B.; Halank, M.; Bruch, L.; Bollmann, T.; Warnke, C.; et al. Pulmonary Hypertension in Patients with Idiopathic Pulmonary Fibrosis—The Predictive Value of Exercise Capacity and Gas Exchange Efficiency. PLoS ONE 2013, 8, e65643. [Google Scholar] [CrossRef][Green Version]
- Armstrong, H.F.; Thirapatarapong, W.; Dussault, N.E.; Bartels, M.N. Distinguishing Pulmonary Hypertension in Interstitial Lung Disease by Ventilation and Perfusion Defects Measured by Cardiopulmonary Exercise Testing. Respirology 2013, 86, 407–413. [Google Scholar] [CrossRef]
- Fukuda, C.Y.; Soares, M.R.; Pereira, C.A.D.C. A score without diffusion capacity of the lung for carbon monoxide for estimating survival in idiopathic pulmonary fibrosis. Medicine 2020, 99, e20739. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.S.; Kim, S.Y.; Kim, D.S.; Kim, Y.W.; Chung, M.P.; Uh, S.T.; Park, C.S.; Park, S.W.; Jeong, S.H.; et al. Comparison of CPI and GAP models in patients with idiopathic pulmonary fibrosis: A nationwide cohort study. Sci. Rep. 2018, 8, 4784. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.; Adamali, H.I.; Millar, A.B. A comparison of published multidimensional indices to predict outcome in idiopathic pulmonary fibrosis. ERJ Open Res. 2017, 3, 96–2016. [Google Scholar] [CrossRef] [PubMed]
- Xaubet, A.; Agusti, C.; Luburich, P.; Roca, J.; Montón, C.; Ayuso, M.C.; Barberá, J.A.; Rodríguez-Roisin, R. Pulmonary Function Tests and CT Scan in the Management of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 1998, 158, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidou, C.; Manali, E.; Lyberopoulos, P.; Kolilekas, L.; Kagouridis, K.; Gyftopoulos, S.; Vougas, K.; Kotanidou, A.; Alchanatis, M.; Karakatsani, A.; et al. The Role of Cardiopulmonary Exercise Test in IPF Prognosis. Pulm. Med. 2013, 2013, 514817. [Google Scholar] [CrossRef] [PubMed]
- Kawut, S.M.; O’Shea, M.K.; Bartels, M.N.; Wilt, J.S.; Sonett, J.R.; Arcasoy, S.M. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir. Med. 2005, 99, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Lama, V.N.; Flaherty, K.R.; Toews, G.B.; Colby, T.V.; Travis, W.D.; Long, Q.; Murray, S.; Kazerooni, E.A.; Gross, B.H.; Lynch, J.P.; et al. Prognostic Value of Desaturation during a 6-Minute Walk Test in Idiopathic Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2003, 168, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.; Arcasoy, S.M.; Wilt, J.S.; D’Ovidio, F.; Sonett, J.R.; Kawut, S.M. Six-Minute-Walk Distance Predicts Waiting List Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 659–664. [Google Scholar] [CrossRef]
- Kiani, A.; Eslaminejad, A.; Shafeipour, M.; Razavi, F.; Seyyedi, S.R.; Sharif-Kashani, B.; Emami, H.; Bakhshayesh-Karam, B.; Abedini, A. Spirometry, cardiopulmonary exercise testing and the six-minute walk test results in sarcoidosis patients. Sarcoidosis Vasc. Diffus. Lung Dis. 2019, 36, 185–194. [Google Scholar]
- Cahalin, L.; Pappagianopoulos, P.; Prevost, S.; Wain, J.; Ginns, L. The Relationship of the 6-Min Walk Test to Maximal Oxygen Consumption in Transplant Candidates With End-Stage Lung Disease. Chest 1995, 108, 452–459. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.; Kolbe, J.; Wilsher, M.L. Comparison of the modified shuttle walk test and cardiopulmonary exercise test in sarcoidosis. Respirology 2014, 19, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Eaton, T.; Young, P.; Milne, D.; Wells, A.U. Six-minute walk, maximal exercise tests: Reproducibility in fibrotic interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Menezes, S.; Dias, C.; Oliveira, J.; Mainenti, M.; Guimarães, F. Cardiopulmonary exercise testing variables as predictors of long-term outcome in thoracic sarcoidosis. Braz. J. Med. Biol. Res. 2012, 45, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Teopompi, E.; Tzani, P.; Aiello, M.; Ramponi, S.; Visca, D.; Gioia, M.R.; Marangio, E.; Serra, W.; Chetta, A. Ventilatory Response to Carbon Dioxide Output in Subjects With Congestive Heart Failure and in Patients with COPD With Comparable Exercise Capacity. Respir. Care 2013, 59, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Muhle, A.; Obst, A.; Winkler, J.; Ewert, R. Cardiopulmonary exercise testing in chronic obstructive pulmonary disease (COPD)—breath-functional characterization and disease severity assessment. Pneumologie 2015, 69, 534–544. [Google Scholar] [PubMed]
- Alencar, M.C.; Arbex, F.F.; Souza, A.; Mazzuco, A.; Sperandio, P.A.; Rocha, A.; Hirai, D.M.; Mancuso, F.; Berton, D.C.; Borghi-Silva, A.; et al. Does Exercise Ventilatory Inefficiency Predict Poor Outcome in Heart Failure Patients With COPD? J. Cardiopulm. Rehabil. Prev. 2016, 36, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Thirapatarapong, W.; Armstrong, H.F.; Bartels, M.N. Comparing Cardiopulmonary Exercise Testing in Severe COPD Patients with and without Pulmonary Hypertension. Heart Lung Circ. 2014, 23, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Puente-Maestu, L.; Palange, P.; Casaburi, R.; Laveneziana, P.; Maltais, F.; Neder, J.A.; O’Donnell, D.E.; Onorati, P.; Porszasz, J.; Rabinovich, R.; et al. Use of exercise testing in the evaluation of interventional efficacy: An official ERS statement. Eur. Respir. J. 2016, 47, 429–460. [Google Scholar] [CrossRef]
- Radtke, T.; Vogiatzis, I.; Urquhart, D.S.; Laveneziana, P.; Casaburi, R.; Hebestreit, H. Standardisation of cardiopulmonary exercise testing in chronic lung diseases: Summary of key findings from the ERS task force. Eur. Respir. J. 2019, 54, 1901441. [Google Scholar] [CrossRef]
- Wallaert, B.; Guetta, A.; Wemeau-Stervinou, L.; Terce, G.; Valette, M.; Neviere, R.; Aguilaniu, B. Prognostic value of clinical exercise testing in idiopathic pulmonary fibrosis. Rev. Mal. Respir 2011, 28, 290–296. [Google Scholar] [CrossRef]
- Fell, C.D.; Martinez, F.J.; Liu, L.X.; Murray, S.; Han, M.K.; Kazerooni, E.A.; Gross, B.H.; Myers, J.; Travis, W.D.; Colby, T.V.; et al. Clinical Predictors of a Diagnosis of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Davis, R.; Sharp, C.; Pauling, J.D. The prognostic value of cardiopulmonary exercise testing in interstitial lung disease: A systematic review. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Gunther, A.; Ammenwerth, W.; Bittmann, I.; Bonnet, R.; Buhl, R.; Eickelberg, O.; Ewert, R.; Gläser, S.; Gottlieb, J. German guideline for diagnosis and management of idiopathic pulmonary fibrosis. Pneumologie 2013, 67, 81–111. [Google Scholar] [PubMed]
- Cottin, V.; Nunes, H.; Brillet, P.-Y.; Delaval, P.; Devouassaoux, G.; Tillie-Leblond, I.; Israel-Biet, D.; Court-Fortune, I.; Valeyre, D.; Cordier, J.-F. Combined pulmonary fibrosis and emphysema: A distinct underrecognised entity. Eur. Respir. J. 2005, 26, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V. Combined pulmonary fibrosis and emphysema: Bad and ugly all the same? Eur. Respir. J. 2017, 50, 1700846. [Google Scholar] [CrossRef] [PubMed]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir Crit. Care Med. 1999, 160, 736–755. [Google Scholar]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef] [PubMed]
- Belloli, E.A.; Beckford, R.; Hadley, R.; Flaherty, K.R. Idiopathic non-specific interstitial pneumonia. Respirology 2015, 21, 259–268. [Google Scholar] [CrossRef]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; Du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef]
- Skolnik, K.; Ryerson, C.J. Unclassifiable interstitial lung disease: A review. Respirology 2015, 21, 51–56. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- ECotes, J.; Chinn, D.J.; Quanjer, P.H.; Roca, J.; Yernault, J.C. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993, 16, 41–52. [Google Scholar]
- Criée, C.-P.; Berdel, D.; Heise, D.; Kardos, P.; Köhler, D.; Leupold, W.; Magnussen, H.; Marek, W.; Merget, R.; Mitfessel, H.; et al. Empfehlungen der Deutschen Atemwegsliga zur Spirometrie; Dustri-Verlag Dr. Karl Feistle: Munich, Germany; Orlando, FL, USA, 2006. [Google Scholar]
- Criée, C.-P.; Berdel, D.; Heise, D.; Kardos, P.; Köhler, D.; Leupold, W.; Magnussen, H.; Marek, W.; Merget, R.; Mitfessel, H.; et al. Empfehlungen der Deutschen Atemwegsliga und der Deutschen Gesellschaft fur Pneumologie und Beatmungsmedizin. Empfehlungen zur Ganzkörperplethysmographie (Bodyplethysmographie); Dustri-Verlag Dr. Karl Feistle: Munich, Germany; Orlando, FL, USA, 2009. [Google Scholar]
- Glaser, S.; Ittermann, T.; Schaper, C.; Obst, A.; Dorr, M.; Spielhagen, T.; Felix, S.B.; Völzke, H.; Bollmann, T.; Opitz, C.F.; et al. The Study of Health in Pomerania (SHIP) reference values for cardiopulmonary exercise testing. Pneumologie 2013, 67, 58–63. [Google Scholar] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Respir. J. 2015, 46, 1855–1856. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Behr, J.; Ewert, R.; Ghofrani, H.A.; Grunig, E.; Halank, M.; Leuchte, H.H.; Olschewski, H.; Schmeisser, A.; Speich, R.; et al. Right heart catheterization in pulmonary hypertension. Dtsch. Med. Wochenschr. 2011, 136, 2601–2616. [Google Scholar] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Weihs, W. Standardised echocardiography. J. Kardiol. 2018, 25, 299–305. [Google Scholar]
- Benzo, R.P.; Paramesh, S.; Patel, S.A.; Slivka, W.A.; Sciurba, F.C. Optimal Protocol Selection for Cardiopulmonary Exercise Testing in Severe COPD. Chest 2007, 132, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Ewert, R.; Glaser, S.; Winkler, J.; Schrader, H.; Trumper, B.G.; Haase, P.U.; Obst, A.; Hoheisel, G. Cardiopulmonary Exercise Testing (CPET) in severe COPD—A multicentre comparison of two test protocols. Pneumologie 2012, 66, 402–407. [Google Scholar] [PubMed]
- Goos, T.; De Sadeleer, L.; Yserbyt, J.; Verleden, G.; Vermant, M.; Verleden, S.; Wuyts, W. Progression in the Management of Non-Idiopathic Pulmonary Fibrosis Interstitial Lung Diseases, Where Are We Now and Where We Would Like to Be. J. Clin. Med. 2021, 10, 1330. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.F.; Raghu, G. Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2019, 28, 190022. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Liang, J.; Cottin, V.; Ryerson, C. Diagnostic Features in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review. B42. ILD Epidemiol. I 2020, 17, 1333–1336. [Google Scholar] [CrossRef]
- Girgis, R.E.; Hoeper, M.M. Pulmonary hypertension in fibrosing idiopathic interstitial pneumonia: Uncertainties, challenges and opportunities. J. Heart Lung Transplant. 2021, 40, 872–881. [Google Scholar] [CrossRef] [PubMed]
- King, C.S.; Shlobin, O.A. The Trouble With Group 3 Pulmonary Hypertension in Interstitial Lung Disease: Dilemmas in Diagnosis and the Conundrum of Treatment. Chest 2020, 158, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Jankowich, M.D.; Rounds, S.I. Combined Pulmonary Fibrosis and Emphysema Syndrome: A Review. Chest 2012, 141, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Armstrong, H.F.; Kim, H.P.; Meza, K.S.; D’Ovidio, F.; Arcasoy, S.M. Cardiopulmonary exercise factors predict survival in patients with advanced interstitial lung disease referred for lung transplantation. Respir. Med. 2017, 126, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wallaert, B.; Wemeau-Stervinou, L.; Salleron, J.; Tillie-Leblond, I.; Perez, T. Do We Need Exercise Tests to Detect Gas Exchange Impairment in Fibrotic Idiopathic Interstitial Pneumonias? Pulm. Med. 2012, 2012, 657180. [Google Scholar] [CrossRef]
- Zou, R.H.; Wallace, W.D.; Nouraie, S.M.; Chan, S.Y.; Risbano, M.G. Lower DLco% identifies exercise pulmonary hypertension in patients with parenchymal lung disease referred for dyspnea. Pulm. Circ. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Molgat-Seon, Y.; Schaeffer, M.R.; Ryerson, C.J.; Guenette, J.A. Cardiopulmonary Exercise Testing in Patients With Interstitial Lung Disease. Front. Physiol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Nagel, C.; Kovacs, G.; Bollmann, T.; Halank, M.; Winkler, J.; Hellmich, M.; Grünig, E.; Olschewski, H.; Ewert, R.; et al. Cardiopulmonary exercise testing for detecting pulmonary arterial hypertension in systemic sclerosis. Heart 2017, 103, 774–782. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Oudiz, R.J.; Karpouzas, G.; Hovanesyan, A.; Jayasinghe, A.; Hansen, J.E.; Rosenkranz, S.; Wasserman, K. Developing Pulmonary Vasculopathy in Systemic Sclerosis, Detected with Non-Invasive Cardiopulmonary Exercise Testing. PLoS ONE 2010, 5, e14293. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, C.J.; Nathan, S.D.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. Prevalence and Outcomes of Pulmonary Arterial Hypertension in Advanced Idiopathic Pulmonary Fibrosis. Chest 2006, 129, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Nagai, S.; Tanaka, S.; Handa, T.; Shigematsu, M.; Nagao, T.; Mishima, M.; Kitaichi, M.; Izumi, T. Significance of Pulmonary Arterial Pressure and Diffusion Capacity of the Lung as Prognosticator in Patients with Idiopathic Pulmonary Fibrosis. Chest 2007, 131, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.M.; Lederer, D.; Borczuk, A.C.; Kawut, S.M. Pulmonary Hypertension in Idiopathic Pulmonary Fibrosis. Chest 2007, 132, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Corte, T.J.; Wort, S.J.; Gatzoulis, M.A.; Macdonald, P.; Hansell, D.M.; Wells, A.U. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax 2009, 64, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Vainshelboim, B.; Oliveira, J.; Fox, B.D.; Kramer, M.R. The Prognostic Role of Ventilatory Inefficiency and Exercise Capacity in Idiopathic Pulmonary Fibrosis. Respir. Care 2016, 61, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).