Brain Sex in Transgender Women Is Shifted towards Gender Identity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Image Acquisition and Processing
2.3. Independent Training Sample
2.4. Data Reduction
2.5. Brain Sex Estimation
2.6. Statistical Analysis
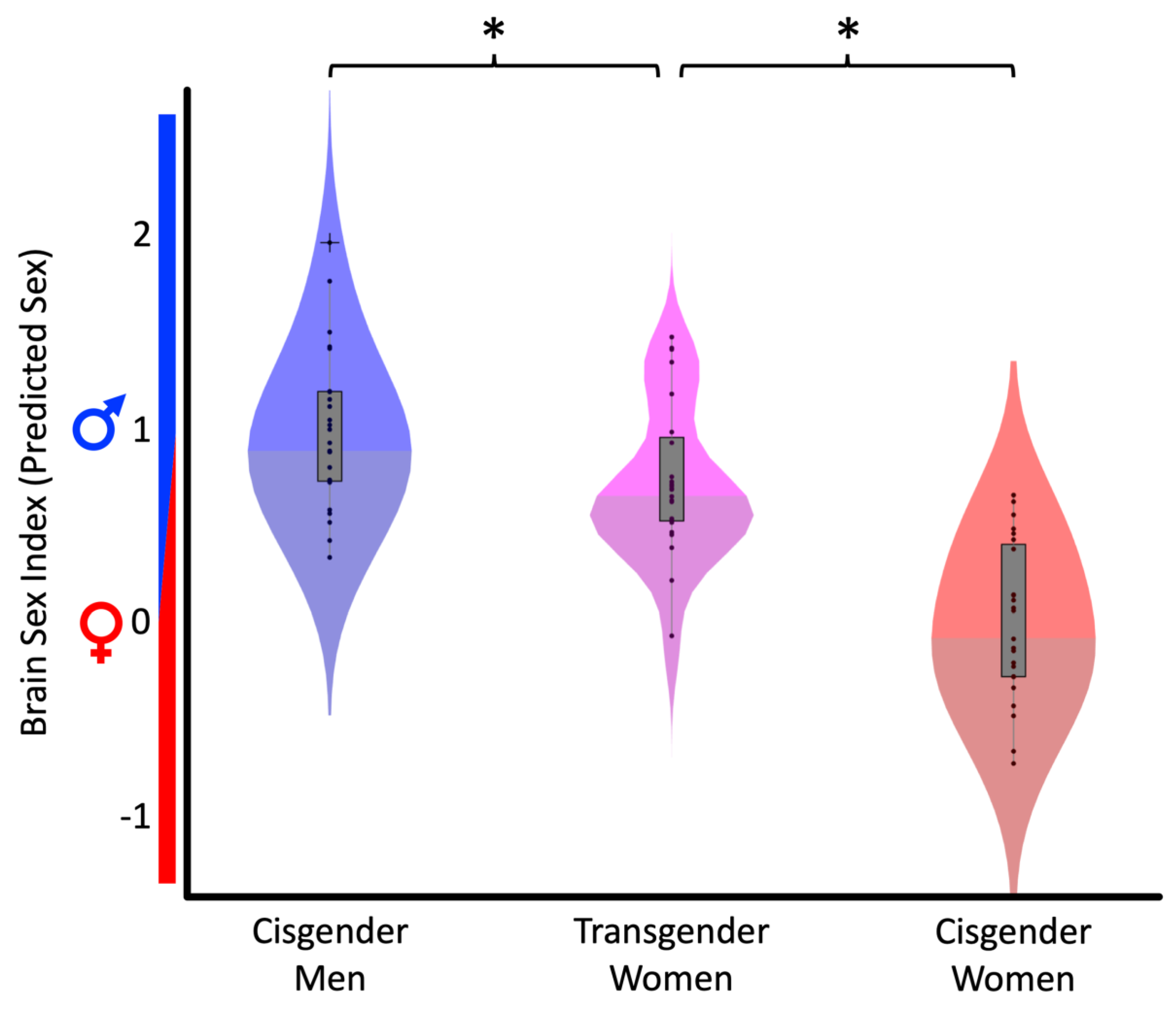
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolan, I.T.; Kuhner, C.J.; Dy, G.W. Demographic and temporal trends in transgender identities and gender confirming surgery. Transl. Androl. Urol. 2019, 8, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Eagly, A.H.; Wood, W. The origins of sex differences in human behavior: Evolved dispositions versus social roles. Am. Psychol. 1999, 54, 408–423. [Google Scholar] [CrossRef]
- Wood, W.; Eagly, A.H. A cross-cultural analysis of the behavior of women and men: Implications for the origins of sex differences. Psychol. Bull. 2002, 128, 699–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coolidge, F.L.; Thede, L.L.; Young, S.E. The heritability of gender identity disorder in a child and adolescent twin sample. Behav.Genet. 2002, 32, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Birth order and ratio of brothers to sisters in transsexuals. Psychol. Med. 2000, 30, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Family cooccurrence of “gender dysphoria”: Ten sibling or parent-child pairs. Arch. Sex. Behav. 2000, 29, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Hare, L.; Bernard, P.; Sanchez, F.J.; Baird, P.N.; Vilain, E.; Kennedy, T.; Harley, V.R. Androgen receptor repeat length polymorphism associated with male-to-female transsexualism. Biol. Psychiatry 2009, 65, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henningsson, S.; Westberg, L.; Nilsson, S.; Lundstrom, B.; Ekselius, L.; Bodlund, O.; Lindstrom, E.; Hellstrand, M.; Rosmond, R.; Eriksson, E.; et al. Sex steroid-related genes and male-to-female transsexualism. Psychoneuroendocrinology 2005, 30, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, R.; Guillamon, A.; Cortes-Cortes, J.; Gomez-Gil, E.; Jacome, A.; Esteva, I.; Almaraz, M.C.; Mora, M.; Aranda, G.; Pasaro, E. Molecular basis of Gender Dysphoria: Androgen and estrogen receptor interaction. Psychoneuroendocrinology 2018, 98, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Sanchez, F.J.; Gaser, C.; Toga, A.W.; Narr, K.L.; Hamilton, L.S.; Vilain, E. Regional gray matter variation in male-to-female transsexualism. Neuroimage 2009, 46, 904–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savic, I.; Arver, S. Sex dimorphism of the brain in male-to-female transsexuals. Cereb. Cortex 2011, 21, 2525–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubiaurre-Elorza, L.; Junque, C.; Gomez-Gil, E.; Segovia, S.; Carrillo, B.; Rametti, G.; Guillamon, A. Cortical thickness in untreated transsexuals. Cereb. Cortex 2013, 23, 2855–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, S.C.; De Cuypere, G.; T’Sjoen, G. Transgender research in the 21st century: A selective critical review from a neurocognitive perspective. Am. J. Psychiatry 2017, 174, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Guillamon, A.; Zubiaurre-Elorza, L.; Junque, C.; Gomez-Gil, E.; Uribe, C.; Khorashad, B.S.; Khazai, B.; Talaei, A.; Habel, U.; et al. The Neuroanatomy of Transgender Identity: Mega-Analytic Findings From the ENIGMA Transgender Persons Working Group. J. Sex. Med. 2021, 18, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Wierckx, K.; Jackson, K.; T’Sjoen, G. Circulating androgens correlate with resting-state MRI in transgender men. Psychoneuroendocrinology 2016, 73, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglund, H.; Lindstrom, P.; Dhejne-Helmy, C.; Savic, I. Male-to-female transsexuals show sex-atypical hypothalamus activation when smelling odorous steroids. Cereb. Cortex 2008, 18, 1900–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.M.; Manzouri, A.H.; Savic, I. Structural connections in the brain in relation to gender identity and sexual orientation. Sci. Rep. 2017, 7, 17954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzouri, A.; Kosidou, K.; Savic, I. Anatomical and Functional Findings in Female-to-Male Transsexuals: Testing a New Hypothesis. Cereb. Cortex 2017, 27, 998–1010. [Google Scholar] [CrossRef] [Green Version]
- Moody, T.D.; Feusner, J.D.; Reggente, N.; Vanhoecke, J.; Holmberg, M.; Manzouri, A.; Sorouri Khorashad, B.; Savic, I. Predicting outcomes of cross-sex hormone therapy in transgender individuals with gender incongruence based on pre-therapy resting-state brain connectivity. Neuroimage Clin. 2021, 29, 102517. [Google Scholar] [CrossRef]
- Savic, I.; Garcia-Falgueras, A.; Swaab, D.F. Sexual differentiation of the human brain in relation to gender identity and sexual orientation. Prog. Brain Res. 2010, 186, 41–62. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Khorashad, B.S.; Feusner, J.D.; Savic, I. Cortical Gyrification in Transgender Individuals. Cereb. Cortex 2021, 31, 3184–3193. [Google Scholar] [CrossRef]
- Zhou, J.N.; Hofman, M.A.; Gooren, L.J.; Swaab, D.F. A sex difference in the human brain and its relation to transsexuality. Nature 1995, 378, 68–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, L.; Kozak, L.R.; Simon, V.; Czobor, P.; Unoka, Z.; Szabo, A.; Csukly, G. Regional grey matter structure differences between transsexuals and healthy controls—A voxel based morphometry study. PLoS ONE 2013, 8, e83947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luders, E.; Sanchez, F.J.; Tosun, D.; Shattuck, D.W.; Gaser, C.; Toga, A.; Vilain, E. Increased Cortical Thickness in Male-to-female Transsexualism. J. Behav. Brain Sci. 2012, 3, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillamon, A.; Junque, C.; Gomez-Gil, E. A Review of the Status of Brain Structure Research in Transsexualism. Arch. Sex. Behav. 2016, 45, 1615–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rametti, G.; Carrillo, B.; Gomez-Gil, E.; Junque, C.; Segovia, S.; Gomez, A.; Guillamon, A. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J. Psychiatr. Res. 2011, 45, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Junque, C.; Gomez-Gil, E.; Abos, A.; Mueller, S.C.; Guillamon, A. Brain network interactions in transgender individuals with gender incongruence. NeuroImage 2020, 211, 116613. [Google Scholar] [CrossRef]
- Kruijver, F.P.; Zhou, J.N.; Pool, C.W.; Hofman, M.A.; Gooren, L.J.; Swaab, D.F. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J. Clin. Endocrinol. Metab. 2000, 85, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Kurth, F. Structural differences between male and female brains. In Sex Differences in Neurology and Psychiatry; Lanzenberger, R., Kranz, G.S., Savic, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 175. [Google Scholar]
- Rosenblatt, J.D. Multivariate revisit to “sex beyond the genitalia”. Proc. Natl. Acad. Sci. USA 2016, 113, E1966–E1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, N.E.; Harenski, K.A.; Harenski, C.L.; Koenigs, M.R.; Decety, J.; Calhoun, V.D.; Kiehl, K.A. Machine learning of brain gray matter differentiates sex in a large forensic sample. Hum. Brain Mapp. 2019, 40, 1496–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekroud, A.M.; Ward, E.J.; Rosenberg, M.D.; Holmes, A.J. Patterns in the human brain mosaic discriminate males from females. Proc. Natl. Acad. Sci. USA 2016, 113, E1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunc, B.; Solmaz, B.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Calkins, M.E.; Ruparel, K.; Gur, R.E.; Gur, R.C.; Verma, R. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurth, F.; Gaser, C.; Luders, E. Development of sex differences in the human brain. Cogn. Neurosci. 2020, 12, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hoekzema, E.; Schagen, S.E.; Kreukels, B.P.; Veltman, D.J.; Cohen-Kettenis, P.T.; Delemarre-van de Waal, H.; Bakker, J. Regional volumes and spatial volumetric distribution of gray matter in the gender dysphoric brain. Psychoneuroendocrinology 2015, 55, 59–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, C.; Forster, K.; Koser, S.A.; Konrad, C.; Zwitserlood, P.; Berger, K.; Hermesdorf, M.; Kircher, T.; Nenadic, I.; Krug, A.; et al. Biological sex classification with structural MRI data shows increased misclassification in transgender women. Neuropsychopharmacology 2020, 45, 1758–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldinger-Melich, P.; Urquijo Castro, M.F.; Seiger, R.; Ruef, A.; Dwyer, D.B.; Kranz, G.S.; Klobl, M.; Kambeitz, J.; Kaufmann, U.; Windischberger, C.; et al. Sex Matters: A Multivariate Pattern Analysis of Sex- and Gender-Related Neuroanatomical Differences in Cis- and Transgender Individuals Using Structural Magnetic Resonance Imaging. Cereb. Cortex 2020, 30, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Clemens, B.; Derntl, B.; Smith, E.; Junger, J.; Neulen, J.; Mingoia, G.; Schneider, F.; Abel, T.; Bzdok, D.; Habel, U. Predictive Pattern Classification Can Distinguish Gender Identity Subtypes from Behavior and Brain Imaging. Cereb. Cortex 2020, 30, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P. Sexual differentiation of brain and other tissues: Five questions for the next 50 years. Horm. Behav. 2020, 120, 104691. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Arnold, A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joel, D. Beyond sex differences and a male-female continuum: Mosaic brains in a multidimensional space. Handb. Clin. Neurol. 2020, 175, 13–24. [Google Scholar] [CrossRef] [PubMed]
- De Vries, G.J.; Rissman, E.F.; Simerly, R.B.; Yang, L.Y.; Scordalakes, E.M.; Auger, C.J.; Swain, A.; Lovell-Badge, R.; Burgoyne, P.S.; Arnold, A.P. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 2002, 22, 9005–9014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, A.P.; Burgoyne, P.S. Are XX and XY brain cells intrinsically different? Trends Endocrinol. Metab. 2004, 15, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009, 30, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carruth, L.L.; Reisert, I.; Arnold, A.P. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002, 5, 933–934. [Google Scholar] [CrossRef] [PubMed]
- Kranz, G.S.; Hahn, A.; Kaufmann, U.; Tik, M.; Ganger, S.; Seiger, R.; Hummer, A.; Windischberger, C.; Kasper, S.; Lanzenberger, R. Effects of testosterone treatment on hypothalamic neuroplasticity in female-to-male transgender individuals. Brain Struct. Funct. 2018, 223, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiger, R.; Hahn, A.; Hummer, A.; Kranz, G.S.; Ganger, S.; Woletz, M.; Kraus, C.; Sladky, R.; Kautzky, A.; Kasper, S.; et al. Subcortical gray matter changes in transgender subjects after long-term cross-sex hormone administration. Psychoneuroendocrinology 2016, 74, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Landre, L.; Wierckx, K.; T’Sjoen, G. A Structural Magnetic Resonance Imaging Study in Transgender Persons on Cross-Sex Hormone Therapy. Neuroendocrinology 2017, 105, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.M.; Manzouri, A.H.; Dhejne, C.; Bergström, K.; Arver, S.; Feusner, J.D.; Savic-Berglund, I. Testosterone Effects on the Brain in Transgender Men. Cereb. Cortex 2018, 28, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Zubiaurre-Elorza, L.; Cerdan, S.; Uribe, C.; Perez-Laso, C.; Marcos, A.; Rodriguez Del Cerro, M.C.; Fernandez, R.; Pasaro, E.; Guillamon, A. The Effects of Testosterone on the Brain of Transgender Men. Androg. Clin. Res. Ther. 2021, 2, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Hulshoff Pol, H.E.; Cohen-Kettenis, P.T.; Van Haren, N.E.M.; Peper, J.S.; Brans, R.G.H.; Cahn, W.; Schnack, H.G.; Gooren, L.J.G.; Kahn, R.S. Changing your sex changes your brain: Influences of testosterone and estrogen on adult human brain structure. Eur. J. Endocrinol. 2006, 155 (Suppl. S1), S107–S114. [Google Scholar]
- Smith, E.S.; Junger, J.; Derntl, B.; Habel, U. The transsexual brain--A review of findings on the neural basis of transsexualism. Neurosci. Biobehav. Rev. 2015, 59, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.K.; Jain, M.; Natarajan, S.; Frasier, S.D.; Vilain, E. Familial mutation in the testis-determining gene SRY shared by an XY female and her normal father. J Clin. Endocrinol. Metab. 2002, 87, 3428–3432. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Gingnell, M.; Poromaa, I.S.; Engman, J.; Kurth, F.; Gaser, C. Potential Brain Age Reversal after Pregnancy: Younger Brains at 4–6Weeks Postpartum. Neuroscience 2018, 386, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Cherbuin, N.; Gaser, C. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. Neuroimage 2016, 134, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Ziegler, G.; Kloppel, S.; Gaser, C. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: Exploring the influence of various parameters. Neuroimage 2010, 50, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Hagemann, G.; Schleussner, E.; Gaser, C. Changes of individual BrainAGE during the course of the menstrual cycle. Neuroimage 2015, 115, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tipping, M.E. Sparse bayesian learning and the relevance vector machine. J. Mach. Learn. Res. 2001, 1, 211–244. [Google Scholar]
- Tipping, M.E. The Relevance Vector Machine. In Advances in Neural Information Processing Systems 12; Solla, S.A., Leen, T.K., Müller, K.-R., Eds.; MIT Press: Cambridge, MA, USA, 2000; pp. 652–658. [Google Scholar]
- Garcia-Falgueras, A.; Swaab, D.F. A sex difference in the hypothalamic uncinate nucleus: Relationship to gender identity. Brain 2008, 131 Pt 12, 3132–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Han, L.; Xi, C.; Xu, Y.; Lai, J.; Lu, S.; Huang, M.; Hu, J.; Wei, N.; Xu, W.; et al. Interactive effects of gender and sexual orientation on cortical thickness, surface area and gray matter volume: A structural brain MRI study. Quant. Imaging Med. Surg. 2020, 10, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Calzo, J.P.; Blashill, A.J. Child Sexual Orientation and Gender Identity in the Adolescent Brain Cognitive Development Cohort Study. JAMA Pediatr. 2018, 172, 1090–1092. [Google Scholar] [CrossRef] [Green Version]
- Bao, A.M.; Swaab, D.F. Sexual differentiation of the human brain: Relation to gender identity, sexual orientation and neuropsychiatric disorders. Front. Neuroendocrinol. 2011, 32, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.; Lindstrom, P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc. Natl. Acad. Sci. USA 2008, 105, 9403–9408. [Google Scholar] [CrossRef] [Green Version]
- Swaab, D.F.; Garcia-Falgueras, A. Sexual differentiation of the human brain in relation to gender identity and sexual orientation. Funct. Neurol. 2009, 24, 17–28. [Google Scholar] [PubMed]
- Skorska, M.N.; Chavez, S.; Devenyi, G.A.; Patel, R.; Thurston, L.T.; Lai, M.C.; Zucker, K.J.; Chakravarty, M.M.; Lobaugh, N.J.; VanderLaan, D.P. A Multi-Modal MRI Analysis of Cortical Structure in Relation to Gender Dysphoria, Sexual Orientation, and Age in Adolescents. J. Clin. Med. 2021, 10, 345. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurth, F.; Gaser, C.; Sánchez, F.J.; Luders, E. Brain Sex in Transgender Women Is Shifted towards Gender Identity. J. Clin. Med. 2022, 11, 1582. https://doi.org/10.3390/jcm11061582
Kurth F, Gaser C, Sánchez FJ, Luders E. Brain Sex in Transgender Women Is Shifted towards Gender Identity. Journal of Clinical Medicine. 2022; 11(6):1582. https://doi.org/10.3390/jcm11061582
Chicago/Turabian StyleKurth, Florian, Christian Gaser, Francisco J. Sánchez, and Eileen Luders. 2022. "Brain Sex in Transgender Women Is Shifted towards Gender Identity" Journal of Clinical Medicine 11, no. 6: 1582. https://doi.org/10.3390/jcm11061582
APA StyleKurth, F., Gaser, C., Sánchez, F. J., & Luders, E. (2022). Brain Sex in Transgender Women Is Shifted towards Gender Identity. Journal of Clinical Medicine, 11(6), 1582. https://doi.org/10.3390/jcm11061582





