2. Methods
2.1. Patients and Setting
The study was conducted at Maccabi Healthcare Services, the second-largest publicly funded health maintenance organization (HMO) in Israel, and the Endocrine Institute of Rabin Medical Center, a large tertiary hospital in Israel. It was approved by the Ethics Review Boards of both facilities.
All patients referred to the Endocrine Institute of Rabin Medical Center and diagnosed with CD between 2000 and 2020 were included. The recruitment of the patients treated for CD at Maccabi Healthcare Services has been previously described [
10]. The computerized database of the HMO was screened for all individuals who fulfilled the following criteria:
The recorded biochemical data were compatible with a CD diagnosis as stipulated in the Endocrine Society Clinical Guidelines [
11].
A diagnosis of overt CD was established by an expert endocrinologist at the time of presentation.
The diagnosis was retrospectively ascertained by the study authors at the time of data collection based on the documented biochemical and imaging tests, as well as management and follow-up details.
Histologic documentation of an ACTH-secreting tumor: in cases in which the histological reports were unavailable or inconclusive, biochemical and clinical resolution of the hypercortisolism after surgical resection was used as diagnostic confirmation.
The results of second-line biochemical tests performed for the differential diagnosis of ACTH-dependent Cushing syndrome were retrieved and analyzed [
7,
8]. For each patient, the year in which the first elevated 24 h urinary free cortisol (UFC) result was recorded (2000–2017) was considered the time of diagnosis.
Postsurgical remission was defined as a low (<138 nmol/L) or undetectable serum cortisol level 18–24 h after the last dose of oral cortisone acetate or hydrocortisone during the first week of surgery; clinical adrenal insufficiency; the need for glucocorticoid replacement; disappearance of clinical features of hypercortisolism over the course of the follow–up; and/or normal UFC levels throughout the first year following the pituitary surgery. Recurrence was defined as the reappearance of clinical symptoms and signs of hypercortisolism, associated with abnormal screening tests for hypercortisolemia.
Measurements of all adenoma diameters were obtained by radiological examination of the pituitary, and tumors in which the largest diameter was at least 10 mm were considered to be macroadenomas. When no visible tumor was seen on magnetic resonance imaging (MRI) following a neuroradiologist review, the tumor was a microadenoma.
Twenty-four-hour UFC was measured using a commercial radioimmunoassay (DiaSorin, Saluggia, Italy, or Siemens Healthcare Diagnostics).
The proliferative potential of corticotroph adenomas was investigated by the presence of Ki-67, a cell cycle antigen and marker of cell proliferation and aggression in various neoplasms [
12]. The Ki-67 labeling index was expressed as a percentage of Ki-67-positive tumor nuclei per total tumor nuclei counted/specimen.
2.2. Study Procedure
The following data were collected by careful review of the patient files: demographic and clinical characteristics at diagnosis of CS, including age, sex, and body mass index; diagnosis of hypertension, diabetes mellitus or impaired fasting glucose, and osteoporosis; reason(s) for referral for Cushing syndrome screening tests; performance (yes/no) and results of diagnostic tests for Cushing syndrome, including UFC, morning cortisol level after overnight 1 mg dexamethasone, late-night salivary cortisol level, and dehydroepiandrosterone sulfate (DHEAS). Data regarding the clinical course after CD diagnosis were also retrieved: performance (yes/no) and time of surgery, medical therapy for hypercortisolism (yes/no), evidence of persistent/recurrent disease, time of disease recurrence, post-surgery glucocorticoid replacement administration (yes/no), and duration.
The Institutional Review Board of Rabin Medical Center and Maccabi Health Services approved the study.
2.3. Systematic Review
We performed a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement of studies [
13]. The literature was searched from inception up to 31 December 2021 for relevant peer-reviewed articles written in English, using Medline Ovid, Medline (PubMed), Web of Science, and Google Scholar databases. The terms included in the search string were combined with Boolean operators as follows: ((“Cushing” OR “Corticotroph”) AND (“Pituitary”) AND (“Macroadenoma” OR “Size” OR “Diameter”)). We supplemented the electronic search by cross-referencing included papers, relevant sections of clinical practice guidelines, relevant systematic and narrative reviews. Two authors (A.A. and I.D.) conducted the search, and articles were first assessed by title, second by abstract, and third by full text. Another author (M.F.) reviewed all studies included. Original studies in adults ≥ 18 years of age with observational design (cross-sectional, case-control, and cohort) reporting data on clinical and biochemical characteristics of pituitary microadenomas vs. macroadenomas were included. Studies in children and adolescents, non-English articles, preprint articles, case reports, articles without pertinent data, non-research articles, and articles without full-text availability were excluded (
Figure 1). The trial characteristics extracted included sample size and number of patients with macroadenoma and biochemical differences between patients with microadenoma and those with macroadenoma, including 24 h UFC, low dose and high dose DST, salivary cortisol, and ACTH.
2.4. Statistical Analysis
The statistical analysis was generated using SAS Software, Version 9.4 (SAS, Cary, NC, USA).
Continuous variables were presented by mean ± SD or median and IQR, and categorical variables were presented by (N,%); t-test was used to compare the value of continuous normal variables between study groups and Wilcoxon for skewed continuous variables, and Fisher’s exact test was used to compare the value of categorical variables between study groups. Two-sided p values less than 0.05 were considered statistically significant. Kruskal–Wallis test was used for non-normal continuous variables.
3. Results
3.1. Study Cohort
We identified 116 patients with CD. Following the exclusion of 11 patients with no data on pituitary MRI findings, the final study cohort consisted of 105 patients (82 women, 78%; mean age ± SD, 41.5 ± 14.5 years): 68 patients from the Maccabi Healthcare services clinics and 37 patients from the Endocrine Institute of Rabin Medical Center. Mean follow-up for the whole cohort was 5.7 ± 4.4 years.
Pituitary MRI revealed a microadenoma in 68 patients (64.8%) and a macroadenoma in 25 patients (23.8%). In 12 patients, no adenoma was visible on pituitary MRI (11.4%) and further investigation, including inferior petrosal sinus sampling (IPSS), confirmed a pituitary source for excess ACTH, thus were classified as having possible microadenomas. Of note, bilateral IPSS was completed for 18 patients, including those without a visible adenoma and patients with a small adenoma (<6 mm), and confirmed the diagnosis of pituitary source for hypercortisolism in all cases.
Baseline characteristics, as shown in
Table 1, were similar between patients with ACTH-secreting microadenomas and macroadenomas, including age (mean age, 42.3 ± 14.9 vs. 39.0 ± 13.2 years, respectively), gender (women: 77.5% vs. 80.0%, respectively), body mass index (mean, 30.8 ± 7.5 vs. 29.4 ± 4.9 kg/m
2, respectively), and prevalence of hypertension, osteoporosis/osteopenia, or menstrual irregularities. No significant difference was seen regarding disorders of glucose metabolism, but impaired fasting glucose or diabetes mellitus were slightly more common among those with microadenomas (
Table 2).
Among both groups, the most common reason for completing an investigation for possible CS was weight gain: 37 patients (46.3%) with microadenomas and 12 patients (48.0%) with macroadenomas. Suspected Cushingoid features were the reason for investigation in more than a quarter of patients with microadenomas (27.5%) and in one of five patients with macroadenomas (20.0%). Virilization and/or oligomenorrhea were reported as the cause for investigation for nine patients (40.0%) with macroadenomas, compared with 15 patients (23.8%) with microadenomas.
3.2. Biochemical Data
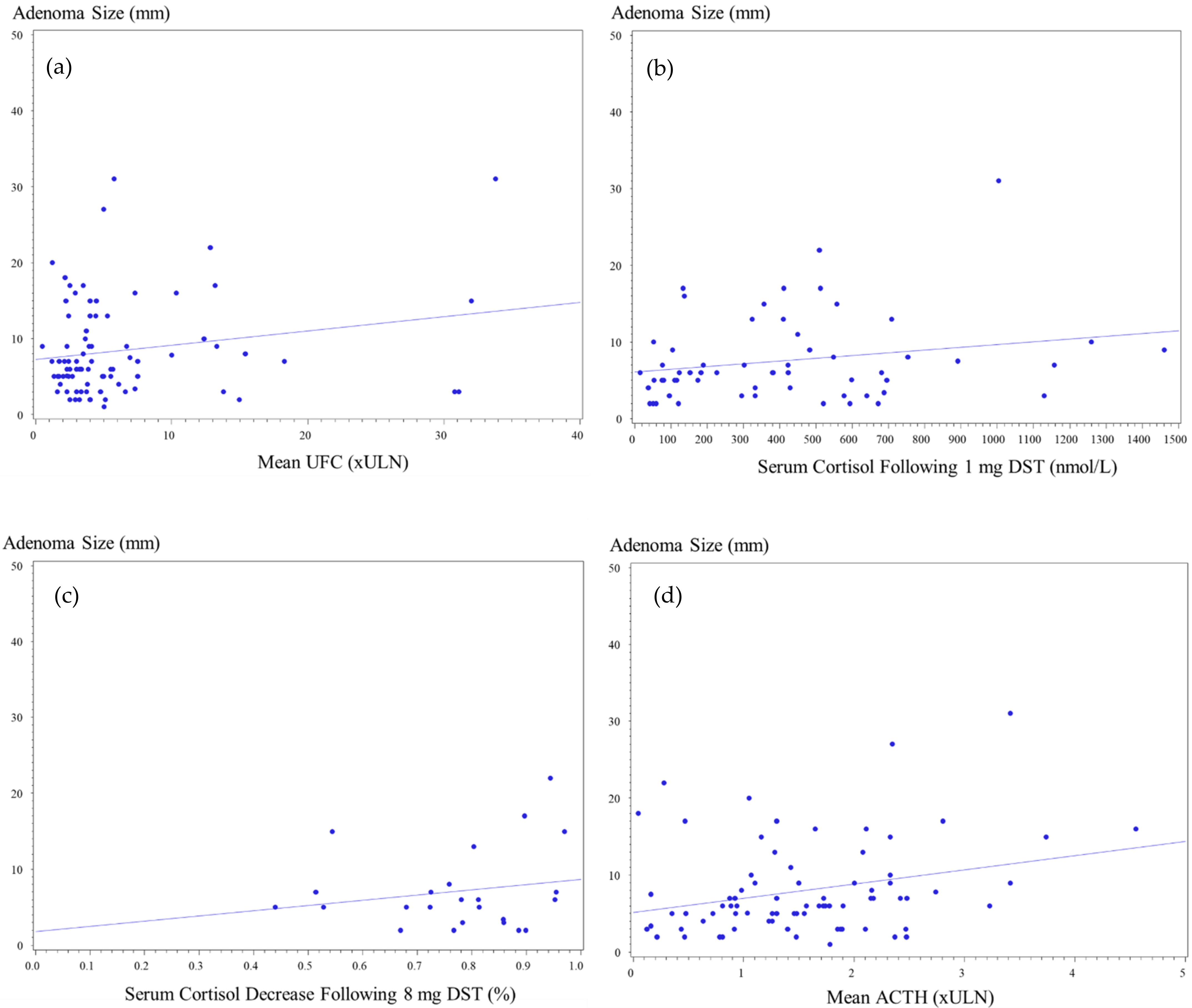
Data on UFC were available for 101 patients (96.2%), 78 (97.5%) patients with pituitary microadenoma and 23 (92.0%) with macroadenomas. Patients with microadenomas had UFC mean of 5.2 ± 5.4 × ULN (range, 0.5–31.1 × ULN), while in patients with macroadenoma, mean was 7.8 ± 8.7 × ULN (range, 1.2–33.8 × ULN) (
p = 0.13). UFC levels > 4 × ULN were recorded in 31 patients (39.7%) with microadenoma and in 12 patients (52.2%) with macroadenoma (
p = NS). Mean UFC levels < 2 × ULN were reported in one patient with a macroadenoma (4.0%), compared with 11 patients (14.1%) with microadenomas. Analysis of the maximal UFC levels indicated higher values for patients with macroadenomas (mean, 8.6 ± 8.7 × ULN; range, 1.2–34.7 × ULN) than microadenomas (6.2 ± 5.5 × ULN; range, 0.8–32.2 × ULN), but the difference was not statistically significant (
p = NS) (
Figure 2).
Data on 1 mg overnight DST results were available for 69 patients: 55 (74.3%) patients with microadenomas and 14 (56.0%) with macroadenomas, with abnormal serum cortisol levels following low-dose dexamethasone reported for 94.2% (65 of 69 patients) of the whole cohort. Mean serum cortisol levels following low-dose DST were higher among patients with macroadenomas than microadenomas (487.6 ± 329.8 vs. 372.0 ± 324.5 nmol/L, respectively;
p = 0.19), but there was an overlap between ACTH-secreting microadenomas (range, 14–1461 nmol/L) and macroadenomas (range, 53–1260 nmol/L). Normal results, defined as serum cortisol < 50 nmol/L following 1 mg dexamethasone, were recorded for four patients with microadenomas but for none of those with macroadenomas (7.3% vs. 0%, respectively). Mildly elevated serum cortisol levels following DST, defined as serum cortisol between 50 and 138 nmol/L, were reported in 14 patients with a microadenoma, compared with three patients with a macroadenoma (25.4% vs. 21.4%, respectively). Serum cortisol levels > 138 nmol/L were reported in most patients with microadenomas or macroadenomas (67.3% vs. 78.6%, respectively) (
Figure 2).
Late-night salivary cortisol results were available only for 14 patients, with elevated levels reported in all three patients (100%) with macroadenoma and in 9 out of 11 patients with microadenomas (81.8%).
Serum ACTH levels were available for 93 patients: 74 (92.5%) with micro and for 19 (76.0%) with macroadenomas. Mean ACTH was 1.3 ± 0.8 × ULN for microadenomas (range, 0.1–3.4 × ULN), compared with a mean of 1.9 ± 1.2 × ULN among those with macroadenomas (range, 0.3–4.6 × ULN) (
p = 0.03). Sixteen out of nineteen (84%) patients with macroadenomas and available data on ACTH concentrations had serum ACTH levels above the normal range, compared with 46 out of 74 (62%) patients with microadenomas. ACTH levels > 2 × ULN were recorded more frequently in patients with macroadenomas than microadenomas (53.0% vs. 20.0%, respectively) (
Figure 2).
Overnight 8 mg DST results were available for 29 patients, including 24 patients with microadenomas and 5 patients with macroadenomas. The degree of cortisol suppressibility was similar for macroadenomas and microadenomas (83.1 ± 17.3% vs. 73.7 ± 20.2%, respectively). All five patients (100%) with macroadenoma had >50% reduction in plasma cortisol, as did 22 out of 24 (81.5%) patients with microadenoma (
Figure 2).
Data on plasma DHEAs concentrations were available for 50 patients: 44 patients with microadenomas and 6 patients with macroadenomas; mean DHEAs levels were similar for both groups (mean, 8.95 ± 19.4 ng/mL vs. 8.94 ± 6.3 ng/mL, respectively).
3.3. Macroadenoma Extension and Biochemical Profile
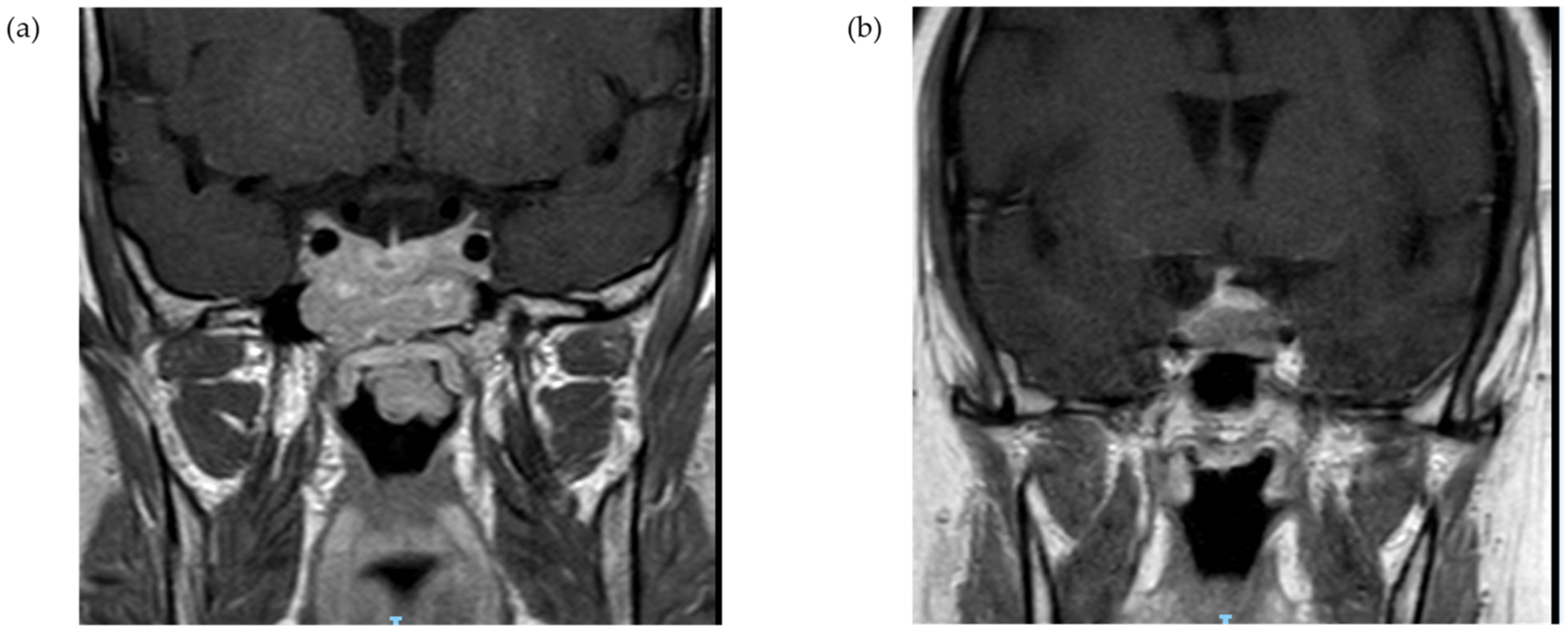
Among patients with corticotroph macroadenoma, data on the radiographic characteristics were available for 23 out of 25 tumors. Suprasellar extension was reported for 15 patients (65.2%) with optic chiasm compression in six cases (26.1%). Cavernous sinus invasion was evident in 10 cases (43.5%), and sphenoid sinus invasion was documented for five patients (21.7%) (illustrative MR imaging,
Figure 3). From a clinical standpoint, six patients (26.1%) reported headaches, and five patients (20.0%) had visual complaints, including two patients with bitemporal hemianopsia (
Table 3). Median UFC values were highest for tumors with sphenoid sinus invasion (median, 5.8 × ULN; IQR, 3.1–23.5 × ULN) or cavernous sinus invasion (median, 5.8 × ULN; IQR, 3.2–11.6 × ULN), followed by tumors with suprasellar extension with no cavernous sinus invasion (median, 4.4 × ULN; IQR, 2.3–11.0 × ULN) or intrasellar macroadenomas (median, 4.6 × ULN; IQR, 3.6–8.8 × ULN) (
p = 0.67). Urinary free cortisol levels >4 × ULN were reported for four out of five patients (80%) with sphenoid sinus invasion and for six out of ten patients (60%) with cavernous sinus invasion, compared with four out of eight (50%) patients with suprasellar extension with no cavernous sinus invasion and two out of four patients (50%) with intrasellar tumors. Similarly, serum cortisol levels following low-dose DST were higher among patients with tumors invading the sphenoid sinus (reported in two patients: 513 and 1004 nmol/L) or the cavernous sinus (reported in three patients: 410, 509, and 1004 nmol/L), compared with intrasellar tumors (median, 324 nmol/L; IQR, 93–984 nmol/L) (
p = 0.57). Furthermore, median ACTH concentrations were highest for patients with sphenoid sinus invasion (median, 2.0 × ULN; IQR, 1.2–3.1 × ULN), or cavernous sinus invasion (median, 2.0 × ULN; IQR, 1.0–2.3 × ULN), followed by the suprasellar extension (median, 1.7 × ULN; IQR, 0.9–2.3 × ULN), and lowest for those with intrasellar macroadenomas (median, 0.5 × ULN; IQR, 0.3–1.9 × ULN) (
p = 0.32).
3.4. Surgical Treatment Outcomes
Most patients in both groups underwent transsphenoidal surgery, including 71 out of 74 (96.0%) with microadenomas and 23 out of 25 (92.0%) with macroadenomas. Data on the Ki-67 proliferation index were available for 15 patients with microadenomas and for 9 with macroadenoma; mean of 3.7 ± 3.1% vs. 5.7 ± 4.8%, respectively. Ki-67 labeling index ≥5% was reported for six out of nine macroadenomas (66%), compared with six out of fifteen microadenomas (40%).
Postoperatively, rates of persistent or recurrent disease following surgery were 30.4% (7 out of 23 patients) among patients with macroadenomas (mean follow-up period of 7.3 ± 4.6 years), compared with rates of 35.2% (25 out of 71 patients) among those with microadenomas (mean follow-up period of 5.1 ± 4.1 years) (p = NS). The persistent disease was reported for three patients (12.0%) with a macroadenoma, compared with 19 patients (26.8%) with a microadenoma. Recurrent disease was reported for five (21.7%) and six (8.5%) patients, respectively (p = NS). Four out of twelve patients (33%) with no visible adenoma on MRI had persistent disease following surgery. The mean time to recurrence after surgery was similar for microadenomas and macroadenomas (43.0 ± 34.6 months vs. 45.3 ± 25.2 months, respectively).
Data on glucocorticoid replacement following surgery were available for 18 patients with macroadenomas, of whom 15 (83.3%) required treatment, as did 52 out of 62 (83.9%) patients with microadenomas. The mean duration of glucocorticoid replacement following surgery was similar for macroadenomas and microadenomas (9.7 ± 11.9 months vs. 9.4 ± 8.3 months, respectively).
3.5. Systematic Review
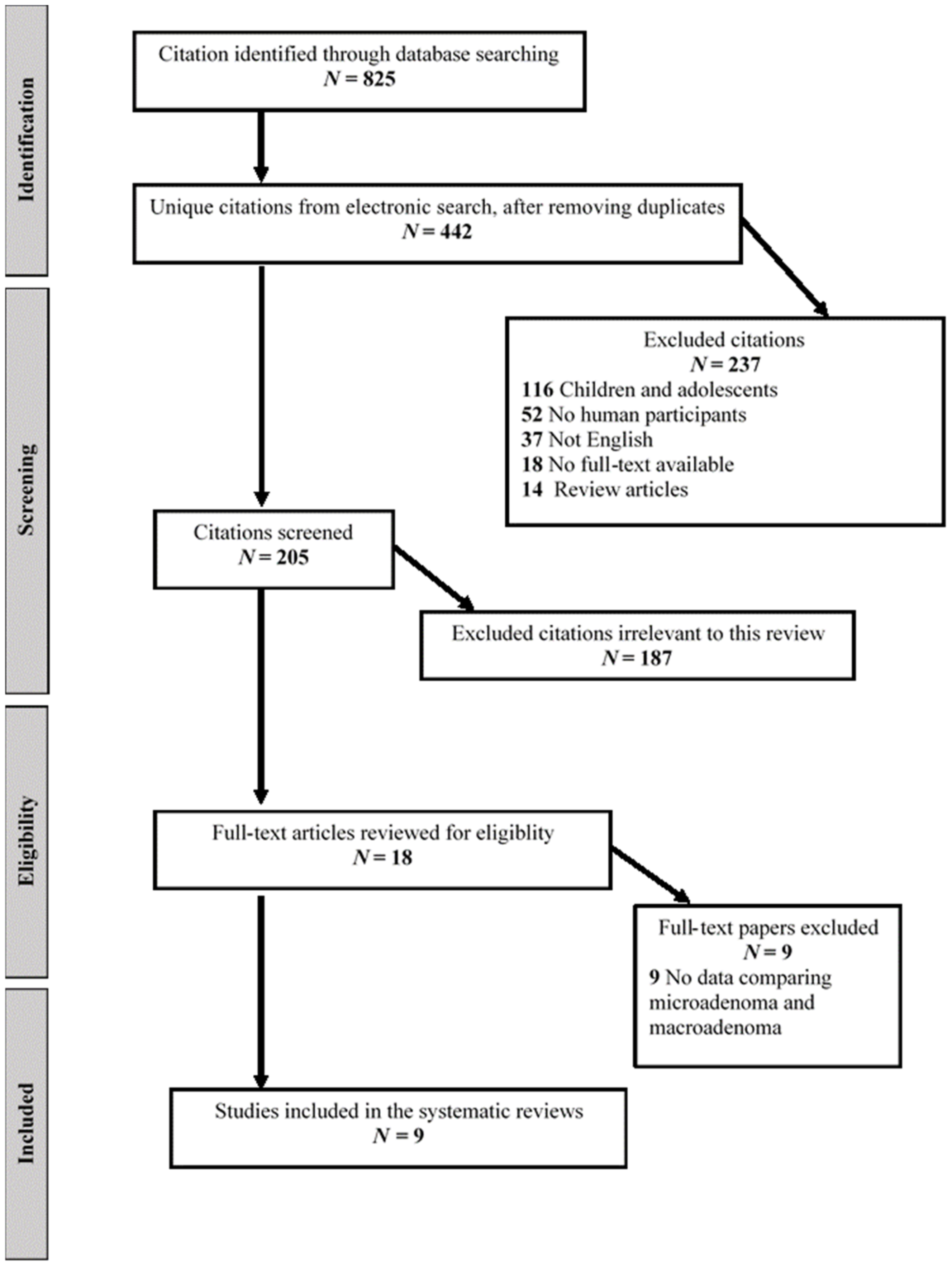
As detailed in the flow diagram (
Figure 1), we retrieved a total of 825 citations from our electronic searches, ultimately yielding 442 unique citations after removing any duplicates. References from the hand search were all included in the electronic database searches. We reviewed 18 full-text papers for eligibility, and nine trials published between 1998 and 2021 met the inclusion criteria and were included in the systematic review [
6,
7,
8,
14,
16,
17,
18,
19,
20]. We excluded trials with no available data on the biochemical profile of corticotroph microadenoma vs. macroadenoma. A summary of trials included in the systematic review is shown in
Table 3. Of the included studies, four were conducted in Europe [
6,
8,
14,
19], two in the United States [
7,
17], two in Asia [
9,
20], and one study in Brazil [
18]. The number of participants ranged from 7 to 74 patients with corticotroph macroadenoma [
6,
7,
8,
14,
16,
17,
18,
19,
20].
Data on UFC were not available in three studies [
6,
14,
19], but five studies reporting on UFC showed no difference in levels between microadenoma and macroadenoma [
7,
8,
16,
17,
19], and only Machado et al. reported on significant difference in UFC, with higher levels reported for patients with microadenoma [
18]. Four studies provided data on cortisol levels following 1 mg dexamethasone with no difference in all studies between microadenoma and macroadenoma [
6,
17,
18,
19]. Data on serum cortisol levels following high dose DST were available from seven studies, three reporting higher serum cortisol among patients with macroadenoma [
6,
7,
8] and four suggesting no difference between patients with microadenomas and macroadenomas [
9,
17,
18,
19]. All studies provided data on ACTH levels, with seven studies showing higher levels for macroadenomas [
6,
7,
8,
14,
18,
19,
20], but two studies reported no difference in ACTH levels between the groups [
9,
17] (
Table 3).
4. Discussion
We show here in the first systematic review and confirmed by our study that patients with Cushing’s disease secondary to ACTH-secreting microadenomas and macroadenomas present with similar clinical and biochemical characteristics, except for higher plasma ACTH concentrations among those with corticotroph macroadenomas. This suggests there is a lack of association between tumor size per se with cortisol secretion values or clinical characteristics. Tumor location or extension may be more important than merely tumor size, as tumors invading the sphenoid or cavernous sinus were associated with both higher plasma ACTH and cortisol secretion.
More than two decades ago, Katznelson et al. [
7] and Selvais et al. [
8] compared corticotroph macro with microadenomas and suggested that macroadenomas were less suppressible after 1 mg dexamethasone administration and after 48 h high dose dexamethasone suppression test and that decrease in UFC post dexamethasone was lower among patients with macroadenoma. Furthermore, patients with macroadenoma were more likely to have baseline plasma ACTH concentrations above the normal range, and ACTH values were higher in patients with macro compared with those with microadenomas [
6]. On the other hand, a more recent study showed no significant differences in plasma ACTH, serum cortisol, or UFC between patients with ACTH-secreting microadenomas and macroadenomas [
9] (
Table 3).
Differences in ACTH assays over time could also influence these results [
16,
17,
19,
20].
In our study, patients with corticotroph microadenomas and macroadenomas shared similar clinical features, with no difference in rates of hypertension, osteoporosis, impaired fasting glucose, or diabetes mellitus at presentation. Interestingly, there was no significant age or gender difference between groups, with a female predominance in both groups. Furthermore, weight gain and Cushingoid features were the most common reasons for completing an investigation for Cushing’s syndrome in both groups. Prior studies to assess clinical differences between microadenomas and macroadenomas patients showed inconsistent data [
9], but in most series, rates of hypertension, diabetes, osteoporosis, and BMI were similar in both groups [
8,
15,
16,
17,
18].
Previous studies [
7,
8] suggested that patients with macroadenomas were more likely to have baseline plasma ACTH concentrations above the normal range (83% vs. 45%). Woo et al. [
6] similarly showed plasma ACTH values were higher for 18 patients with corticotroph macro vs. 183 with microadenomas. In line with these previous reports and others [
6,
7,
8,
14,
16,
17] but in contrast with other series [
9,
15], our study shows higher plasma ACTH among patients with corticotroph macroadenoma, compared with microadenomas, and likewise, more patients with baseline plasma ACTH values above the normal range in macroadenoma patients, compared with microadenoma ones (84% vs. 62%). The correlation between adenoma size and the severity of hormonal hypersecretion is well-established for prolactin-secreting tumors, and our findings support a linear correlation between ACTH and maximum adenoma diameter. It has been postulated for other pituitary adenomas that a correlation between hormonal hypersecretion and tumor size is due to the fact that the vast majority of cases are secondary to a monoclonal adenoma, composed of an identical histological type [
21].
Unlike the association between tumor size with plasma ACTH concentrations, no association was seen with UFC, low-dose DST, or high-dose DST. It should be noted that while serum cortisol following low- or high-dose DST was not significantly different between groups, all false-negative results of low-dose DST were documented among patients with microadenoma, while all patients with macroadenomas had abnormal results. Furthermore, more than 80% of patients with macroadenomas had significantly increased serum cortisol levels following DST (>138 nmol/L), while two-thirds of patients with microadenomas had only mildly elevated serum cortisol levels (50–138 nmol/L). These findings suggest that low-dose DST may perform better in corticotroph macroadenomas than in microadenomas, and a positive low DST should be followed by additional confirmatory testing. However, contrary to prior reports showing reduced suppressibility during high dose DST in macroadenomas, in our study, all patients with macroadenoma who underwent high dose DST had >50% reduction in plasma cortisol. High-dose DST has limited value in diagnosis overall [
1,
22], but our data do not support the theory that corticotroph macroadenomas are less suppressible after 8 mg dexamethasone administration [
6,
7,
8,
14]. However, the limited number of patients with a macroadenoma in our study precludes any definitive conclusion.
Surprisingly, our data show that patients with corticotroph macroadenomas exhibit lower cortisol secretion for the degree of ACTH elevation. While it is possible that the lack of difference is secondary to the limited sample size, there are several other potential explanations. First, large pituitary adenomas may be less differentiated and less efficient at the processing of proopiomelanocortin to ACTH, resulting in the production of biologically less potent ACTH precursors and fragments that may cross-react in the ACTH assay [
23]. Undoubtedly, a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for biologically active intact ACTH can provide a more precise estimation of ACTH values [
24]. Second, while mean ACTH concentrations were significantly higher among patients with macroadenomas, there was a significant overlap of ACTH values between the groups, suggesting that large tumors may produce a similar amount of ACTH as small tumors; thus, these findings may be partly due to low level of hormone production per tumor mass or due to disturbances in the regulated exocytotic pathway [
25]. Third, variations in ACTH pulsatility and action on the adrenal gland may affect cortisol secretion [
15].
Interestingly, we found for the first time that tumors with sphenoid or cavernous sinus invasion were associated with higher levels of ACTH, UFC, and serum cortisol following low-dose DST, compared to patients with suprasellar or intrasellar macroadenomas, suggesting that tumor location may have a more significant impact on the biochemical profile of patients with CD. However, the limited sample size prohibited statistical analysis.
Surgical remission rates were similar in patients with microadenomas and macroadenomas, similar to other series [
9,
26], ranging between 65% and 70%, also in line with previous reports [
27]. These findings suggest that surgical intervention success is not dependent merely on tumor size and that tumor invasion and surgical expertise are more important factors in achieving remission.
While there was a trend for a higher Ki-67 labeling index in corticotroph macroadenomas (5.7 ± 4.8% vs. 3.7 ± 3.1%, respectively), the difference was not statistically significant, although Ki-67 labeling index ≥5% was more common among macroadenoma patients. As suggested by Losa and colleagues, it is possible that the difference in the proliferation activity is the main factor underlying the different patterns of growth among corticotroph microadenoma and macroadenomas [
14]. Despite last WHO recommendations [
28] suggesting no need to measure Ki-67 for pituitary adenomas, it might be useful to continue it for assessing corticotroph macroadenomas prognosis.
While previous studies have investigated the clinical and biochemical differences between microadenomas and macroadenomas in patients with Cushing’s disease, the data are limited and inconsistent. Our systematic review identified nine previous studies on this topic; six of these did not provide any data on UFC levels, three of these reports were published more than 20 years ago, and most studies included less than 20 patients with macroadenoma. Our study provides a more up-to-date assessment and takes into account advances in imaging technologies and laboratory testing. Moreover, our cohort included a relatively large number of patients with macroadenoma, with data on UFC levels for almost all patients.
Our study had several important limitations, including the retrospective design, missing or unavailable biochemical data as only 68 patients had no missing values in the main hormonal variables (ACTH, serum cortisol after 1 mg DST, and UFC), fewer patients with macroadenoma, as well as low number of patients who underwent cortisol salivary testing. However, we have more patients with macroadenomas compared with previous reports, which may be explained by our stricter exclusion criteria (e.g., unspecified hypercortisolism, more likely to harbor microadenomas). A potential referral bias, as those who have larger tumors are more likely to be referred to our specialized center, could also play a role; however, the other studies were also done in pituitary centers. In addition, a meta-analysis was not performed due to the heterogeneity of the studies included in terms of patients recruited, imaging modalities, laboratory techniques, and outcomes reported.
Our study suggests classifying corticotroph tumors solely according to the tumor size may not be as clinically important as is tumor invasion; the latter is impacting the potential for complete tumor removal and biochemical remission. Furthermore, a low threshold for CS screening in patients with all macroadenomas is also very important as patients with larger adenomas can sometimes present with mild hypercortisolism.