Clinical Outcomes of an Innovative Cefazolin Delivery Program for MSSA Infections in OPAT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Population
2.3. Data Collection and Definitions
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. MSSA Treatment
3.3. Clinical Outcomes
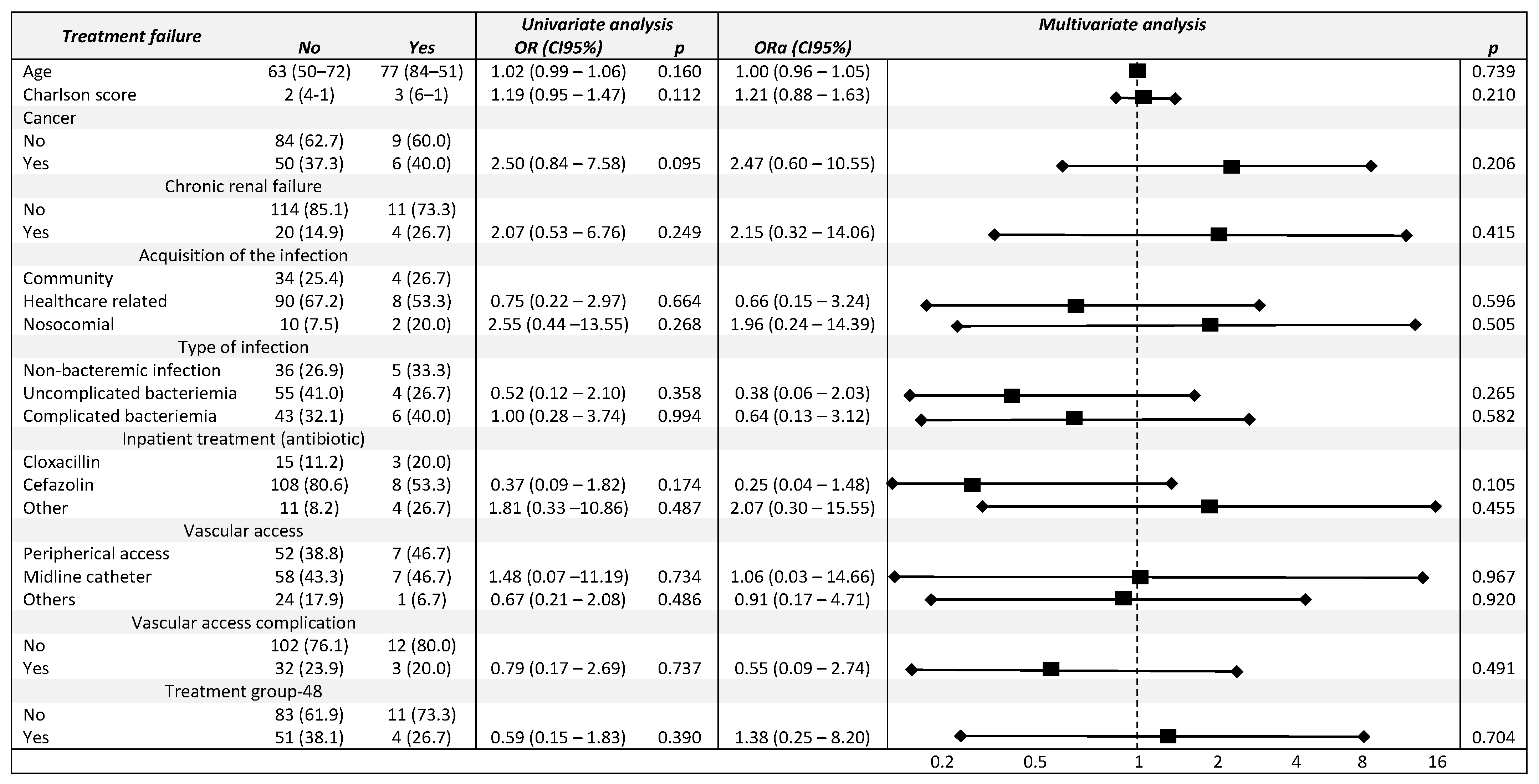
3.4. Univariate and Multivariable Analysis Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clinical Microbiology Reviews. Am. Soc. Microbiol. 2015, 28, 603–661. [Google Scholar]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDanel, J.S.; Roghmann, M.C.; Perencevich, E.N.; Ohl, M.E.; Goto, M.; Livorsi, D.J.; Jones, M.; Albertson, J.P.; Nair, R.; O’Shea, A.M.J.; et al. Comparative Effectiveness of Cefazolin Versus Nafcillin or Oxacillin for Treatment of Methicillin-Susceptible Staphylococcus aureus Infections Complicated by Bacteremia: A Nationwide Cohort Study. Clin. Infect. Dis. 2017, 65, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Patel, N.J.; O’Donnell, N. Optimal treatment of mssa bacteraemias: A meta-analysis of cefazolin versus antistaphylococcal penicillins. J. Antimicrob. Chemother. 2018, 73, 2643–2651. [Google Scholar] [CrossRef]
- Sriskandarajah, S.; Hobbs, J.; Roughead, E.; Ryan, M.; Reynolds, K. Safety and effectiveness of ‘hospital in the home’ and ‘outpatient parenteral antimicrobial therapy’ in different age groups: A systematic review of observational studies. Int. J. Clin. Pract. 2018, 8, e13216. [Google Scholar] [CrossRef]
- Psaltikidis, E.M.; Silva, E.N.D.; Bustorff-Silva, J.M.; Moretti, M.L.; Resende, M.R. Economic evaluation of outpatient parenteral antimicrobial therapy: A systematic review. Expert Rev. Pharm. Outcomes Res. 2017, 17, 355–375. [Google Scholar] [CrossRef]
- Candel, F.J.; Julián-Jiménez, A.; González, J.; Castillo-Del Javier, F.; González, C. Current status in outpatient parenteral antimicrobial therapy: A practical view. Rev. Esp. Quim. 2016, 29, 55–68. [Google Scholar]
- Norris, A.H.; Shrestha, N.K.; Allison, G.M.; Keller, S.C.; Bhavan, K.P.; Zurlo, J.J.; Hersh, A.L.; Gorski, L.A.; Bosso, J.A.; Rathore, M.H.; et al. 2018 IDSA Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapy. Clin. Infect. Dis. 2018, 68, e1–e35. [Google Scholar] [CrossRef]
- Shah, A.B.; Norris, A.H. Handbook of Outpatient Parenteral Antimicrobial Therapy for Infectious Diseases, 3rd ed.; Shah, A.B., Norris, A.H., Eds.; CRG Publishing: Arlington, VI, USA, 2016. [Google Scholar]
- López Cortés, L.E.; Mujal Martínez, A.; Fernández Martínez de Mandojana, M.; Martín, N.; Gil Bermejo, M.; Solà Aznar, J.; Villegas Bruguera, E.; Peláez Cantero, M.J.; Retamar Gentil, P.; Delgado Vicente, M.; et al. Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC), the Sociedad Española de Hospitalización a Domicilio (SEHAD) Group. Executive summary of outpatient parenteral antimicrobial therapy: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases and the Spanish Domiciliary Hospitalisation Society. Enferm. Infecc. Microbiol. Clin. 2019, 37, 405–409. [Google Scholar] [CrossRef]
- Dimitrova, M.; Gilchrist, M.; Seaton, R.A. Outpatient parenteral antimicrobial therapy (OPAT) versus inpatient care in the UK: A health economic assessment for six key diagnoses. BMJ Open 2021, 11, e049733. [Google Scholar] [CrossRef]
- Youngster, I.; Shenoy, E.S.; Hooper, D.C.; Nelson, S.B. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin. Infect. Dis. 2014, 59, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birrell, M.T.; Fuller, A. Twice daily cefazolin is effective for treatment of serious methicillin-sensitive Staphylococcus aureus infection in an outpatient parenteral antimicrobial therapy program. Ther. Adv. Infect. Dis. 2019, 6, 204993611988284. [Google Scholar] [CrossRef] [PubMed]
- Hamad, Y.; Connor, L.; Bailey, T.C.; George, I.A. Outcomes of outpatient parenteral antimicrobial therapy with ceftriaxone for methicillin-susceptible Staphylococcus aureus bloodstream infections-a single-center observational study. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2020; Volume 7. [Google Scholar]
- Winans, S.A.; Luce, A.M.; Hasbun, R. Outpatient parenteral antimicrobial therapy for the treatment of methicillin-susceptible Staphylococcus aureus: A comparison of cefazolin and ceftriaxone. Infection 2013, 41, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Tam, I.; Weigel, B.; Breeze, J.L.; Paulus, J.K.; Nelson, J.; Allison, G.M. Comparative outcomes of β-lactam antibiotics in outpatient parenteral antibiotic therapy: Treatment success, readmissions and antibiotic switches. J. Antimicrob. Chemother. 2015, 70, 2389–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eljaaly, K.; Alshehri, S.; Erstad, B.L. Systematic Review and Meta-analysis of the Safety of Antistaphylococcal Penicillins Compared to Cefazolin. Antimicrob. Agents Chemother. 2018, 62, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Echevarria, K.L.; Traugott, K.A. β-Lactam Therapy for Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Comparative Review of Cefazolin versus Antistaphylococcal Penicillins. Pharmacotherapy 2017, 37, 346–360. [Google Scholar] [CrossRef]
- Gupta, V.D.; Stewart, K.R. Quantitation of Carbenicillin Disodium, Cefazolin Sodium, Cephalothin Sodium, Nafcillin Sodium, and Ticarcillin Disodium by High-pressure Liquid Chromatography. J. Pharm. Sci. 1980, 69, 1264–1267. [Google Scholar] [CrossRef]
- Gupta, V.D. Chemical Stability of Cefazolin Sodium after Reconstituting in 0.9% Sodium Chloride Injection and Storage in Polypropylene Syringes for PediatricUse. Int. J. Pharm. Compd. 2003, 7, 152. [Google Scholar]
- Gil-Navarro, M.V.; Luque-Marquez, R.; Báez-Gutiérrez, N.; Álvarez-Marín, R.; Navarro-Amuedo Ma, D.; Praena-Segovia, J.; Carmona-Caballero, J.M.; Fraile-Ramos, E.; López-Cortés, L.E. Antifungal treatment administered in OPAT programs is a safe and effective option in selected patients. Enferm. Infecc. Microbiol. Clin. 2020, 38, 479–484. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for Reporting Observational Studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef]
- Garner, J.S.; William, M.N.; Jarwis, W.R.; Emori, T.G.; Horan, T.C.; Huges, J.M. CDC definitions for nosocomial infections. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Jung, N.; Rieg, S. Essentials in the management of S. aureus bloodstream infection. Infection 2018, 46, 441–442. [Google Scholar] [CrossRef]
- Fowler, V.G.; Olsen, M.K.; Corey, G.R.; Woods, C.W.; Cabell, C.H.; Reller, L.B.; Cheng, A.C.; Dudley, T.; Oddone, E.Z. Clinical Identifiers of Complicated Staphylococcus aureus Bacteremia. Arch. Intern. Med. 2003, 163, 2066–2072. [Google Scholar] [CrossRef] [Green Version]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care-associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Grau, D.; Clarivet, B.; Lotthé, A.; Bommart, S.; Parer, S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: A prospective cohort study. Antimicrob. Resist. Infect. Control 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, K.E.; Cramer, R.A.; Bell, A.M.; Wagner, J.L.; Stover, K.R. Ceftriaxone as an Alternative Therapy for the Treatment of Methicillin-Susceptible Staphylococcus aureus Bacteremia after Initial Clearance of Bloodstream Infection. Case Rep. Infect. Dis. 2021, 2021, 8884685. [Google Scholar] [CrossRef]
- Wieland, B.W.; Marcantoni, J.R.; Bommarito, K.M.; Warren, D.K.; Marschall, J. A retrospective comparison of ceftriaxone versus oxacillin for osteoarticular infections due to methicillin-susceptible Staphylococcus aureus. Clin. Infect. Dis. 2012, 54, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Patel, U.C.; McKissic, E.L.; Kasper, D.; Lentino, J.R.; Pachucki, C.T.; Lee, T.; Lopanrsi, B.K. Outcomes of ceftriaxone use compared to standard of therapy in methicillin susceptible staphylococcal aureus (MSSA) bloodstream infections. Int. J. Clin. Pharm. 2014, 36, 1282–1289. [Google Scholar] [CrossRef]
- López-Cortés, L.E.; Gil-Navarro, M.V.; Luque-Marquez, R. When antimicrobial stewardship programmes reach the home. Enferm. Infecc. Microbiol. Clin. 2021, 39, 269–270. [Google Scholar] [CrossRef]
- Mahoney, M.V.; Childs-Kean, L.M.; Khan, P.; Rivera, C.G.; Stevens, R.W.; Ryan, K.L. Recent Updates in Antimicrobial Stewardship in Outpatient Parenteral Antimicrobial Therapy. Curr. Infect. Dis. Rep. 2021, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.C.W.; Seaton, R.A. Recent innovations and new applications of outpatient parenteral antimicrobial therapy. Expert Rev. Anti-Infect. Ther. 2021, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Morrisette, T.; Miller, M.A.; Montague, B.T.; Barber, G.R.; McQueen, R.B.; Krsak, M. On-and off-label utilization of dalbavancin and oritavancin for Gram-positive infections. J. Antimicrob. Chemother. 2019, 74, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, I.J.E.; van Leerdam, E.J.; Gompelman, M.; Tuinte, R.A.M.; Aarntzen, E.H.J.G.; Berrevoets, M.A.H.; Maat, I.; Bleeker-Rovers, C.P.; van Crevel, R.; ten Oever, J. Intravenous to Oral Switch in Complicated Staphylococcus aureus Bacteremia without Endovascular Infection: A Retrospective Single-Center Cohort Study. Clin. Infect. Dis. 2021, 73, 895–898. [Google Scholar] [CrossRef]
- Boclé, H.; Lavigne, J.P.; Cellier, N.; Crouzet, J.; Kouyoumdjian, P.; Sotto, A.; Loubet, P. Effectiveness of early switching from intravenous to oral antibiotic therapy in Staphylococcus aureus prosthetic bone and joint or orthopedic metalware-associated infections. BMC Musculoskelet. Disord. 2021, 22, 315. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.T.; Sousa, A.; Moreno-Flores, A.; Longueira, R.; Diéguez, P.; Suárez, M.; Lima, O.; Vasallo, F.J.; Álvarez-Fernández, M.; Crespo, M. The benefits and safety of oral sequential antibiotic therapy in non-complicated and complicated Staphylococcus aureus bacteremia. Int. J. Infect.Dis. 2021, 102, 554–560. [Google Scholar] [CrossRef]
- Dagher, M.; Fowler, V.G.; Wright, P.W.; Staub, M.B. A Narrative Review of Early Oral Stepdown Therapy for the Treatment of Uncomplicated Staphylococcus aureus Bacteremia: Yay or Nay. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Willekens, R.; Puig-Asensio, M.; Ruiz-Camps, I.; Larrosa, M.N.; González-López, J.J.; Rodríguez-Pardo, D.; Fernández-Hidalgo, N.; Pigrau, C.; Almirante, B. Early Oral Switch to Linezolid for Low-risk Patients with Staphylococcus aureus Bloodstream Infections: A Propensity-matched Cohort Study. Clin. Infect. Dis. 2010, 69, 381–887. [Google Scholar] [CrossRef] [Green Version]
- Kaasch, A.J.; Fätkenheuer, G.; Prinz-Langenohl, R.; Paulus, U.; Hellmich, M.; Weiß, V.; Jung, N.; Rieg, S.; Kern, W.V.; Seifert, H. Early oral switch therapy in low-risk Staphylococcus aureus bloodstream infection (SABATO): Study protocol for a randomized controlled trial. Trials. 2015, 16, 450. [Google Scholar] [CrossRef] [Green Version]
- López-Cortés, L.E.; Gálvez-Acebal, J.; Rodríguez-Baño, J. Therapy of Staphylococcus aureus bacteremia: Evidences and challenges. Enferm. Infecc. Microbiol. Clin. 2020, 38, 489–497. [Google Scholar] [CrossRef]
- Miller, W.R.; Seas, C.; Carvajal Lina, P.; Diaz, L.; Echeverri, A.M.; Ferro, C.; Rios, R.; Porras, P.; Luna, C.; Gotuzzo, E.; et al. The Cefazolin Inoculum Effect Is Associated With Increased Mortality in Methicillin-Susceptible Staphylococcus aureus Bacteremia. In Open Forum Infectious Diseases; IDSA: Arlington, VA, USA, 2018; Volume 5. [Google Scholar]
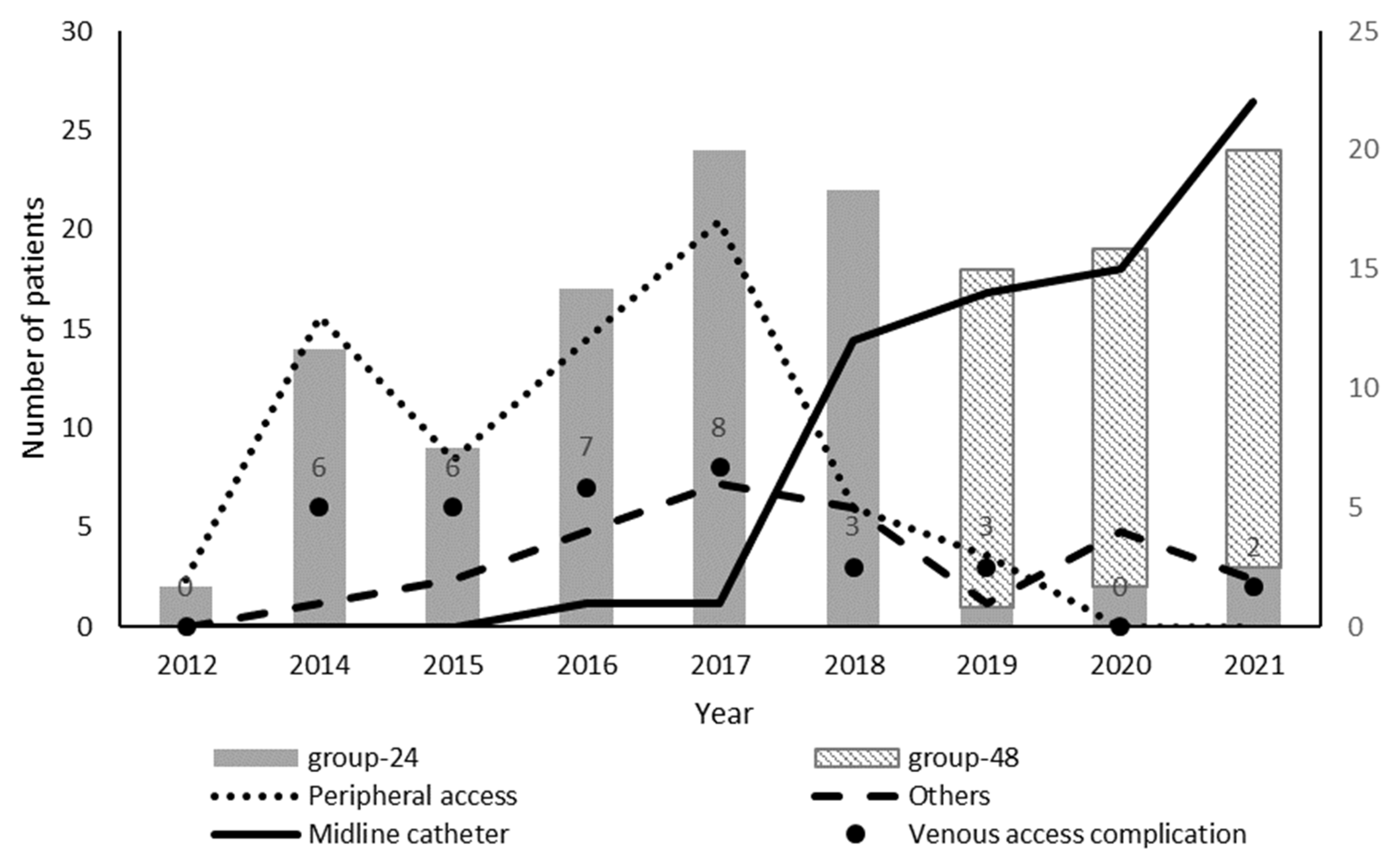
| Baseline Characteristics | Overall (n = 149) | Treatment | p | |
|---|---|---|---|---|
| Group 24 (n = 94) | Group 48 (n = 55) | |||
| Age (Median—IQR) | 63 (74–50) | 65 (77–52) | 57 (44–70) | 0.023 |
| Male gender | 104 (69.8) | 66 (70.2) | 38 (69.1) | 0.886 |
| Charlson score (Median—IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.570 |
| Comorbidities | ||||
| Cancer | 56 (37.6) | 29 (30.9) | 27 (49.1) | 0.027 |
| Cardiac insufficiency | 50 (33.6) | 35 (37.2) | 15 (27.3) | 0.214 |
| Diabetes mellitus | 44 (29.5) | 32 (34.0) | 12 (21.8) | 0.114 |
| Chronic obstructive pulmonary disease | 27 (18.1) | 19 (20.2) | 8 (14.5) | 0.386 |
| Chronic renal failure | 24 (16.1) | 20 (21.3) | 4 (7.3) | 0.025 |
| Liver disease | 10 (6.7) | 5 (5.3) | 5 (9.1) | 0.375 |
| MSSA infection | ||||
| Acquisition of the infection | ||||
| Community acquired | 38 (25.5) | 27 (28.7) | 11 (20.0) | 0.087 |
| Nosocomial acquisition | 13 (8.7) | 11 (11.7) | 2 (3.6) | |
| Health care-associated infection | 98 (65.8) | 56 (59.6) | 42 (76.4) | |
| Type of infection | ||||
| Non-bacteremic infection | 41 (27.1) | 29 (30.9) | 12 (21.8) | 0.412 |
| Uncomplicated bacteriemia | 59 (39.6) | 34 (36.2) | 25 (45.5) | |
| Complicated bacteriemia | 49 (32.9) | 31 (33.0) | 18 (32.7) | |
| Diagnosis | ||||
| Catheter-related bloodstream infection | 66 (44.4) | 39 (41.5) | 27 (49.1) | 0.078 |
| Primary bacteriemia | 18 (12.1) | 6 (6.4) | 12 (21.8) | |
| Prosthetic articular joint infection | 11 (7.4) | 9 (9.6) | 2 (3.6) | |
| Septic arthritis | 7 (4.7) | 6 (6.4) | 1 (1.8) | |
| Endocarditis | 5 (3.4) | 5 (5.3) | 0 (0.0) | |
| Osteomyelitis | 5 (3.4) | 2 (2.1) | 3 (5.5) | |
| Pyelonephritis | 5 (3.4) | 3 (3.2) | 2 (3.6) | |
| Skin and soft tissue infection | 4 (2.7) | 3 (3.2) | 1 (1.8) | |
| Pneumonia | 3 (2.0) | 2 (2.1) | 1 (1.8) | |
| Intraabdominal infection | 3 (2.0) | 3 (3.2) | 0 (0.0) | |
| Diabetic foot infection | 2 (1.3) | 2 (2.1) | 0 (0.0) | |
| Others | 20 (13.4) | 14 (14.9) | 6 (10.9) | |
| Treatment Characteristics | Overall (n = 149) | Treatment | p | |
|---|---|---|---|---|
| Group-24 (n = 94) | Group-48 (n = 55) | |||
| Inpatient Treatment (Days) | 7 (5–11) | 7 (4–10) | 7 (6–13) | 0.208 |
| Inpatient treatment (antibiotic) | ||||
| Cefazolin | 116 (77.9) | 67 (71.3) | 49 (89.1) | 0.036 |
| Cloxacillin | 18 (12.1) | 14 (14.9) | 4 (7.3) | |
| Other or unknown | 15 (10.1) | 13 (13.8) | 2 (3.6) | |
| OPAT treatment (days) | 9 (6–14) | 9 (5–13) | 10 (7–16) | 0.163 |
| Vascular access | ||||
| Peripheral access | 59 (39.6) | 56 (59.6) | 3 (5.5) | <0.000 |
| Midline catheter | 65 (43.6) | 20 (21.3) | 45 (81.8) | |
| Central access with peripheral insertion | 15 (10.1) | 9 (9.6) | 6 (10.9) | |
| Central access | 6 (4.0) | 6 (6.4) | 0 (0.0) | |
| Reservoir | 4 (2.7) | 3 (3.2) | 1 (1.8) | |
| Clinical outcomes | ||||
| Treatment success (composite endpoint) | 134 (89.9) | 83 (88.3) | 51 (92.7) | 0.386 |
| Treatment failure (composite endpoint) | 15 (10.1) | 11 (11.7) | 4 (7.3) | 0.386 |
| Cause of treatment failure (% of treatment failure) | ||||
| Infection relapse or progression | 9 (60.0) | 6 (54.5) | 3 (75.0) | 0.816 |
| Death during OPAT or readmission | 4 (26.7) | 3 (27.3) | 1 (25.0) | |
| Antibiotic side effects | 1 (6.7) | 1 (9.1) | 0 (0.0) | |
| Lack of familiar support | 1 (6.7) | 1 (9.1) | 0 (0.0) | |
| Unplanned readmission | 11 (7.4) | 8 (8.5) | 3 (5.5) | 0.491 |
| Vascular access complication | 35 (23.3) | 31 (33.0) | 4 (7.3) | <0.001 |
| Type of complication (% of vascular access complication) | ||||
| Phlebitis | 8 (23.5) | 8 (26.7) | 0 (0.0) | 0.247 |
| Extravasation, malfunction, or catheter loss | 26 (76.5) | 23 (74.2) | 4 (100.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Hidalgo, L.; Luque-Márquez, R.; de Alarcon, A.; Guisado-Gil, A.B.; Gutierrez-Gutierrez, B.; Navarro-Amuedo, M.D.; Praena-Segovia, J.; Carmona-Caballero, J.M.; Fraile-Ramos, E.; Gutierrez-Valencia, A.; et al. Clinical Outcomes of an Innovative Cefazolin Delivery Program for MSSA Infections in OPAT. J. Clin. Med. 2022, 11, 1551. https://doi.org/10.3390/jcm11061551
Herrera-Hidalgo L, Luque-Márquez R, de Alarcon A, Guisado-Gil AB, Gutierrez-Gutierrez B, Navarro-Amuedo MD, Praena-Segovia J, Carmona-Caballero JM, Fraile-Ramos E, Gutierrez-Valencia A, et al. Clinical Outcomes of an Innovative Cefazolin Delivery Program for MSSA Infections in OPAT. Journal of Clinical Medicine. 2022; 11(6):1551. https://doi.org/10.3390/jcm11061551
Chicago/Turabian StyleHerrera-Hidalgo, Laura, Rafael Luque-Márquez, Aristides de Alarcon, Ana Belén Guisado-Gil, Belen Gutierrez-Gutierrez, Maria Dolores Navarro-Amuedo, Julia Praena-Segovia, Juan Manuel Carmona-Caballero, Elena Fraile-Ramos, Alicia Gutierrez-Valencia, and et al. 2022. "Clinical Outcomes of an Innovative Cefazolin Delivery Program for MSSA Infections in OPAT" Journal of Clinical Medicine 11, no. 6: 1551. https://doi.org/10.3390/jcm11061551
APA StyleHerrera-Hidalgo, L., Luque-Márquez, R., de Alarcon, A., Guisado-Gil, A. B., Gutierrez-Gutierrez, B., Navarro-Amuedo, M. D., Praena-Segovia, J., Carmona-Caballero, J. M., Fraile-Ramos, E., Gutierrez-Valencia, A., Lopez-Cortes, L. E., & Gil-Navarro, M. V. (2022). Clinical Outcomes of an Innovative Cefazolin Delivery Program for MSSA Infections in OPAT. Journal of Clinical Medicine, 11(6), 1551. https://doi.org/10.3390/jcm11061551






