Prevalence and Rate of Resolution of Left Atrial Thrombus in Patients with Non-Valvular Atrial Fibrillation: A Two-Center Retrospective Real-World Study
Abstract
1. What Is New
2. Introduction
- the prevalence of LAA thrombus in an unselected cohort of patients undergoing TEE;
- the prevalence of LAA thrombus despite recommended anticoagulant therapy;
- the rate of LAA thrombus resolution on repeat TEE after at least three weeks of anticoagulation;
- the efficacy of different anticoagulant regimens (including VKA, heparin, and NOACs);
- clinical and echocardiographic determinants of LAA thrombus persistence.
3. Methods
4. Statistical Analysis
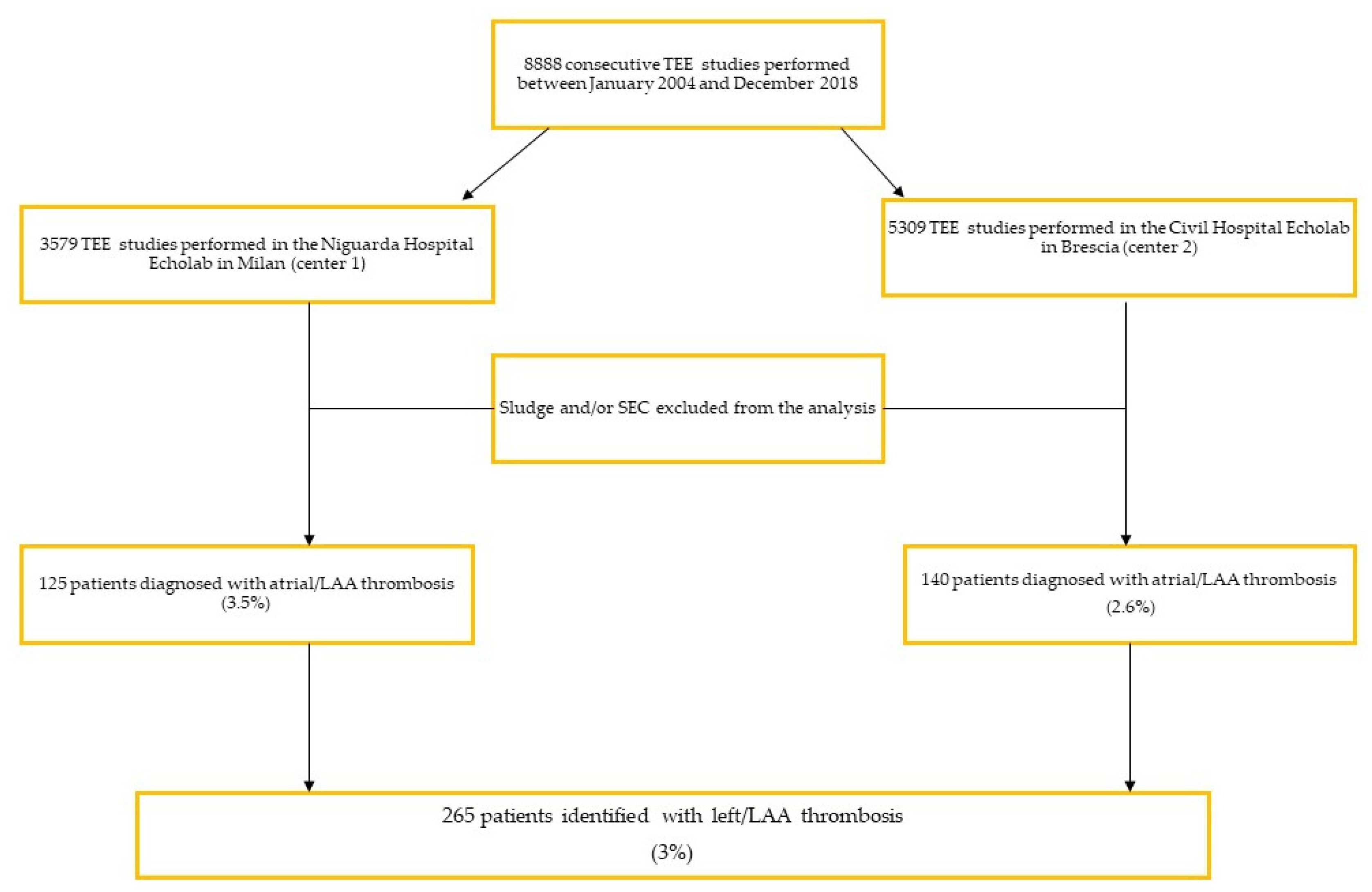
5. Results
5.1. Study Population Characteristics
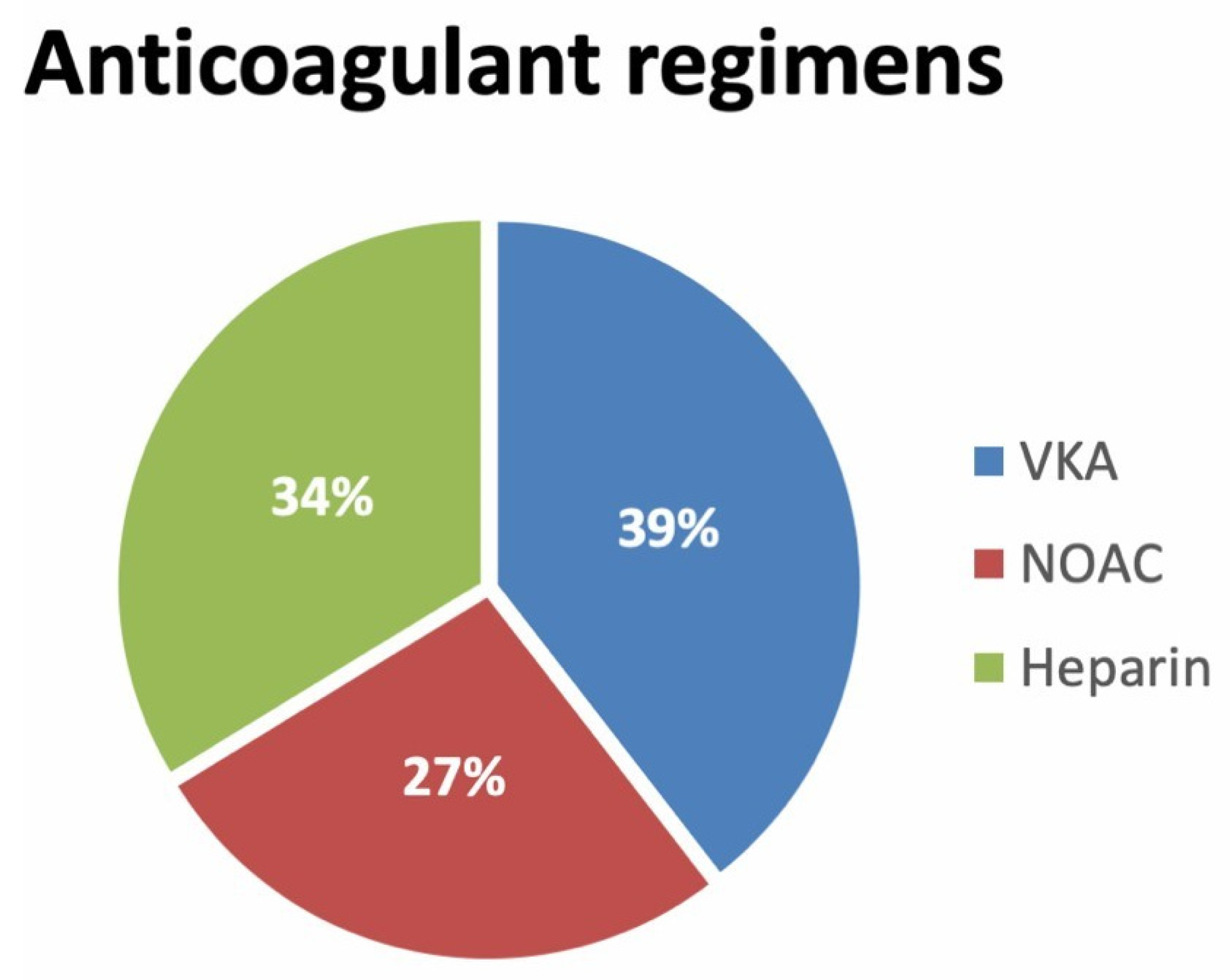
5.2. Anticoagulant Treatment
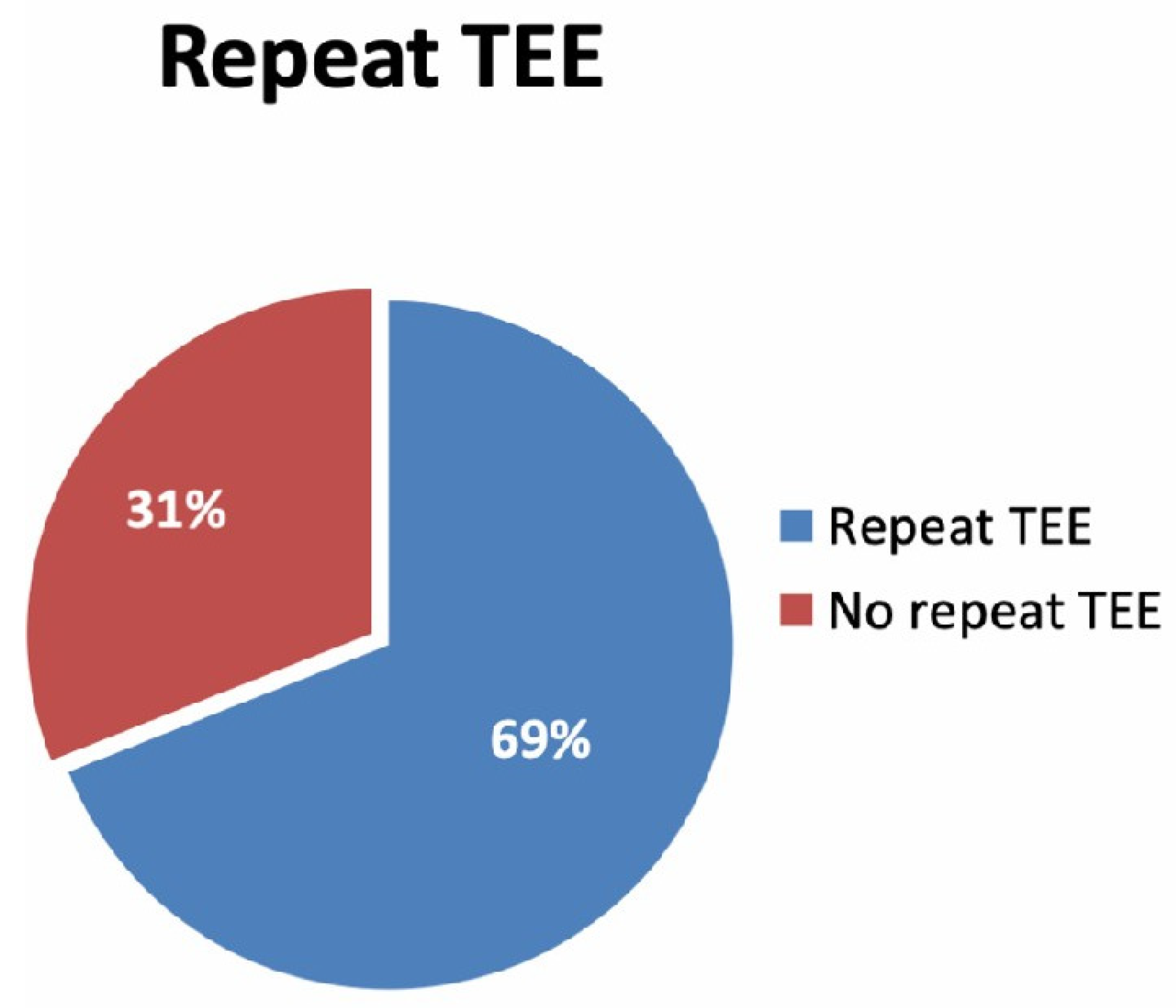
5.3. LAA Thrombus Resolution Rate
5.4. Predictors of LAA Thrombus Resolution after Anticoagulant Treatment
6. Discussion
Study Limitations and Strengths
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of Atrial Fibrillation to Incidence and Outcome of Ischemic Stroke. Stroke 2005, 36, 1115–1119. [Google Scholar] [CrossRef]
- Jaakkola, S.; Kiviniemi, T.O.; Airaksinen, K.E.J. Cardioversion for atrial fibrillation—How to prevent thromboembolic complications? Ann. Med. 2018, 50, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Benedetto, V.; Gichuru, P.; Burnell, J.; Antoniou, S.; Schilling, R.J.; Strain, W.D.; Ryan, R.; Watkins, C.; Marshall, T.; et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: A population-based study. Heart 2020, 106, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Farinha, J.M.; Jones, I.D.; Lip, G.Y.H. Optimizing adherence and persistence to non-vitamin K antagonist oral anticoagulant therapy in atrial fibrillation. Eur. Heart J. Suppl. 2022, 24 (Suppl. A), A42–A55. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef] [PubMed]
- Nuotio, I.; Hartikainen, J.E.K.; Grönberg, T.; Biancari, F.; Airaksinen, K.E.J. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA 2014, 312, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Kupczynska, K.; Michalski, B.W.; Miskowiec, D.; Kasprzak, J.D.; Wejner-Mik, P.; Wdowiak-Okrojek, K.; Lipiec, P. Association between left atrial function assessed by speckle-tracking echocardiography and the presence of left atrial appendage thrombus in patients with atrial fibrillation. Anatol. J. Cardiol. 2017, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Jaber, W.A.; Prior, D.L.; Thamilarasan, M.; Grimm, R.A.; Thomas, J.D.; Klein, A.L.; Asher, C.R. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: A transesophageal echocardiographic study. Am. Heart J. 2000, 140, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Tadeo, G.; Beretta, S.; Tagliagambe, L.M.; Manzillo, G.F.; Spata, M.; Santarone, M. Atrial thrombi resolution after prolonged anticoagulation in patients with atrial fibrillation: A transesophageal echocardiographic study. Chest 1999, 115, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Nagarakanti, R.; Ezekowitz, M.D.; Oldgren, J.; Yang, S.; Chernick, M.; Aikens, T.H.; Flaker, G.; Brugada, J.; Kamenský, G.; Parekh, A.; et al. Dabigatran versus warfarin in patients with atrial fibrillation: An analysis of patients undergoing cardioversion. Circulation 2011, 123, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zylla, M.M.; Pohlmeier, M.; Hess, A.; Mereles, D.; Kieser, M.; Bruckner, T.; Scholz, E.; Zitron, E.; Schweizer, P.A.; Katus, H.A.; et al. Prevalence of intracardiac thrombi under phenprocoumon, direct oral anticoagulants (dabigatran and rivaroxaban), and bridging therapy in patients with atrial fibrillation and flutter. Am. J. Cardiol. 2015, 115, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Ferner, M.; Wachtlin, D.; Konrad, T.; Deuster, O.; Meinertz, T.; Von Bardeleben, S.; Münzel, T.; Seibert-Grafe, M.; Breithardt, G.; Rostock, T. Rationale and design of the RE-LATED AF—AFNET 7 trial: REsolution of Left atrial-Appendage Thrombus—Effects of Dabigatran in patients with Atrial Fibrillation. Clin. Res. Cardiol. 2016, 105, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cappato, R.; Ezekowitz, M.D.; Klein, A.L.; Camm, A.J.; Ma, C.-S.; Le Heuzey, J.-Y.; Talajic, M.; Scanavacca, M.; Vardas, P.E.; Kirchhof, P.; et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur. Heart J. 2014, 35, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Hammerstingl, C.; Marin, F.; Cappato, R.; Meng, I.L.; Kirsch, B.; van Eickels, M.; Cohen, A. Left atrial thrombus resolution in atrial fibrillation or flutter: Results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am. Heart J. 2016, 178, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, M.D.; Pollack, C.V.; Halperin, J.L.; England, R.D.; Nguyen, S.V.P.; Spahr, J.; Sudworth, M.; Cater, N.B.; Breazna, A.; Oldgren, J.; et al. Apixaban compared to heparin/Vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: The EMANATE trial. Eur. Heart J. 2018, 39, 2959–2971. [Google Scholar] [CrossRef]
- Flaker, G.; Lopes, R.D.; Al-Khatib, S.M.; Hermosillo, A.G.; Hohnloser, S.H.; Tinga, B.; Zhu, J.; Mohan, P.; Garcia, D.; Bartunek, J.; et al. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: Insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). J. Am. Coll. Cardiol. 2014, 63, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, H.L.; Nagabandi, A.; Brown, K.; Ayyala, D.N.; Sharma, G.K. Prevalence and clinical characteristics associated with left atrial thrombus detection: Apixaban. World J. Cardiol. 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Merino, J.L.; Ezekowitz, M.D.; Zamoryakhin, D.; Melino, M.; Jin, J.; Mercuri, M.F.; Grosso, M.A.; Fernandez, V.; Al-Saady, N.; et al. Edoxaban versus enoxaparin–warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): A randomised, open-label, phase 3b trial. Lancet 2016, 388, 1995–2003. [Google Scholar] [CrossRef]
- Murtaza, G.; Turagam, M.K.; Atti, V.; Garg, J.; Boda, U.; Velagapudi, P.; Akella, K.; Natale, A.; Gopinathannair, R.; Lakkireddy, D. Warfarin vs non-vitamin K oral anticoagulants for left atrial appendage thrombus: A meta-analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 1822–1827. [Google Scholar] [CrossRef]
- Farkowski, M.M.; Jubele, K.; Marín, F.; Gandjbakhch, E.; Ptaszynski, P.; Merino, J.L.; Lenarczyk, R.; Potpara, T.S. Diagnosis and management of left atrial appendage thrombus in patients with atrial fibrillation undergoing cardioversion or percutaneous left atrial procedures: Results of the European Heart Rhythm Association survey. EP Eur. 2020, 22, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, H.; Nagai, T.; Watanabe, A.; Takatsuki, S.; Okumura, K. Left atrial thrombus formation and resolution during dabigatran therapy: A Japanese Heart Rhythm Society report. J. Arrhythm. 2015, 31, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, R.; Zaborska, B.; Baran, J.; Sikora-Frac, M.; Kulakowski, P. Rivaroxaban twice daily for lysis of left atrial appendage thrombus: A potential new therapeuticoption. Pol. Arch. Med. Wewn. 2016, 126, 430–431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, T.; Shinoda, Y.; Ikeoka, K.; Minamisaka, T.; Fukuoka, H.; Inui, H.; Hoshida, S. Dabigatran therapy resulting in the resolution of rivaroxaban-resistant left atrial appendage thrombi in patients with atrial fibrillation. Intern. Med. 2017, 56, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Minamishima, T.; Sato, T.; Sakata, K.; Yoshino, H.; Soejima, K. Resolution of a warfarin and dabigatran-resistant left atrial appendage thrombus with apixaban. J. Arrhythm. 2016, 32, 233–235. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Entire Cohort |
|---|---|
| Number of patients | 265 |
| Age, years—median (25–75th) | 71 (65–78) |
| Male, n (%) | 175 (66) |
| Atrial Fibrillation, n (%) | 256 (97) |
| Paroxysmal | 10 (4.0) |
| Persistent | 163 (64.0) |
| Permanent | 83 (32.0) |
| Sinus rhythm, n (%) | 9(4) |
| Smoking, n (%) | 44(17) |
| COPD, n (%) | 46 (17.4) |
| Active cancer, n (%) | 27 (10) |
| Diabetes, n (%) | 67 (25.3) |
| Arterial Hypertension, n (%) | 204 (77) |
| HF, n (%) | 190 (72) |
| HFpEF | 42 (22.1) |
| HFmrEF | 39 (20.5) |
| HFrEF | 107 (56.3) |
| CHA2DS2VASc score—median (25–75th) | 4(3-5) |
| History of cardiac disease, n (%) | 208 (78.5) |
| Chronic Coronary Syndromes, n (%) | 79 (38) |
| Valvular, n (%) | 53 (26) |
| Hypertrophic CM, n (%) | 5 (3) |
| Dilated CM, n (%) | 26 (13) |
| Tachycardiomyopathy, n (%) | 25 (12) |
| Echocardiographic Parameters | Entire Cohort (265) |
|---|---|
| Left atrial dimensions (median) (25–75th) | |
| AP (mm) | 53 (46–61) |
| Area (cm2) | 30 (26–36.7) |
| Volume (mL) | 110 (86–152) |
| E/e’ | 12 (9–16) |
| LAA morphology | |
| Chicken wing, n (%) | 26 (21) |
| Other, n (%) | 100 (79) |
| Cactus, n (%) | 17 (13) |
| Cauliflower, n (%) | 42 (33.3) |
| Windsock, n (%) | 41 (32.5) |
| LAA emptying velocity (cm/sec)-(median) (25–75th) | 20 (15–20) |
| LVEF (%)-(median) (25–75th) | 43 (30–55) |
| >50%, n (%) | 85 (41) |
| 40–49%, n (%) | 27 (13) |
| <40%, n (%) | 93 (46) |
| Moderate or severe mitral regurgitation n, (%) | 75 (28) |
| Moderate or severe mitral stenosis n, (%) | 13 (5) |
| Mitral valve replacement n, (%) | 33 (12.5) |
| 1. Mechanical valve | 26 (79) |
| 2. Tissue valve | 71 (21.2) |
| 3. Transcatheter MV intervention (Mitraclip) | 4 (1.5) |
| N (%) | |
|---|---|
| anticoagulated | 258 (97.4) |
| VKA | 158 (52.1) |
| NOAC | 71 (27) |
| RIVAROXABAN | 18 (7) |
| APIXABAN | 24 (9.1) |
| DABIGATRAN | 24 (9.1) |
| EDOXABAN | 5 (1.9%) |
| HEPARIN | 49 (18.5) |
| Non-anticoagulated | 7 (2.6) |
| Variables in the Equation with Y = Resolution | |||||||
|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | Sig. (p) | OR | 95% C.I for Lower | EXP (B) Upper | |
| Age | −0.047 | 0.060 | 0.617 | 0.432 | 0.954 | 0.848 | 1.073 |
| Gender | 1.041 | 1.151 | 0.818 | 0.366 | 2.832 | 0.297 | 27.001 |
| CHADS2-VASC2 | 0.839 | 0.429 | 3.828 | 0.050 | 2.315 | 0.999 | 5.367 |
| Left atrium diameter (AP) | 0.020 | 0.030 | 0.421 | 0.517 | 1.020 | 0.961 | 1.082 |
| Left atrium volume | 0.012 | 0.012 | 1.125 | 0.289 | 1.013 | 0.990 | 1.036 |
| EF % | −0.050 | 0.046 | 1.214 | 0.270 | 0.951 | 0.0870 | 1.040 |
| Severe MR | 0.132 | 1.009 | 0.017 | 0.896 | 1.141 | 0.158 | 8.249 |
| LAA emptying velocity | 0.167 | 0.069 | 5.789 | 0.016 | 1.182 | 1.031 | 1.354 |
| Constant | −5.410 | 5.098 | 1.126 | 0.289 | 0.004 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faggiano, P.; Dinatolo, E.; Moreo, A.; De Chiara, B.; Sbolli, M.; Musca, F.; Curnis, A.; Belli, O.; Giannattasio, C.; Tomasi, C.; et al. Prevalence and Rate of Resolution of Left Atrial Thrombus in Patients with Non-Valvular Atrial Fibrillation: A Two-Center Retrospective Real-World Study. J. Clin. Med. 2022, 11, 1520. https://doi.org/10.3390/jcm11061520
Faggiano P, Dinatolo E, Moreo A, De Chiara B, Sbolli M, Musca F, Curnis A, Belli O, Giannattasio C, Tomasi C, et al. Prevalence and Rate of Resolution of Left Atrial Thrombus in Patients with Non-Valvular Atrial Fibrillation: A Two-Center Retrospective Real-World Study. Journal of Clinical Medicine. 2022; 11(6):1520. https://doi.org/10.3390/jcm11061520
Chicago/Turabian StyleFaggiano, Pompilio, Elisabetta Dinatolo, Antonella Moreo, Benedetta De Chiara, Marco Sbolli, Francesco Musca, Antonio Curnis, Oriana Belli, Cristina Giannattasio, Cesare Tomasi, and et al. 2022. "Prevalence and Rate of Resolution of Left Atrial Thrombus in Patients with Non-Valvular Atrial Fibrillation: A Two-Center Retrospective Real-World Study" Journal of Clinical Medicine 11, no. 6: 1520. https://doi.org/10.3390/jcm11061520
APA StyleFaggiano, P., Dinatolo, E., Moreo, A., De Chiara, B., Sbolli, M., Musca, F., Curnis, A., Belli, O., Giannattasio, C., Tomasi, C., Metra, M., & Santangelo, G. (2022). Prevalence and Rate of Resolution of Left Atrial Thrombus in Patients with Non-Valvular Atrial Fibrillation: A Two-Center Retrospective Real-World Study. Journal of Clinical Medicine, 11(6), 1520. https://doi.org/10.3390/jcm11061520






