Recent Non-Invasive Parameters to Identify Subjects at High Risk of Sudden Cardiac Death
Abstract
1. Introduction
2. Electrocardiographic Features Associated with the Risk of Arrhythmias
2.1. QRS Fragmentation
2.2. Early Repolarization (ER)
2.3. Brugada Syndrome (BrS)
2.4. T-Wave Morphology in Long QT Syndrome (LQTS)
2.5. Electrocardiographic Markers in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C)
3. Echocardiographic Markers for Arrhythmias
3.1. Left Ventricular Hypertrophy (LVH)
3.2. Global and Segmental Longitudinal Strain
3.3. Mechanical Dispersion
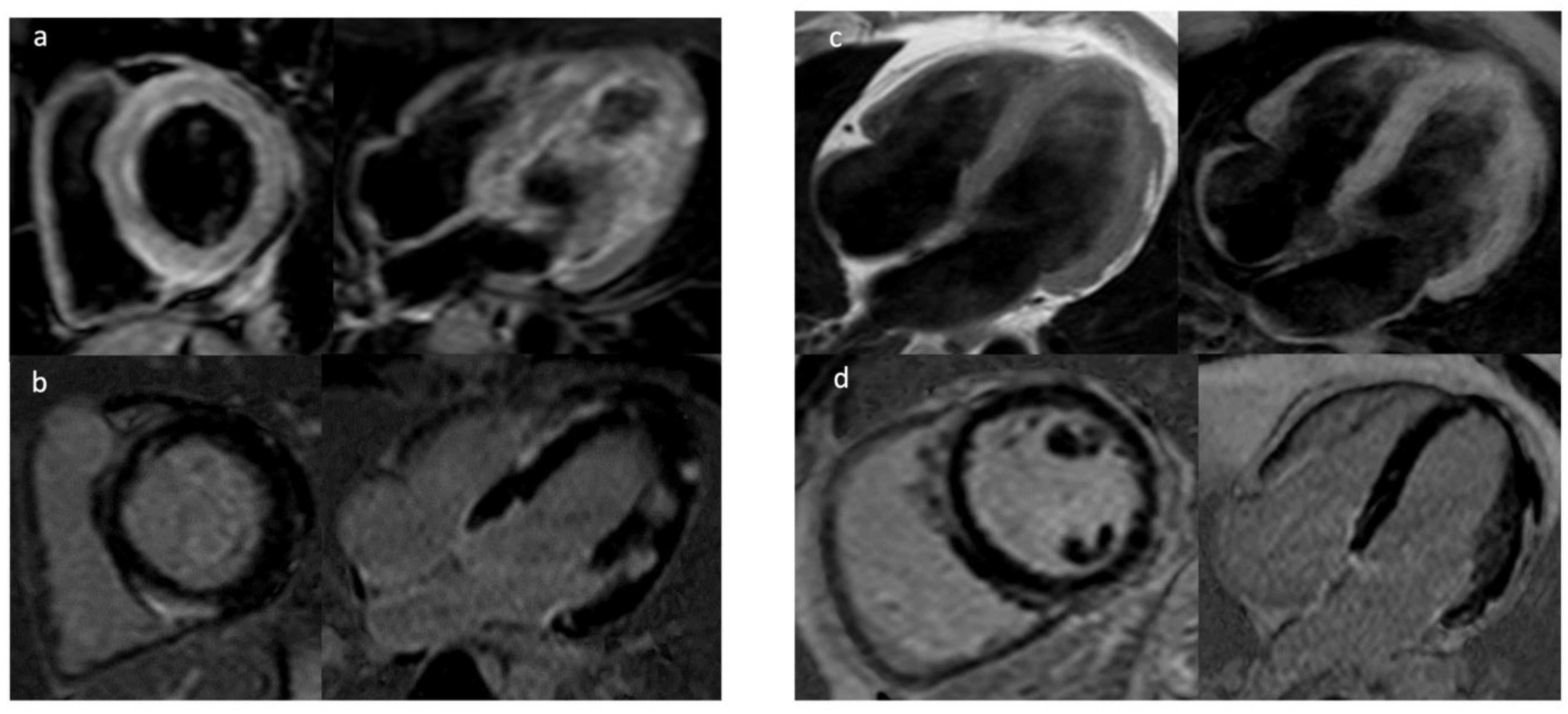
4. Cardiac Magnetic Resonance (CMR)
4.1. Late Gadolinium Enhancement
4.2. Mapping Techniques and Extracellular Volume
4.3. Feature Tracking (FT)
4.4. CMR in Mitral Valve Prolapse
5. Genetic Testing
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Al Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, 210–271. [Google Scholar]
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Abdelghani, S.A.; Rosenthal, T.M.; Morin, D.P. Surface Electrocardiogram Predictors of Sudden Cardiac Arrest. Ochsner J. 2016, 16, 280–289. [Google Scholar] [PubMed]
- Hathaway, W.R.; Peterson, E.D.; Wagner, G.S.; Granger, C.B.; Zabel, K.M.; Pieper, K.S.; Clark, K.A.; Woodlief, L.H.; Califf, R.M. Prognostic significance of the initial electrocardiogram in patients with acute myocardial infarction. GUSTO-I Investigators. Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries. JAMA 1998, 279, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Petrina, M.; Goodman, S.G.; Eagle, K.A. The 12-lead electrocardiogram as a predictive tool of mortality after acute myocardial infarction: Current status in an era of revascularization and reperfusion. Am. Heart J. 2006, 152, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Flowers, N.C.; Horan, L.G.; Thomas, J.R.; Tolleson, W.J. The anatomic basis for high-frequency components in the electrocardiogram. Circulation 1969, 39, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Khan, B.; Jacob, S.; Kumar, A.; Mahenthiran, J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006, 113, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Suradi, H.; Maskoun, W.; Michael, M.A.; Shen, C.; Peng, J.; Dandamudi, G.; Mahenthiran, J. Fragmented wide QRS on a 12-lead ECG: A sign of myocardial scar and poor prognosis. Circ. Arrhythm. Electrophysiol. 2008, 1, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; SubaÊius, H.; Patel, T.; Cunnane, R.; Kadish, A.H. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Derval, N.; Sacher, F.; Jesel, L.; Deisenhofer, I.; de Roy, L.; Pasquié, J.L.; Nogami, A.; Babuty, D.; Yli-Mayry, S.; et al. Sudden cardiac arrest associated with early repolarization. N. Engl. J. Med. 2008, 358, 2016–2023. [Google Scholar] [CrossRef]
- Rizzo, C.; Monitillo, F.; Iacoviello, M. 12-lead electrocardiogram features of arrhythmic risk: A focus on early repolarization. World J. Cardiol. 2016, 8, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.J.; Sager, S.J.; Freiser, M.; McGonagle, S.; Castellanos, A.; Myerburg, R.J. Inferolateral early repolarization in athletes. J. Interv. Card. Electrophysiol. 2011, 31, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P. Normal resting electrocardiographic variants in young athletes. Phys. Sportsmed. 2008, 36, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Osborn, J.J. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am. J. Physiol. 1953, 175, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, W.; Kaess, B.M.; Debiec, R.; Nelson, C.P.; Stark, K.; Tobin, M.D.; Macfarlane, P.W.; Tomaszewski, M.; Samani, N.J.; Hengstenberg, C. Heritability of early repolarization: A population-based study. Circ. Cardiovasc. Genet. 2011, 4, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.X.; Antzelevitch, C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST- segment elevation. Circulation 1999, 100, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, J.T.; Anttonen, O.; Junttila, M.J.; Aro, A.L.; Kerola, T.; Rissanen, H.A.; Reunanen, A.; Huikuri, H.V. Long-term outcome associated with early repolarization on electrocardiography. N. Engl. J. Med. 2009, 361, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, J.T.; Junttila, M.J.; Anttonen, O.; Aro, A.L.; Luttinen, S.; Kerola, T.; Sager, S.J.; Rissanen, H.A.; Myerburg, R.J.; Reunanen, A.; et al. Early repolarization: Electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 2011, 123, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Rosso, R.; Adler, A.; Halkin, A.; Viskin, S. Risk of sudden death among young individuals with J waves and early repolarization: Putting the evidence into perspective. Heart Rhythm 2011, 8, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Rosso, R.; Kogan, E.; Belhassen, B.; Rozovski, U.; Scheinman, M.M.; Zeltser, D.; Halkin, A.; Steinvil, A.; Heller, K.; Glikson, M.; et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J. Am. Coll. Cardiol. 2008, 52, 1231–1238. [Google Scholar] [CrossRef]
- Sarkozy, A.; Chierchia, G.B.; Paparella, G.; Boussy, T.; De Asmundis, C.; Roos, M.; Henkens, S.; Kaufman, L.; Buyl, R.; Brugada, R.; et al. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2009, 2, 154–161. [Google Scholar] [CrossRef]
- Kawata, H.; Morita, H.; Yamada, Y.; Noda, T.; Satomi, K.; Aiba, T.; Isobe, M.; Nagase, S.; Nakamura, K.; Fukushima Kusano, K.; et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: A novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm 2013, 10, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Bayoumy, A.; Gong, M.Q.; Christien Li, K.H.; Wong, S.H.; Wu, W.K.; Li, G.P.; Bazoukis, G.; Letsas, K.P.; Wong, W.T.; Xia, Y.L.; et al. Spontaneous type 1 pattern, ventricular arrhythmias and sudden cardiac death in Brugada syndrome: An updated systematic review and meta-analysis. J. Geriatr. Cardiol. 2017, 14, 639–643. [Google Scholar] [PubMed]
- Shi, S.; Barajas-Martinez, H.; Liu, T.; Sun, Y.; Yang, B.; Huang, C.; Hu, D. Prevalence of spontaneous Brugada ECG pattern recorded at standard intercostal leads: A meta-analysis. Int. J. Cardiol. 2017, 254, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Probst, V.; Veltmann, C.; Eckardt, L.; Meregalli, P.G.; Gaita, F.; Tan, H.L.; Babuty, D.; Sacher, F.; Giustetto, C.; Schulze-Bahr, E.; et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation 2010, 121, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Gasparini, M.; Napolitano, C.; Della Bella, P.; Ottonelli, A.G.; Sassone, B.; Giordano, U.; Pappone, C.; Mascioli, G.; Rossetti, G.; et al. Riskstratification in Brugadasyndrome: Results of the PRELUDE (PRogrammedELectricalstimUlationpreDictivevaluE) registry. J. Am. Coll. Cardiol. 2012, 59, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Letsas, K.P.; Baranchuk, A.; Shao, Q.; Tse, G.; Zhang, N.; Zhang, Z.; Hu, D.; Li, G.; Liu, T. Meta-analysis of fragmented QRS as an electrocardiographic predictor for arrhythmic events in patients with Brugada syndrome. Front. Physiol. 2017, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Watanabe, I.; Okumura, Y.; Ashino, S.; Kofune, M.; Nagashima, K.; Kofune, T.; Nakai, T.; Kunimoto, S.; Kasamaki, Y.; et al. Prolonged QRS duration in lead V2 and risk of life-threatening ventricular arrhythmia in patients with Brugada syndrome. Int. Heart J. 2011, 52, 98–102. [Google Scholar] [CrossRef] [PubMed]
- BabaiBigi, M.A.; Aslani, A.; Shahrzad, S. aVR sign as a risk factor for life-threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm 2007, 4, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Letsas, K.P.; Efremidis, M.; Asvestas, D.; Vlachos, K.; Georgopoulos, S.; Tse, G.; Liu, T.; Bazoukis, G.; Sideris, A.; Baranchuk, A.; et al. Right ventricular outflow tract electroanatomical abnormalities predict ventricular fibrillation inducibility in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2018, 11, e005928. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.; Giustetto, C.; Martino, A.; Sciarra, L.; Cerrato, N.; Marziali, M.; Rauzino, J.; Carlino, G.; de Ruvo, E.; Guerra, F.; et al. New Electrocardiographic Marker of Sudden Death in Brugada Syndrome: The S-Wave in Lead I. J. Am. Coll. Cardiol. 2016, 67, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Castro Hevia, J.; Antzelevitch, C.; TornésBárzaga, F.; Dorantes Sánchez, M.; DorticósBalea, F.; Zayas Molina, R.; Quiñones Pérez, M.A.; Fayad Rodríguez, Y. Tpeak-tend and Tpeak-tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J. Am. Coll. Cardiol. 2006, 47, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Maury, P.; Sacher, F.; Gourraud, J.B.; Pasquié, J.L.; Raczka, F.; Bongard, V.; Duparc, A.; Mondoly, P.; Sadron, M.; Chatel, S.; et al. Increased Tpeak-Tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm 2015, 12, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Zumhagen, S.; Zeidler, E.M.; Stallmeyer, B.; Ernsting, M.; Eckardt, L.; Schulze-Bahr, E. Tpeak-Tend interval and Tpeak-Tend/QT ratio in patients with Brugada syndrome. Europace 2016, 18, 1866–1872. [Google Scholar] [PubMed]
- Migliore, F.; Testolina, M.; Zorzi, A.; Bertaglia, E.; Silvano, M.; Leoni, L.; Bellin, A.; Basso, C.; Thiene, G.; Allocca, G.; et al. First-degree atrioventricular block on basal electrocardiogram predicts future arrhythmic events in patients with Brugada syndrome: A long-term follow-up study from the Veneto region of Northeastern Italy. Europace 2019, 21, 322–331. [Google Scholar] [CrossRef]
- Morita, H.; Watanabe, A.; Kawada, S.; Miyamoto, M.; Morimoto, Y.; Nakagawa, K.; Nishii, N.; Nakamura, K.; Ito, H. Identification of electrocardiographic risk markers for the initial and recurrent episodes of ventricular fibrillation in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2018, 29, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur. Heart J. 2015, 36, 2757–2759. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; McNitt, S.; Polonsky, B.; Rosero, S.Z.; Kutyifa, V.; Huang, A.; Moss, A.J.; Zareba, W. Risk Stratification of Type 2 Long-QT Syndrome Mutation Carriers With Normal QTc Interval: The Value of Sex, T-Wave Morphology, and Mutation Type. Circ. Arrhythm. Electrophysiol. 2018, 11, e005918. [Google Scholar] [CrossRef] [PubMed]
- Bosman, L.P.; Sammani, A.; James, C.A.; Cadrin-Tourigny, J.; Calkins, H.; van Tintelen, J.P.; Hauer, R.N.W.; Asselbergs, F.W.; TeRiele, A.S.J.M. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: A systematic review and meta-analysis. Heart Rhythm 2018, 15, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.; Marchlinski, F.; Anastasakis, A.; Bauce, B.; Basso, C.; Brunckhorst, C.; Tsatsopoulou, A.; et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: An international task force consensus statement. Eur. Heart J. 2015, 36, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Orgeron, G.M.; Te Riele, A.; Tichnell, C.; Wang, W.; Murray, B.; Bhonsale, A.; Judge, D.P.; Kamel, I.R.; Zimmerman, S.L.; Tandri, H.; et al. Performance of the 2015 International Task Force Consensus Statement Risk Stratification Algorithm for Implantable Cardioverter-Defibrillator Placement in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2018, 11, e005593. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; Calkins, H.; Hauer, R.N.; Corrado, D.; Svendsen, J.H.; Wichter, T.; Biernacka, E.K.; Saguner, A.M.; TeRiele, A.S.; Zareba, W. High interobserver variability in the assessment of epsilon waves: Implications for diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm 2016, 13, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.G.; van der Zwaag, P.A.; van der Werf, C.; van der Smagt, J.J.; Noorman, M.; Bhuiyan, Z.A.; Wiesfeld, A.C.; Volders, P.G.; van Langen, I.M.; Atsma, D.E.; et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: Pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation 2011, 123, 2690–2700. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Stecker, E.C.; Vickers, C.; Waltz, J.; Socoteanu, C.; John, B.T.; Mariani, R.; McAnulty, J.H.; Gunson, K.; Jui, J.; Chugh, S.S. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. J. Am. Coll. Cardiol. 2006, 47, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, A.; Suleiman, M.; Laish-Farkash, A.; Samania, N.; Kazatsker, M.; Goldenberg, I.; Glikson, M.; Beinart, R. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: From the Israeli ICD Registry. Heart Rhythm 2015, 12, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- De Haan, S.; Knaapen, P.; Beek, A.M.; de Cock, C.C.; Lammertsma, A.A.; van Rossum, A.C.; Allaart, C.P. Risk stratification for ventricular arrhythmias in ischaemic cardiomyopathy: The value of non-invasive imaging. Europace 2010, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Konety, S.H.; Koene, R.J.; Norby, F.L.; Wilsdon, T.; Alonso, A.; Siscovick, D.; Sotoodehnia, N.; Gottdiener, J.; Fox, E.R.; Chen, L.Y.; et al. Echocardiographic Predictors of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. Circ. Cardiovasc. Imaging. 2016, 9, e004431. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Khan, H.; Kurl, S.; Willeit, P.; Karppi, J.; Ronkainen, K.; Di Angelantonio, E. Left ventricular mass and the risk of sudden cardiac death: A population-based study. J. Am. Heart Assoc. 2014, 3, e001285. [Google Scholar] [CrossRef] [PubMed]
- Verheule, S.; Schotten, U. Electrophysiological Consequences of Cardiac Fibrosis. Cells 2021, 10, 3220. [Google Scholar] [CrossRef]
- Haider, A.W.; Larson, M.G.; Benjamin, E.J.; Levy, D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J. Am. Coll. Cardiol. 1998, 32, 1454–1459. [Google Scholar] [CrossRef]
- Reinier, K.; Dervan, C.; Singh, T.; Uy-Evanado, A.; Lai, S.; Gunson, K.; Jui, J.; Chugh, S.S. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm 2011, 8, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.; Bertini, M.; Borleffs, C.J.; Delgado, V.; Boersma, E.; Piers, S.R.; Thijssen, J.; Nucifora, G.; Shanks, M.; Ewe, S.H.; et al. Predictors of death and occurrence of appropriate implantable defibrillator therapies in patients with ischemic cardiomyopathy. Am. J. Cardiol. 2010, 106, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Ersbøll, M.; Valeur, N.; Andersen, M.J.; Mogensen, U.M.; Vinther, M.; Svendsen, J.H.; Møller, J.E.; Kisslo, J.; Velazquez, E.J.; Hassager, C.; et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Knappe, D.; Pouleur, A.C.; Claggett, B.; Wang, P.J.; Moss, A.J.; Solomon, S.D.; Kutyifa, V. Regional Longitudinal Deformation Improves Prediction of Ventricular Tachyarrhythmias in Patients With Heart Failure With Reduced Ejection Fraction: A MADIT-CRT Substudy (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). Circ. Cardiovasc. Imaging 2017, 10, e005096. [Google Scholar] [PubMed]
- Wu, T.J.; Ong, J.J.; Hwang, C.; Lee, J.J.; Fishbein, M.C.; Czer, L.; Trento, A.; Blanche, C.; Kass, R.M.; Mandel, W.J.; et al. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: Role of increased fibrosis in the generation of reentry. J. Am. Coll. Cardiol. 1998, 32, 187–196. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.; Patil, S.; Marx, C.; Horsfall, M.; Chew, D.P.; Sree Raman, K.; Daril, N.D.M.; Tiver, K.; Joseph, M.X.; Ganesan, A.N.; et al. Advanced Echocardiographic Imaging for Prediction of SCD in Moderate and Severe LV Systolic Function. JACC Cardiovasc. Imaging 2020, 13, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Debonnaire, P.; Thijssen, J.; Leong, D.P.; Joyce, E.; Katsanos, S.; Hoogslag, G.E.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Delgado, V.; et al. Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int. J. Cardiovasc. Imaging 2014, 30, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef]
- Alizade, E.; Yesin, M.; Tabakci, M.M.; Avci, A.; Bulut, M.; Acar, G.; Şimşek, Z.; Izci, S.; Barutçu, S.; Pala, S. Utility of speckle tracking echocardiography imaging in patients with asymptomatic and symptomatic arrhythmogenic right ventricular cardiomyopathy. Echocardiography 2016, 33, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, S.I.; Haugaa, K.H.; Anfinsen, O.G.; Leren, T.P.; Smiseth, O.A.; Kongsgaard, E.; Amlie, J.P.; Edvardsen, T. Right ventricular mechanical dispersion is related to malignant arrhythmias: A study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur. Heart J. 2011, 32, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.; Arzanauskaite, M.; Vassiliou, V.S.; Lota, A.; Izgi, C.; Tayal, U.; et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017, 135, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Piers, S.R.; Everaerts, K.; van der Geest, R.J.; Hazebroek, M.R.; Siebelink, H.M.; Pison, L.A.; Schalij, M.J.; Bekkers, S.C.; Heymans, S.; Zeppenfeld, K. Myocardial scar predicts monomorphic ventricular tachycardia but not polymorphic ventricular tachycardia or ventricular fibrillation in nonischemic dilated cardiomyopathy. Heart Rhythm 2015, 12, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Cheong, B.Y.; Muthupillai, R.; Wilson, J.M.; Sung, A.; Huber, S.; Amin, S.; Elayda, M.A.; Lee, V.V.; Flamm, S.D. Prognostic significance of delayed-enhancement magnetic resonance imaging: Survival of 857 patients with and without left ventricular dysfunction. Circulation 2009, 120, 2069–2076. [Google Scholar] [CrossRef]
- Klem, I.; Weinsaft, J.W.; Bahnson, T.D.; Hegland, D.; Kim, H.W.; Hayes, B.; Parker, M.A.; Judd, R.M.; Kim, R.J. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J. Am. Coll. Cardiol. 2012, 60, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.Y.; Chan, A.K.; Brown, K.A.; Chan, C.W.; Reynolds, H.G.; Tsang, S.; Davis, R.B. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006, 113, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-term follow-up of biopsy-proven viral myocarditis: Predictors of mortality and incomplete recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Schuleri, K.H.; Centola, M.; Evers, K.S.; Zviman, A.; Evers, R.; Lima, J.A.; Lardo, A.C. Cardiovascular magnetic resonance characterization of peri-infarct zone remodeling following myocardial infarction. J. Cardiovasc. Magn. Reson. 2012, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Demirel, F.; Adiyaman, A.; Timmer, J.R.; Dambrink, J.H.; Kok, M.; Boeve, W.J.; Elvan, A. Myocardial scar characteristics based on cardiac magnetic resonance imaging is associated with ventricular tachyarrhythmia in patients with ischemic cardiomyopathy. Int. J. Cardiol. 2014, 177, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Halley, C.M.; Carrigan, T.P.; Zysek, V.; Popovic, Z.B.; Setser, R.; Schoenhagen, P.; Starling, R.C.; Flamm, S.D.; Desai, M.Y. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: A delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc. Imaging 2009, 2, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Yee, R.; Gula, L.; Krahn, A.D.; Skanes, A.; Leong-Sit, P.; Klein, G.J.; Stirrat, J.; Fine, N.; Pallaveshi, L.; et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: Evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ. Cardiovasc. Imaging 2012, 5, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Azevedo, C.F.; Cheng, A.; Gupta, S.N.; Bluemke, D.A.; Foo, T.K.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007, 115, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, R.; Chaudhry, U.; van der Pals, J.; Engblom, H.; Arheden, H.; Heiberg, E.; Wu, K.C.; Borgquist, R.; Carlsson, M. Cardiovascular Magnetic Resonance to Predict Appropriate Implantable Cardioverter Defibrillator Therapy in Ischemic and Nonischemic Cardiomyopathy Patients Using Late Gadolinium Enhancement Border Zone: Comparison of Four Analysis Methods. Circ. Cardiovasc. Imaging 2017, 10, e006105. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.T.; Shayne, A.J.; Brown, K.A.; Gupta, S.N.; Chan, C.W.; Luu, T.M.; Di Carli, M.F.; Reynolds, H.G.; Stevenson, W.G.; Kwong, R.Y. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 2006, 114, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Reed, E.; Sheppard, M.N.; Elkington, A.G.; Ho, S.Y.; Burke, M.; Petrou, M.; Pennell, D.J. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sohal, M.; Voigt, T.; Sammut, E.; Tobon-Gomez, C.; Child, N.; Jackson, T.; Shetty, A.; Bostock, J.; Cooklin, M.; et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm 2015, 12, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Flett, A.S.; Hayward, M.P.; Ashworth, M.T.; Hansen, M.S.; Taylor, A.M.; Elliott, P.M.; McGregor, C.; Moon, J.C. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation 2010, 122, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Spieker, M.; Haberkorn, S.; Gastl, M.; Behm, P.; Katsianos, S.; Horn, P.; Jacoby, C.; Schnackenburg, B.; Reinecke, P.; Kelm, M.; et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2017, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [PubMed]
- Leyva, F.; Zegard, A.; Acquaye, E.; Gubran, C.; Taylor, R.; Foley, P.W.X.; Umar, F.; Patel, K.; Panting, J.; Marshall, H.; et al. Outcomes of Cardiac Resynchronization Therapy With or without Defibrillation in Patients with Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 70, 1216–1227. [Google Scholar] [CrossRef]
- Overhoff, D.; Ansari, U.; Hohneck, A.; Tülümen, E.; Rudic, B.; Kuschyk, J.; Lossnitzer, D.; Baumann, S.; Froelich, M.F.; Waldeck, S.; et al. Prediction of cardiac events with non-contrast magnetic resonance feature tracking in patients with ischaemic cardiomyopathy. ESC Heart Fail. 2022, 9, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Dukkipati, S.R.; Turagam, M.; Liao, S.L.; Adams, D.H.; Reddy, V.Y. Arrhythmic Mitral Valve Prolapse: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.F.; Ackerman, M.J.; McLeod, C.J.; Kapa, S.; Mulpuru, S.K.; Sriram, C.S.; Cannon, B.C.; Asirvatham, S.J.; Noseworthy, P.A. Sites of Successful Ventricular Fibrillation Ablation in Bileaflet Mitral Valve Prolapse Syndrome. Circ. Arrhythm. Electrophysiol. 2016, 9, e004005. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nezafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.; Lancefield, T.; Voskoboinik, A.; Taylor, A.J. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. EurHeart J. Cardiovasc. Imaging 2015, 16, 634–641. [Google Scholar]
- Ichinose, A.; Otani, H.; Oikawa, M.; Takase, K.; Saito, H.; Shimokawa, H.; Takahashi, S. MRI of cardiac sarcoidosis: Basal and subepicardial localization of myocardial lesions and their effect on left ventricular function. AJR 2008, 191, 862–869. [Google Scholar] [CrossRef]
- Crawford, T.; Mueller, G.; Sarsam, S.; Prasitdumrong, H.; Chaiyen, N.; Gu, X.; Schuller, J.; Kron, J.; Nour, K.A.; Cheng, A.; et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ. Arrhythm. Electrophysiol. 2014, 7, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Klein, M.R.; Chandra, S.; Spencer, K.T.; Decara, J.M.; Lang, R.M.; Burke, M.C.; Garrity, E.R.; Hogarth, D.K.; Archer, S.L.; et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur. J. Heart Fail. 2011, 13, 1231–1237. [Google Scholar] [CrossRef]
- Smedema, J.P.; van Geuns, R.J.; Ainslie, G.; Ector, J.; Heidbuchel, H.; Crijns, H. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC HeartFail. 2017, 4, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Sikkema-Raddatz, B.; Johansson, L.F.; de Boer, E.N.; Almomani, R.; Boven, L.G.; van den Berg, M.P.; van Spaendonck-Zwarts, K.Y.; van Tintelen, J.P.; Sijmons, R.H.; Jongbloed, J.D.; et al. Targeted next-generation sequencing can replace Sanger sequencing in clinical diagnostics. Hum. Mutat. 2013, 34, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Fokstuen, S.; Makrythanasis, P.; Nikolaev, S.; Santoni, F.; Robyr, D.; Munoz, A.; Bevillard, J.; Farinelli, L.; Iseli, C.; Antonarakis, S.E.; et al. Multiplex targeted high-throughput sequencing for Mendelian cardiac disorders. Clin. Genet. 2014, 85, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Osman, J.; Tan, S.C.; Lee, P.Y.; Low, T.Y.; Jamal, R. Sudden Cardiac Death (SCD)—Risk stratification and prediction with molecular biomarkers. J. Biomed. Sci. 2019, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Mazzarotto, F.; Tayal, U.; Buchan, R.J.; Midwinter, W.; Wilk, A.; Whiffin, N.; Govind, R.; Mazaika, E.; de Marvao, A.; Dawes, T.J.W.; et al. Reevaluating the Genetic Contribution of Monogenic Dilated Cardiomyopathy. Circulation 2020, 141, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Dal Ferro, M.; Severini, G.M.; Gigli, M.; Mestroni, L.; Sinagra, G. Genetics of Dilated Cardiomyopathy: Current Knowledge and Future Perspectives. In Dilated Cardiomyopathy: From Genetics to Clinical Management; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Verdonschot, J.A.J.; Hazebroek, M.R.; Krapels, I.P.C.; Henkens, M.T.H.M.; Raafs, A.; Wang, P.; Merken, J.J.; Claes, G.R.F.; Vanhoutte, E.K.; van den Wijngaard, A.; et al. Implications of Genetic Testing in Dilated Cardiomyopathy. Circ. Genomic Precis. Med. 2020, 13, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Pasotti, M.; Klersy, C.; Pilotto, A.; Marziliano, N.; Rapezzi, C.; Serio, A.; Mannarino, S.; Gambarin, F.; Favalli, V.; Grasso, M.; et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J. Am. Coll. Cardiol. 2008, 52, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Bécane, H.M.; Bonne, G.; Varnous, S.; Muchir, A.; Ortega, V.; Hammouda, E.H.; Urtizberea, J.A.; Lavergne, T.; Fardeau, M.; Eymard, B.; et al. High incidence of sudden death with conduction system and myocardial disease due to lamins A and C gene mutation. Pacing Clin. Electrophysiol. 2000, 23, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Van Berlo, J.H.; de Voogt, W.G.; van der Kooi, A.J.; van Tintelen, J.P.; Bonne, G.; Yaou, R.B.; Duboc, D.; Rossenbacker, T.; Heidbüchel, H.; de Visser, M.; et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: Do lamin A/C mutations portend a high risk of sudden death? J. Mol. Med. 2005, 83, 79–83. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Hazebroek, M.R.; Derks, K.W.J.; BarandiaránAizpurua, A.; Merken, J.J.; Wang, P.; Bierau, J.; van den Wijngaard, A.; Schalla, S.M.; Abdul Hamid, M.A.; et al. Titin cardiomyopathy leads to altered mitochondrial energetics, increased fibrosis and long-term life-threatening arrhythmias. Eur. Heart J. 2018, 39, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.N.; Caleshu, C.; Reuter, C.; Lazzeroni, L.C.; Ingles, J.; Garcia, J.; McCaleb, K.; Adesiyun, T.; Sedaghat-Hamedani, F.; Kumar, S.; et al. Regional variation in RBM20 causes a highly penetrant arrhythmogenic cardiomyopathy. Circ. Heart Fail. 2019, 12, e005371. [Google Scholar] [CrossRef] [PubMed]
- McNair, W.P.; Sinagra, G.; Taylor, M.R.; Di Lenarda, A.; Ferguson, D.A.; Salcedo, E.E.; Slavov, D.; Zhu, X.; Caldwell, J.H.; Mestroni, L. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J. Am. Coll. Cardiol. 2011, 57, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, M.; Muser, D.; Vitali-Serdoz, L.; Buiatti, A.; Morgera, T. Arrhythmias in Dilated Cardiomyopathy: Diagnosis and Treatment. In Dilated Cardiomyopathy: From Genetics to Clinical Management; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Ortiz-Genga, M.F.; Cuenca, S.; Dal Ferro, M.; Zorio, E.; Salgado-Aranda, R.; Climent, V.; Padrón-Barthe, L.; Duro-Aguado, I.; Jiménez-Jáimez, J.; Hidalgo-Olivares, V.M.; et al. Truncating FLNC mutations are associated with high-risk dilated and Arrhythmogenic cardiomyopathies. J. Am. Coll. Cardiol. 2016, 68, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Begay, R.L.; Graw, S.L.; Sinagra, G.; Asimaki, A.; Rowland, T.J.; Slavov, D.B.; Gowan, K.; Jones, K.L.; Brun, F.; Merlo, M.; et al. Filamin C truncation mutations are associated with arrhythmogenic dilated cardiomyopathy and changes in the cell-cell adhesion structures. JACC Clin. Electrophysiol. 2018, 4, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Muresan, I.D.; Agoston-Coldea, L. Phenotypes of hypertrophic cardiomyopathy: Genetics, clinics, and modular imaging. Heart Fail. Rev. 2021, 26, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Havndrup, O.; Bundgaard, H.; Andersen, P.S.; Larsen, L.A.; Vuust, J.; Kjeldsen, K.; Christiansen, M. The Val606Met mutation in the cardiac beta-myosin heavy chain gene in patients with familial hypertrophic cardiomyopathy is associated with a high risk of sudden death at young age. Am. J. Cardiol. 2001, 87, 1315–1317. [Google Scholar] [CrossRef]
- Konno, T.; Shimizu, M.; Ino, H.; Fujino, N.; Uchiyama, K.; Mabuchi, T.; Sakata, K.; Kaneda, T.; Fujita, T.; Masuta, E.; et al. A novel mutation in the cardiac myosin-binding protein C gene is responsible for hypertrophic cardiomyopathy with severe ventricular hypertrophy and sudden death. Clin. Sci. 2006, 110, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Rigato, I.; Bauce, B.; Rampazzo, A.; Zorzi, A.; Pilichou, K.; Mazzotti, E.; Migliore, F.; Marra, M.P.; Lorenzon, A.; De Bortoli, M.; et al. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ. Cardiovasc. Genet. 2013, 6, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Bhonsale, A.; Groeneweg, J.A.; James, C.A.; Dooijes, D.; Tichnell, C.; Jongbloed, J.D.; Murray, B.; teRiele, A.S.; van den Berg, M.P.; Bikker, H.; et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur. Heart J. 2015, 36, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Hoorntje, E.T.; TeRijdt, W.P.; James, C.A.; Pilichou, K.; Basso, C.; Judge, D.P.; Bezzina, C.R.; van Tintelen, J.P. Arrhythmogenic cardiomyopathy: Pathology, genetics, and concepts in pathogenesis. Cardiovasc. Res. 2017, 113, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- McKenna, W.J.; Asaad, N.A.; Jacoby, D.L. Prediction of ventricular arrhythmia and sudden death in arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2019, 40, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, J.; Splawski, I.; Atkinson, D.; Li, Z.; Robinson, J.L.; Moss, A.J.; Towbin, J.A.; Keating, M.T. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 1995, 80, 805–811. [Google Scholar] [CrossRef]
- Chen, Q.; Kirsch, G.E.; Zhang, D.; Brugada, R.; Brugada, J.; Brugada, P.; Potenza, D.; Moya, A.; Borggrefe, M.; Breithardt, G.; et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 1998, 392, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.J.; Alshinawi, C.; Kyndt, F.; Probst, V.; Hoorntje, T.M.; Hulsbeek, M.; Wilde, A.A.; Escande, D.; Mannens, M.M.; Le Marec, H. Cardiac conduction defects associate with mutations in SCN5A. Nat. Genet. 1999, 23, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Rook, M.B.; Groenewegen, W.A.; Herfst, L.J.; van der Wal, A.C.; Lam, J.; Jongsma, H.J.; Wilde, A.A.; Mannens, M.M. Compound heterozygosity for mutations (W156X and R225W) in SCN5A associated with severe cardiac conduction disturbances and degenerative changes in the conduction system. Circ. Res. 2003, 92, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Perez Riera, A.R.; Antzelevitch, C.; Schapacknik, E.; Dubner, S.; Ferreira, C. Is there an overlap between Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy/dysplasia? J. Electrocardiol. 2005, 38, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Makita, N.; Xing, Y.; Watanabe, S.; Futatani, T.; Ye, F.; Saito, K.; Ibuki, K.; Watanabe, K.; Hirono, K.; et al. SCN5A variants in Japanese patients with left ventricular noncompaction and arrhythmia. Mol. Genet. Metab. 2008, 93, 468–474. [Google Scholar] [CrossRef]
- Gacita, A.M.; McNally, E.M. Genetic spectrum of arrhythmogenic cardiomyopathy. Circ. Heart Fail. 2019, 12, e005850. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Napolitano, C.; Tiso, N.; Memmi, M.; Vignati, G.; Bloise, R.; Sorrentino, V.; Danieli, G.A. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001, 103, 196–200. [Google Scholar] [CrossRef]
- Van der Werf, C.; Wilde, A.A. Catecholaminergic polymorphic ventricular tachycardia: From bench to bedside. Heart 2013, 99, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Morin, D.P.; Link, M.S. Sudden cardiac death in Long QT syndrome (LQTS), Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia (CPVT). Prog. Cardiovasc. Dis. 2019, 62, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Napolitano, C.; Memmi, M.; Colombi, B.; Drago, F.; Gasparini, M.; DeSimone, L.; Coltorti, F.; Bloise, R.; Keegan, R.; et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002, 106, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: This document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 2011, 13, 1077–1109. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Wilde, A.A.; Horie, M.; Cho, Y.; Behr, E.R.; Berul, C.; Blom, N.; Brugada, J.; Chiang, C.E.; Huikuri, H.; et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm 2013, 10, e85–e108. [Google Scholar] [CrossRef] [PubMed]
- Ormondroyd, E.; Oates, S.; Parker, M.; Blair, E.; Watkins, H. Pre-symptomatic genetic testing for inherited cardiac conditions: A qualitative exploration of psychosocial and ethical implications. Eur. J. Hum. Genet. 2014, 22, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [PubMed]
- Priori, S.G. Genetic testing to predict sudden cardiac death: Current perspectives and future goals. Indian Heart J. 2014, 66, S58–S60. [Google Scholar] [CrossRef] [PubMed][Green Version]

| ECG Abnormality | Pathophysiologic Background | Clinical Setting |
|---|---|---|
| QRSf | Conduction delay from inhomogeneous activation of the ventricles due to myocardial scar | DCM, IDCM HCM, BrS LQTS, ARVD Cardiac Sarcoidosis |
| ER (“J-waves” or “J-point elevation”) | Altered ion channel function (alterations in sodium, potassium and calcium currents) | Young African men Athletes HCM |
| TW Inversion | Changes during phase three of the action potential | ARVD |
| Echocardiography | Pathophysiologic Background | Clinical Setting | Information |
|---|---|---|---|
| LVEF | Left ventricular systolic function | ICM NICM Myocarditis ARVC LVNC | Severe LV systolic dysfunction, of any cause, identified by measuring the LVEF, is associated with an increased risk of SCD (LVEF < 35%) |
| LV GLS/RLS | Measure of LV systolic function (indirect reflector of myocardial fibrosis/scar) | ICM NICM HCM | GLS is associated with SCD, appropriate ICD therapy and VA |
| Mechanical dispersion | Slow and heterogeneous electrical conduction of the LV myocardium (indirect reflector of myocardial fibrosis/scar) | ICM NICM HCM ARVC | Predictor of VA in patients with moderate and severe LV systolic dysfunction (despite the etiology of LV dysfunction) and in HCM patients. Predictor of VT/VF (in patients with ARVC) |
| LV wall thickness | Left ventricular hypertrophy | HCM Myocarditis | Independent predictor of SCD |
| RVEF, RV diameter, regional RV akinesia, dyskinesia or aneurism | RV remodeling and dysfunction | ARVC | Correlated with more frequent sustained ventricular arrhythmias and ICD appropriate shocks |
| CMR | Pathophysiologic Background | Clinical Setting | Information |
|---|---|---|---|
| LGE | Fibrosis | ICM, NICM HCM, Myocarditis ARVC, LVNC Mitral valve prolapse | Independent predictor for VA and SCD |
| T1 and ECV | Tissue edema and diffuse fibrosis | ICM, NICM HCM, Myocarditis | Higher native T1 values associated with VA |
| T2 | Myocardial edema | Myocarditis | Abnormal T2 mapping is involved in predicting major adverse events including cardiac death |
| LVEF | Left ventricular systolic function | ICM, NICM Myocarditis, ARVC LVNC | LV systolic dysfunction is associated with an increased risk of SCD |
| RVEF | Right ventricular systolic function | ARVDC | Overall increase in VA in RV dysfunction |
| Strain Imaging and MD | Myocardial deformation and function | ICM, NICM | Impaired strain associated with SCD |
| Inherited Cardiomyopathy | Genes Associated with SCD | Protein Encoded |
|---|---|---|
| DCM | TTN | Titin |
| LMNA | Lamin A/C | |
| FLNC | Filamin C | |
| SCN5A | Sodium voltage-gated channel alpha subunit 5 | |
| HCM | MYH7 | B-Myosin Heavy Chain 7 |
| MYBPC3 | Myosin-Binding Protein C 3 | |
| BrS | SCN5A | Nav1.5—Cardiac sodium channel α subunit |
| ARVD | PLN R14del | Phospholamban |
| LMNA | Lamin A/C | |
| SCN5A | Sodium voltage-gated channel alpha subunit 5 | |
| RBM20 | RNA binding motif protein 20 | |
| TMEM43 | Transmembrane Protein 43 | |
| LQTS | SCN5A | Nav1.5—Cardiac sodium channel α subunit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbo, M.D.; Vitale, E.; Pesolo, M.; Casavecchia, G.; Gravina, M.; Pellegrino, P.; Brunetti, N.D.; Iacoviello, M. Recent Non-Invasive Parameters to Identify Subjects at High Risk of Sudden Cardiac Death. J. Clin. Med. 2022, 11, 1519. https://doi.org/10.3390/jcm11061519
Corbo MD, Vitale E, Pesolo M, Casavecchia G, Gravina M, Pellegrino P, Brunetti ND, Iacoviello M. Recent Non-Invasive Parameters to Identify Subjects at High Risk of Sudden Cardiac Death. Journal of Clinical Medicine. 2022; 11(6):1519. https://doi.org/10.3390/jcm11061519
Chicago/Turabian StyleCorbo, Maria Delia, Enrica Vitale, Maurizio Pesolo, Grazia Casavecchia, Matteo Gravina, Pierluigi Pellegrino, Natale Daniele Brunetti, and Massimo Iacoviello. 2022. "Recent Non-Invasive Parameters to Identify Subjects at High Risk of Sudden Cardiac Death" Journal of Clinical Medicine 11, no. 6: 1519. https://doi.org/10.3390/jcm11061519
APA StyleCorbo, M. D., Vitale, E., Pesolo, M., Casavecchia, G., Gravina, M., Pellegrino, P., Brunetti, N. D., & Iacoviello, M. (2022). Recent Non-Invasive Parameters to Identify Subjects at High Risk of Sudden Cardiac Death. Journal of Clinical Medicine, 11(6), 1519. https://doi.org/10.3390/jcm11061519







