Safety of Obtaining an Extra Biobank Kidney Biopsy Core
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Selection
2.2. Clinical Variables
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Population
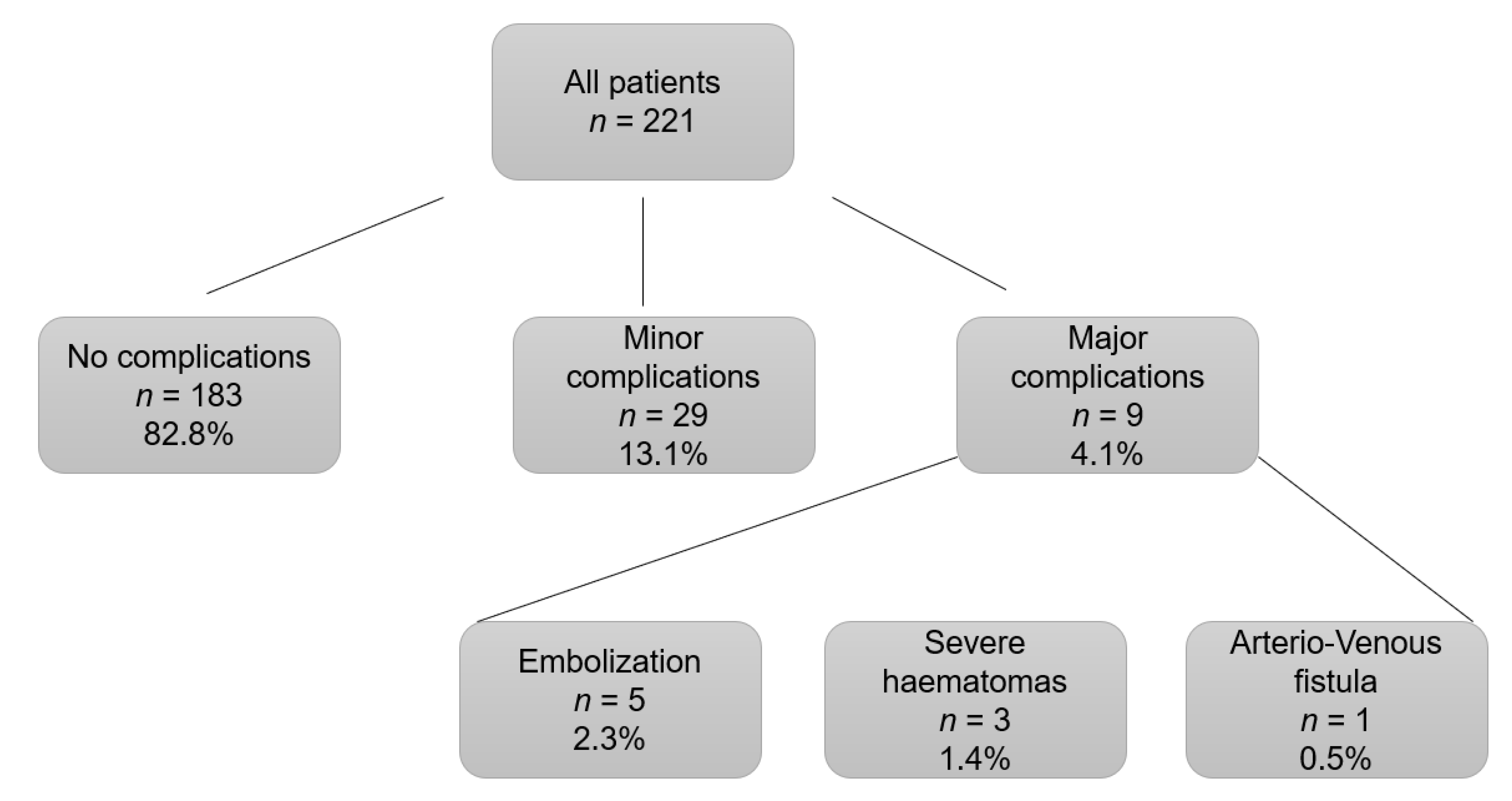
3.2. Post-Kidney Biopsy Complications
3.3. Risk Factors for Complications Associated with Kidney Biopsy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luciano, R.L.; Moeckel, G.W. Update on the Native Kidney Biopsy: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Palsson, R.; Short, S.A.P.; Kibbelaar, Z.A.; Amodu, A.; Stillman, I.E.; Rennke, H.G.; McMahon, G.M.; Waikar, S.S. Bleeding Complications After Percutaneous Native Kidney Biopsy: Results From the Boston Kidney Biopsy Cohort. Kidney Int. Rep. 2020, 5, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Koirala, A.; Ashley Jefferson, J. How safe is a native kidney biopsy? Clin. J. Am. Soc. Nephrol. 2020, 15, 1541–1542. [Google Scholar] [CrossRef]
- Stojimirovic, B. Aspiration biopsy of the kidney. Med. Glas. 1959, 13, 437–440. [Google Scholar] [PubMed]
- Kark, R.M.; Muehrcke, R.C. Biopsy of Kidney in Prone Position. Lancet 1954, 263, 1047–1049. [Google Scholar] [CrossRef]
- Poggio, E.D.; McClelland, R.L.; Blank, K.N.; Hansen, S.; Bansal, S.; Bomback, A.S.; Canetta, P.A.; Khairallah, P.; Kiryluk, K.; Lecker, S.H.; et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin. J. Am. Soc. Nephrol. 2020, 15, 1595–1602. [Google Scholar] [CrossRef]
- Hogan, J.J.; Mocanu, M.; Berns, J.S. The native kidney biopsy: Update and evidence for best practice. Clin. J. Am. Soc. Nephrol. 2016, 11, 354–356. [Google Scholar] [CrossRef]
- Rathod, K.R.; Popat, B.A.; Pandey, A.; Jamale, T.E.; Hase, N.K.; Deshmukh, H.L. Safety and effectiveness of transjugular renal biopsy: A single center study. Indian J. Nephrol. 2017, 27, 118–123. [Google Scholar] [CrossRef]
- Bolufer, M.; García-Carro, C.; Agraz, I.; Díez Miranda, I.; Jaramillo, J.; Arredondo, K.; Bury, R.; Ramos, N.; Azancot, M.A.; Gabaldón, A.; et al. Biopsia renal transyugular. La alternativa a la biopsia percutánea en pacientes de alto riesgo. Nefrología 2020, 40, 634–639. [Google Scholar] [CrossRef]
- Bolufer Cardona, M.; Soler Romeo, M.J.; McMahon, G.M. Transjugular Kidney Biopsy as a Safe Method to Increase the Etiological Diagnosis in Kidney Disease. Kidney Int. Rep. 2021, 6, 2535–2536. [Google Scholar] [CrossRef]
- St Jeor, J.D.; Reisenauer, C.J.; Andrews, J.C.; Fleming, C.J.; Misra, S.; Takahashi, E.A. Transjugular Renal Biopsy Bleeding Risk and Diagnostic Yield: A Systematic Review. J. Vasc. Interv. Radiol. 2020, 31, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Pombas, B.; Rodríguez, E.; Sánchez, J.; Radosevic, A.; Gimeno, J.; Busto, M.; Barrios, C.; Sans, L.; Pascual, J.; Soler, M.J. Risk factors associated with major complications after ultrasound-guided percutaneous renal biopsy of native kidneys. Kidney Blood Press Res. 2020, 45, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Nasic, S.; Segelmark, M. Clinical parameters predicting complications in native kidney biopsies. Clin. Kidney J. 2020, 13, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Halimi, J.M.; Gatault, P.; Longuet, H.; Barbet, C.; Bisson, A.; Sautenet, B.; Herbert, J.; Buchler, M.; Grammatico-Guillon, L.; Fauchier, L. Major bleeding and risk of death after percutaneous native kidney biopsies a french nationwide cohort study. Clin. J. Am. Soc. Nephrol. 2020, 15, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B. Approach to renal biopsy. Am. J. Kidney Dis. 2003, 42, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Whittier, W.L. Complications of the Percutaneous Kidney Biopsy. Adv. Chronic. Kidney Dis. 2012, 19, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tøndel, C.; Vikse, B.E.; Bostad, L.; Svarstad, E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin. J. Am. Soc. Nephrol. 2012, 7, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Eiro, M.; Katoh, T.W.T. Risk factors for bleeding complications in percutaneous renal biopsy. Clin. Exp. Nephrol. 2005, 9, 40–45. [Google Scholar] [CrossRef]
- Peters, B.; Andersson, Y.; Stegmayr, B.; Mölne, J.; Jensen, G.; Dahlberg, P.; Holm-Gunnarsson, I.; Ekberg, J.; Bjurström, K.; Haux, S.B.; et al. A study of clinical complications and risk factors in 1001 native and transplant kidney biopsies in Sweden. Acta Radiol. 2014, 55, 890–896. [Google Scholar] [CrossRef]
- Manno, C.; Strippoli, G.F.M.; Arnesano, L.; Bonifati, C.; Campobasso, N.; Gesualdo, L.; Schena, F.P. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int. 2004, 66, 1570–1577. [Google Scholar] [CrossRef]
- Diaz-Buxo, J.A.D.J.J. Complications of percutaneous renal biopsy: An analysis of 1000 consecutive biopsies. Clin. Nephrol. 1975, 4, 223–227. [Google Scholar] [PubMed]
- Christensen, J.; Lindequist, S.; Knudsen, D.U.; Pedersen, R.S. Ultrasound-guided renal biopsy with biopsy gun technique-efficacy and complications. Acta Radiol. 1995, 36, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Shidham, G.B.; Siddiqi, N.; Beres, J.A.; Logan, B.; Nagaraja, H.N.; Shidham, S.G.; Piering, W.F. Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology 2005, 10, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Corapi, K.M.; Chen, J.L.T.; Balk, E.M.; Gordon, C.E. Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am. J. Kidney Dis. 2012, 60, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Massak, M.; Czaplicki, M.; Young, E.; Sharma, S.; Chang, A.; Anand, P. Use a smartphone camera at the bedside to assess adequacy of kidney biopsies. J. Am. Soc. Nephrol. 2021, 32, 3024–3026. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Bonifati, C.; Torres, D.D.; Campobasso, N.; Schena, F.P. Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: A randomized controlled trial. Am. J. Kidney Dis. 2011, 57, 850–855. [Google Scholar] [CrossRef]
- Peters, B.; Hadimeri, H.; Mölne, J.; Nasic, S.; Jensen, G.; Stegmayr, B. Desmopressin (Octostim®) before a native kidney biopsy can reduce the risk for biopsy complications in patients with impaired renal function: A pilot study. Nephrology 2018, 23, 366–370. [Google Scholar] [CrossRef]
- Hogan, J.J.; Owen, J.G.; Blady, S.J.; Almaani, S.; Avasare, R.S.; Bansal, S.; Lenz, O.; Luciano, R.L.; Parikh, S.V.; Ross, M.J.; et al. The Feasibility and Safety of Obtaining Research Kidney Biopsy Cores in Patients with Diabetes. Clin. J. Am. Soc. Nephrol. 2020, 15, 1024–1026. [Google Scholar] [CrossRef]
- Leclerc, S.; Nadeau-Fredette, A.C.; Elftouh, N.; Lafrance, J.P.; Pichette, V.; Laurin, L.P. Use of Desmopressin Prior to Kidney Biopsy in Patients With High Bleeding Risk. Kidney Int. Rep. 2020, 5, 1180–1187. [Google Scholar] [CrossRef]
- Sousanieh, G.; Whittier, W.L.; Rodby, R.A.; Peev, V.; Korbet, S.M. Percutaneous Renal Biopsy Using an 18-Gauge Automated Needle Is Not Optimal. Am. J. Nephrol. 2021, 51, 982–987. [Google Scholar] [CrossRef]
- Waldo, B.; Korbet, S.M.; Freimanis, M.G.; Lewis, E.J. The value of post-biopsy ultrasound in predicting complications after percutaneous renal biopsy of native kidneys. Nephrol. Dial. Transplant. 2009, 24, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- de Mier, M.V.P.R.; Espinosa-Hernández, M.; Rodelo-Haad, C.; Motta EE de Gómez-Carrasco, J.; Ortega, R.; Aljama, P. Estudio prospectivo de las complicaciones asociadas a la biopsia percutánea en riñón nativo: Experiencia en un centro. Nefrologia 2014, 34, 383–387. [Google Scholar] [CrossRef]
- Li, Q.; Lin, X.; Zhang, X.; Samir, A.E.; Arellano, R.S. Imaging-Related Risk Factors for Bleeding Complications of US-Guided Native Renal Biopsy: A Propensity Score Matching Analysis. J. Vasc. Interv. Radiol. 2019, 30, 87–94. [Google Scholar] [CrossRef]
- Lees, J.S.; McQuarrie, E.P.; Mordi, N.; Geddes, C.C.; Fox, J.G.; Mackinnon, B. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin. Kidney J. 2017, 10, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.T.; Valdez-Ortiz, R.; González-Parra, C.; Espinoza-Dávila, E.; Morales-Buenrostro, L.E.; Correa-Rotter, R. Percutaneous renal biopsy of native kidneys: Efficiency, safety and risk factors associated with major complications. Arch Med Sci. 2011, 7, 823–831. [Google Scholar] [CrossRef]
- Bonani, M.; Seeger, H.; Weber, N.; Lorenzen, J.M.; Wüthrich, R.P.; Kistler, A.D. Safety of kidney biopsy when performed as an outpatient procedure. Kidney Blood Press Res. 2021, 46, 310–322. [Google Scholar] [CrossRef]
- Whittier, W.L.; Sayeed, K.; Korbet, S.M. Clinical factors influencing the decision to transfuse after percutaneous native kidney biopsy. Clin. Kidney J. 2016, 9, 102–107. [Google Scholar] [CrossRef][Green Version]
- Townsend, R.R.; Guarnieri, P.; Argyropoulos, C.; Blady, S.; Boustany-Kari, C.M.; Devalaraja-Narashimha, K.; Morton, L.; Mottl, A.K.; Patel, U.; Palmer, M.; et al. Rationale and design of the Transformative Research in Diabetic Nephropathy (TRIDENT) Study. Kidney Int. 2020, 97, 10–13. [Google Scholar] [CrossRef]

| Major Complications | Minor Complications |
|---|---|
| Need of transfusion | Pain |
| Embolization | Hematuria |
| Nephrectomy | Hematoma without blood transfusion |
| Death |
| Characteristics | All Patients |
|---|---|
| Patients (n) | 221 |
| Age (years) | 56.6 (±16.8) |
| Sex (Women/Men) (n, %) | 91 (41.2%)/130 (58.8%) |
| Hypertension (>140/90 mmHg) (n, %) | 128 (57.9%) |
| Diabetes Mellitus (n, %) | 52 (23.5%) |
| Weight (kg) | 75.8 (±24.6) |
| BMI (kg/m2) | 27.6 (±5.01) |
| Systolic blood pressure (mmHg) | 136 (±24.6) |
| Diastolic blood pressure (mmHg) | 76 (±15) |
| Creatinine (mg/dL) | 2.24 (±1.94) |
| Proteinuria (g/24 h) | 1.56 (0.506–3.59) |
| Urinary albumin/creatinine ratio (mg/g) | 613 (100.3–2300) |
| Urinary protein/creatinine ratio (mg/g) | 1153.8 (482.8–3156) |
| Platelets (n) | 250.995 (±89.742) |
| INR | 0.99 (±0.1) |
| PT (seconds) | 11.86 (±1.2) |
| Hb pre (g/dL) | 12.03 (±2.3) |
| Hb post (g/dL) | 11.3 (±2.3) |
| Microhematuria (n, %) | 100 (45.2%) |
| Kidney size (cm) | 11.04 (±1.2) |
| Cortical size (cm) | 1.67 (±0.64) |
| Technique (US-guided/transjugular) (n, %) | 213 (96.4%)/8 (3.6%) |
| Number of renal cores (n, %): | |
| 3 | 152 (68.8%) |
| 2 | 62 (28%) |
| 1 | 7 (3.2%) |
| Anticoagulants (n, %): | |
| Acenocoumarol | 7 (3.2%) |
| Heparin | 3 (1.4%) |
| Others | 5 (2.3%) |
| Antiplatelets (n, %): | |
| ASA 100 mg | 30 (13.6%) |
| ASA 300 mg | 1 (0.5%) |
| Clopidogrel | 5 (2.3%) |
| Characteristics | Number of Kidney Cores | p | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Patients (n, %) | 7 (3.2%) | 62 (28.1%) | 151 (68.8%) | - |
| Age (years) | 45.4 (±17.9) | 56 (±17.2) | 57.4 (±16.5) | 0.171 |
| Sex (Women/Men) (n, %) | 2 (28.6%)/ 5 (71.4%) | 29 (46.8%)/ 33 (53.2%) | 60 (39.5%)/ 92 (60.5%) | 0.486 |
| Hypertension (n, %) | 5 (71.4%) | 37 (59.7%) | 86 (56.6%) | 0.699 |
| Diabetes Mellitus (n, %) | 0 (0%) | 15 (24.2%) | 37 (24.3%) | 0.329 |
| Weight (kg) | 68.3 (±19.2) | 74.8 (±15.3) | 76.6 (±15.3) | 0.321 |
| BMI (kg/m2) | 25.4 (±2.8) | 27.1 (±5) | 27.9 (±5.1) | 0.298 |
| Systolic blood pressure (mmHg) | 151.3 (±25.9) | 134.3 (±28.2) | 135.4 (±22.8) | 0.22 |
| Diastolic blood pressure (mmHg) | 87.7 (±19.2) | 76.6 (±15.5) | 75.2 (±15) | 0.101 |
| Creatinine (mg/dL) | 4.5 (±3.3) | 2.5 (±2.3) | 2 (±1.6) | 0.002 |
| Proteinuria (g/24 h) | 2.8 [2.3–7.8] | 3 [2–4] | 2.8 [2.2–3.4] | 0.948 |
| Urinary albumin/creatinine ratio (mg/g) | 387.4 [51.7–826.5] | 1577.2 [950–2204.4] | 1616 [1243–1989.1] | 0.403 |
| Urinary protein/creatinine ratio (mg/g) | 131.1 [57.2–2005.1] | 2730.8 [1757.9–3703.6] | 2740.6 [2080.6–3400.7] | 0.619 |
| Platelets (n) | 260,428 (±58,266) | 238,725 (±103,365) | 255,565 (±84,828) | 0.444 |
| INR | 0.99 (±0.1) | 0.99 (±0.1) | 0.98 (±0.1) | 0.807 |
| PT (seconds) | 11.9 (±1.2) | 11.9 (±1.1) | 11.8 (±1.2) | 0.843 |
| Hb pre (g/dL) | 12.5 (±2.6) | 11.7 (±2.4) | 12.1 (±2.3) | 0.466 |
| Hb post (g/dL) | 11.4 (±2.7) | 11.1 (±2.6) | 11.4 (±2.1) | 0.784 |
| Microhematuria (n. %) | 4 (57.1%) | 26 (41.9%) | 70 (46.4%) | 0.689 |
| Kidney size (cm) | 10.7 (±1.2) | 11.2 (±1.3) | 11 (±1.2) | 0.449 |
| Cortical size (cm) | 1.7 (±0.2) | 1.7 (±0.3) | 1.7 (±0.3) | 0.985 |
| Left kidney/Right kidney (n, %) | 3 (42.9%)/ 4 (57.1%) | 19 (31.1%)/ 42 (68.9%) | 77 (51%)/ 74 (49%) | 0.031 |
| Technique (US-guided/ Transjugular) (n, %) | 7 (100%)/ 0 | 58 (93.5%)/ 4 (6.5%) | 148 (97.4%)/ 4 (2.6%) | 0.348 |
| Transfusion (n, %) | 2 (28.6%) | 9 (14.5%) | 14 (9.2%) | 0.184 |
| Anticoagulants (n. %): | ||||
| Acenocoumarol | 0 | 3 (4.8%) | 4 (2.6%) | 0.701 |
| Heparin | 0 | 1 (1.6%) | 2 (1.3%) | |
| Others | 0 | 3 (4.8%) | 2 (1.3%) | |
| Antiplatelets (n. %): | ||||
| ASA 100 mg | 0 | 6 (9.7%) | 24 (15.8%) | |
| ASA 300 mg | 0 | 0 | 1 (0.7%) | 0.743 |
| Clopidogrel | 0 | 2 (3.2%) | 3 (2%) | |
| Characteristics | Without Complications | With Complications | p |
|---|---|---|---|
| Patients (n, %) | 183 (82.8%) | 38 (17.2%) | - |
| Age (years) | 57.7 (±16.3) | 51.4 (±18.2) | 0.034 |
| Sex (Women/Men) (n, %) | 73 (39.9%)/ 110 (40.1%) | 18 (47.4%)/ 20 (52.6%) | 0.469 |
| Hypertension (n, %) | 108 (59%) | 20 (52.6%) | 0.476 |
| Diabetes Mellitus (n, %) | 10 (26.3%) | 42 (23%) | 0.676 |
| Weight (kg) | 76.9 (±15.4) | 70.5 (±14.5) | 0.022 |
| BMI (kg/m2) | 27.8 (±4.97) | 26.2 (±5.1) | 0.096 |
| Systolic blood pressure (mmHg) | 134.5 (±23.8) | 141 (±27.5) | 0.134 |
| Diastolic blood pressure (mmHg) | 75.5 (±15.6) | 78.5 (±14.2) | 0.262 |
| Creatinine (mg/dL) | 2.26 (±1.9) | 2.15 (±1.97) | 0.751 |
| Proteinuria (g/24 h) | 2.85 (2.27–3.43] | 3.01 (1.88–4.15] | 0.809 |
| Urinary albumin/creatinine ratio (mg/g) | 1556.57 (1214.84–1898.31] | 1622.96 (856.65–2389.27] | 0.875 |
| Urinary protein/creatinine ratio (mg/g) | 2653.11 (2065.71–3240.52] | 2907.7 (1613.22–4202.19] | 0.725 |
| Platelets (n) | 245,295 (±84,011) | 278,447 (±110,613) | 0.038 |
| INR | 0.99 (±0.1) | 0.96 (0.09) | 0.07 |
| PT (seconds) | 11.92 (±1.22) | 11.54 (±1.08) | 0.05 |
| Hb pre (g/dL) | 12.07 (±2.27) | 11.84 (±2.56) | 0.057 |
| Hb post (g/dL) | 11.4 (±2.2) | 10.8 (±2.5) | 0.14 |
| Microhematuria (n, %) | 84 (46.2%) | 16 (42.1%) | 0.722 |
| Kidney size (cm) | 11.08 (±1.25) | 10.84 (±1.09) | 0.277 |
| Cortical size (cm) | 1.67 (±0.69) | 1.64 (±0.32) | 0.807 |
| Left kidney/Right kidney (n, %) | 85 (47%)/ 96 (53%) | 24 (63.2%)/ 14 (36.8%) | 0.286 |
| Technique (US-guided/transjugular) (n, %) | 177 (96.7%)/ 6 (3.3%) | 36 (94.7%)/ 2 (5.3%) | 0.628 |
| Transfusion (n, %) | 15 (8.2%) | 10 (26.3%) | 0.003 |
| Number of renal cores | |||
| (n, %): | |||
| 3 | 130 (71%) | 22 (57.9%) | |
| 2 | 50 (27.3%) | 12 (31.6%) | 0.012 |
| 1 | 3 (1.6%) | 4 (10.5%) | |
| Anticoagulants (n, %): | |||
| Acenocoumarol | 7 (3.8%) | 0 | |
| Heparin | 3 (1.6%) | 0 | 0.342 |
| Others | 5 (2.7%) | 0 | |
| Antiplatelets (n, %): | |||
| AAS 100 mg | 24 (13.1%) | 6 (15.8%) | |
| AAS 300 mg | 1 (0.5%) | 0 | 0.935 |
| Clopidogrel | 4 (2.2%) | 1 (2.6%) |
| Characteristics | Without Complications | Minor Complications | Major Complications | p |
|---|---|---|---|---|
| Patients (n, %) | 183 (82.8%) | 29 (13.1%) | 9 (4.1%) | - |
| Age (years) | 57.7 (±16.3) | 52.9 (±17.8) | 46.6 (±20) | 0.065 |
| Sex (Women/Men) (n, %) | 73 (39.9%)/ 110 (40.1%) | 13 (44.8%)/ 16 (55.2%) | 5 (55.6%)/ 4 (44.4%) | 0.591 |
| Hypertension (n, %) | 108 (59%) | 17 (58.6%) | 3 (33.3%) | 0.312 |
| Diabetes Mellitus (n, %) | 10 (26.3%) | 9 (31%) | 1 (11.1%) | 0.425 |
| Weight (kg) | 76.9 (±15.4) | 71.04 (±14.4) | 68.97 (±15.3) | 0.069 |
| BMI (kg/m2) | 27.8 (±4.97) | 26.93 (±5.36) | 24.13 (±3.73) | 0.097 |
| Systolic blood pressure (mmHg) | 134.5 (±23.8) | 144.24 (±24.5) | 130.67 (±35.4) | 0.114 |
| Diastolic blood pressure (mmHg) | 75.5 (±15.6) | 79.24 (±13.9) | 76.22 (±16) | 0.468 |
| Creatinine (mg/dL) | 2.26 (±1.9) | 1.93 (±1.65) | 2.86 (±2.76) | 0.432 |
| Proteinuria (g/24 h) | 2.85 (2.27–3.43) | 2.85 (1.57–4.2) | 3.47 (0.55–6.39) | 0.896 |
| Urinary albumin/creatinine ratio (mg/g) | 1556.57 (1214.84–1898.31) | 1412.1 (789.6–2034.6) | 2255.6 (671.1–5182.3) | 0.632 |
| Urinary protein/creatinine ratio (mg/g) | 2653.11 (2065.71–3240.52) | 2536.5 (1332–3741.02) | 4114.2 (536.9–8765.2) | 0.562 |
| Platelets (n) | 245,295 (±84,011) | 270,517 (±80,529) | 304,000 (±181,464) | 0.072 |
| INR | 0.99 (±0.1) | 0.94 (±0.07) | 1.02 (±0.11) | 0.028 |
| PT (seconds) | 11.92 (±1.22) | 11.33 (±0.94) | 12.2 (±1.29) | 0.032 |
| Hb pre (g/dL) | 12.07 (±2.27) | 12.26 (±2.67) | 10.49 (±1.64) | 0.115 |
| Hb post (g/dL) | 11.4 (±2.2) | 11.28 (±2.45) | 9.24 (±2.13) | 0.02 |
| Microhematuria (n, %) | 84 (46.2%) | 12 (41.4%) | 4 (44.4%) | 0.89 |
| Kidney size (cm) | 11.08 (±1.25) | 10.93 (±1.1) | 10.57 (±1.1) | 0.413 |
| Cortical size (cm) | 1.67 (±0.69) | 1.6 (±0.32) | 1.75 (±0.33) | 0.829 |
| Left kidney/Right kidney (n, %) | 85 (47%)/ 96 (53%) | 10 (34.5%)/ 19 (65.5%) | 4 (44.4%)/ 5 (55.6%) | 0.455 |
| Technique (US-guided/ Transjugular) (n, %) | 177 (96.7%)/ 6 (3.3%) | 29 (100%)/ 0 | 7 (77.8%)/ 2 (22.2%) | 0.006 |
| Transfusion (n, %) | 15 (8.2%) | 5 (17.2%) | 5 (55.6%) | <0.001 |
| Number of renal cores (n, %): | ||||
| 3 | 130 (71%) | 17 (58.6%) | 5 (55.6%) | |
| 2 | 50 (27.3%) | 9 (31%) | 3 (33.3%) | 0.064 |
| 1 | 3 (1.6%) | 3 (10.3%) | 1 (11.1%) | |
| Anticoagulants (n. %): | ||||
| Acenocoumarol | 7 (3.8%) | 0 | 0 | |
| Heparin | 3 (1.6%) | 0 | 0 | 0.765 |
| Others | 5 (2.7%) | 0 | 0 | |
| Antiplatelets (n. %): | ||||
| ASA 100 mg | 24 (13.1%) | 6 (20.7%) | 0 | |
| ASA 300 mg | 1 (0.5%) | 0 | 0 | 0.378 |
| Clopidogrel | 4 (2.2%) | 0 | 1 (11.1%) |
| Variable | OR | CI (95%) | Lateral Significance (p) |
|---|---|---|---|
| PT (seconds) * | 1.36 | 0.94–1.97 | 0.1 |
| Renal cores (1 core vs. 2/3 cores) | 7.09 | 1.34–37.04 | 0.021 |
| Age (years) * | 1.01 | 0.99–1.04 | 0.299 |
| Platelets (n) * | 1 | 1–1 | 0.130 |
| Weight (kg) * | 1.02 | 0.99–1.04 | 0.245 |
| Hb pre (g/dL) * | 1.13 | 0.94–1.36 | 0.2 |
| Study | Country | Year | Type of Study | Number of Patients | Type of Needles | Number of Kidney Cores | Complications Regarding Number of Kidney Cores |
|---|---|---|---|---|---|---|---|
| Singh S, et al. [25] | United States | 2021 | Prospective | 20 | 18G (95%) 16G (5%) | From 2 to 6 cores (2.9) | No data |
| Manno et al. [26] | Italy | 2011 | Randomized Clinical Trial | 162 | 16G | 2 cores | No data |
| Peters et al. [27] | Sweden | 2018 | Prospective | 576 | 16G or 18G | From 2 to 3 cores (Unknown) | No differences |
| Pombas et al. [12] | Spain | 2020 | Retrospective | 661 | 16G | 2 cores | No data |
| Hogan et al. [28] | United States | 2020 | Prospective | 160 | 16G (54%) 18G (44%) | From 1 to 4 cores (Unknown) | No differences |
| Leclerc et al. [29] | Canada | 2020 | Retrospective | 413 | 16G (98%) 18G (2%) | 2 cores (86%) 1 core (14%) | No data |
| Sousanieh et al. [30] | United States | 2020 | Prospective | 592 | 14G (57%) 16G (43%) | From 1 to 3 cores (2.2–2.3) | No data |
| Waldo et al. [31] | United States | 2009 | Prospective | 162 | 14G | No data | No data |
| Pendon-Ruiz de Mier et al. [32] | Spain | 2014 | Prospective | 241 | 16G | From 1 to 2 cores (unknown) | No differences |
| Li et al. [33] | China | 2018 | Retrospective | 551 | 16G or 18G (unknown) | From 1 to 4 cores (unknown) | No differences |
| Lees et al. [34] | UK | 2017 | Retrospective | 2563 | 16G | From 1 to 3 cores (unknown) | No data |
| Torres et al. [35] | Mexico | 2011 | Retrosepctive | 623 | 16G | No data | No data |
| Current study | Spain | 2021 | Retrospective | 221 | 16G | 1 core (3.2%) 2 cores (28.1%) 3 cores (68.8%) | More complications in patients with one kidney core obtained. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermejo, S.; García-Carro, C.; Mast, R.; Vergara, A.; Agraz, I.; León, J.C.; Bolufer, M.; Gabaldon, M.-A.; Serón, D.; Bestard, O.; et al. Safety of Obtaining an Extra Biobank Kidney Biopsy Core. J. Clin. Med. 2022, 11, 1459. https://doi.org/10.3390/jcm11051459
Bermejo S, García-Carro C, Mast R, Vergara A, Agraz I, León JC, Bolufer M, Gabaldon M-A, Serón D, Bestard O, et al. Safety of Obtaining an Extra Biobank Kidney Biopsy Core. Journal of Clinical Medicine. 2022; 11(5):1459. https://doi.org/10.3390/jcm11051459
Chicago/Turabian StyleBermejo, Sheila, Clara García-Carro, Richard Mast, Ander Vergara, Irene Agraz, Juan Carlos León, Monica Bolufer, Maria-Alejandra Gabaldon, Daniel Serón, Oriol Bestard, and et al. 2022. "Safety of Obtaining an Extra Biobank Kidney Biopsy Core" Journal of Clinical Medicine 11, no. 5: 1459. https://doi.org/10.3390/jcm11051459
APA StyleBermejo, S., García-Carro, C., Mast, R., Vergara, A., Agraz, I., León, J. C., Bolufer, M., Gabaldon, M.-A., Serón, D., Bestard, O., & Soler, M. J. (2022). Safety of Obtaining an Extra Biobank Kidney Biopsy Core. Journal of Clinical Medicine, 11(5), 1459. https://doi.org/10.3390/jcm11051459






