Low Incidence of Hepatocellular Carcinoma after Antiviral Therapy in Patients with Chronic Hepatitis C and Hemophilia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Approved DAAs
2.3. Assessment of Efficacy
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
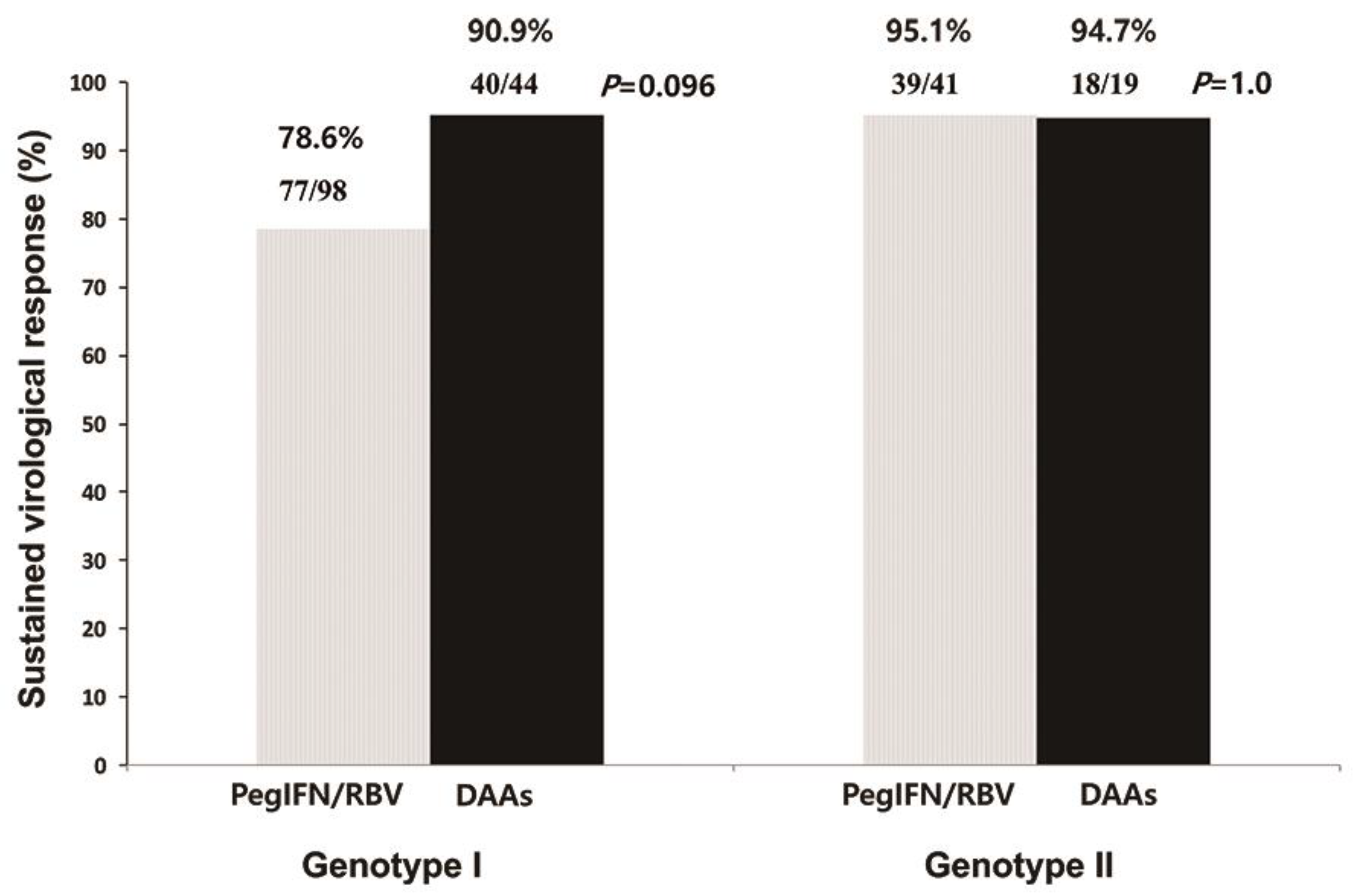
3.2. Antiviral Response
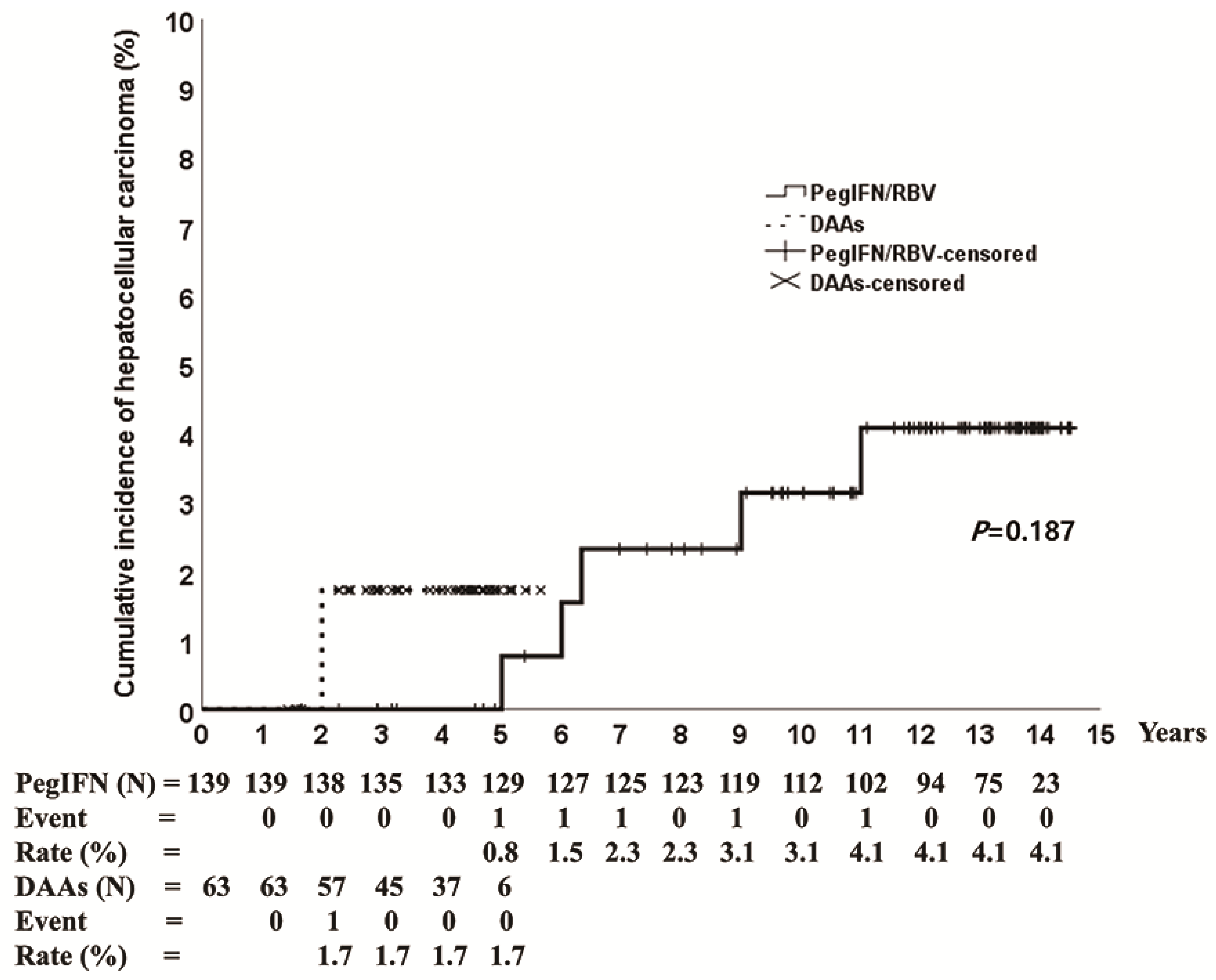
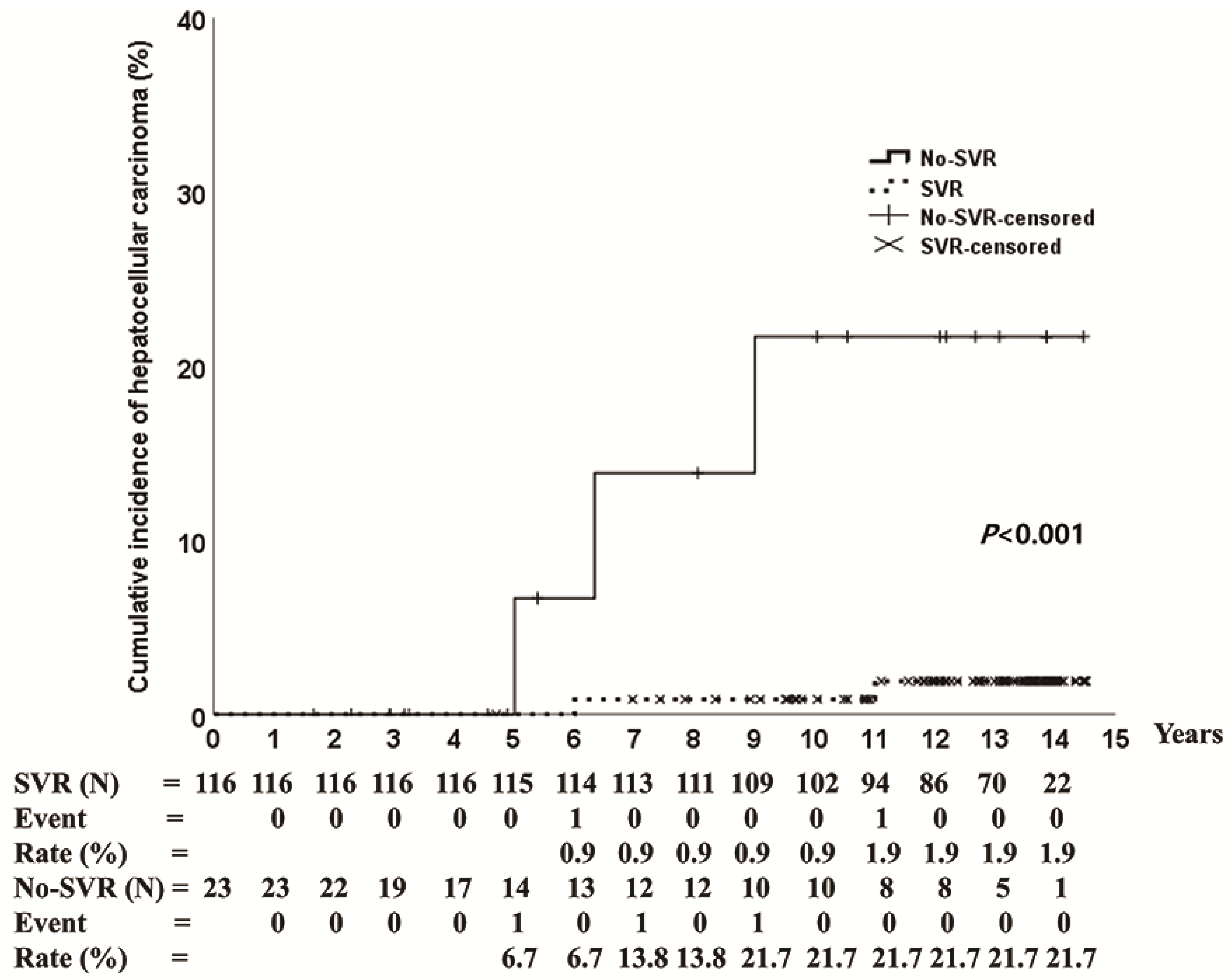
3.3. HCC Incidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Saito, I.; Miyamura, T.; Ohbayashi, A.; Harada, H.; Katayama, T.; Kikuchi, S.; Watanabe, Y.; Koi, S.; Onji, M.; Ohta, Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 1990, 87, 6547–6549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, M.W.; Shiffman, M.L.; Reddy, K.R.; Smith, C.; Marinos, G.; Gonçales, F.L.; Häussinger, D.; Diago, M.; Carosi, G.; Dhumeaux, D.; et al. Peginterferon Alfa-2a plus Ribavirin for Chronic Hepatitis C Virus Infection. N. Engl. J. Med. 2002, 347, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Hadziyannis, S.J.; Sette, H., Jr.; Morgan, T.R.; Balan, V.; Diago, M.; Marcellin, P.; Ramadori, G.; Bodenheimer, H., Jr.; Bernstein, D.; Rizzetto, M.; et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 2004, 140, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Perez-Olmeda, M.; Nunez, M.; Romero, M.; Gonzalez, J.; Castro, A.; Arribas, J.R.; Pedreira, J.; Barreiro, P.; Garcia-Samaniego, J.; Martín-Carbonero, L.; et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. Aids 2003, 17, 1023–1028. [Google Scholar] [CrossRef]
- Manns, M.P.; Pockros, P.J.; Norkrans, G.; Smith, C.I.; Morgan, T.R.; Haussinger, D.; Shiffman, M.L.; Hadziyannis, S.J.; Schmidt, W.N.; Jacobson, I.M.; et al. Long-term clearance of hepatitis C virus following interferon alpha-2b or peginterferon alpha-2b, alone or in combination with ribavirin. J. Viral Hepat. 2013, 20, 524–529. [Google Scholar] [CrossRef]
- Ferenci, P. Treatment of hepatitis C in difficult-to-treat patients. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 284–292. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Degasperi, E.; Colombo, M.; Aghemo, A. Direct-acting antivirals: The endgame for hepatitis C? Curr. Opin. Virol. 2017, 24, 31–37. [Google Scholar] [CrossRef]
- Spengler, U. Direct antiviral agents (DAAs)—A new age in the treatment of hepatitis C virus infection. Pharmacol. Ther. 2018, 183, 118–126. [Google Scholar] [CrossRef]
- Seeff, L.B. Natural history of chronic hepatitis C. Hepatology 2002, 36, S35–S46. [Google Scholar]
- Yee, T.T.; Griffioen, A.; Sabin, C.A.; Dusheiko, G.; Lee, C.A. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut 2000, 47, 845–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Putte, D.E.F.; Makris, M.; Fischer, K.; Yee, T.T.; Kirk, L.; van Erpecum, K.J.; Patch, D.; Posthouwer, D.; Mauser-Bunschoten, E.P. Long-term follow-up of hepatitis C infection in a large cohort of patients with inherited bleeding disorders. J. Hepatol. 2014, 60, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Witkop, M.L.; Peerlinck, K.; Luxon, B.A. Medical co-morbidities of patients with haemophilia: Pain, obesity and hepatitis C. Haemophilia 2016, 22 (Suppl 5), 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Lee, H.W.; Lee, Y.J.; Park, S.J.; Yoo, K.Y.; Kim, H.J. Highly effective peginterferon α-2a plus ribavirin combination therapy for chronic hepatitis C in hemophilia in Korea. Clin. Mol. Hepatol. 2015, 21, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Yoo, K.Y.; Won, J.W.; Kim, H.J. Direct Acting Antiviral Agents in Korean Patients with Chronic Hepatitis C and Hemophilia Who Are Treatment-Naïve or Treatment-Experienced. Gut Liver 2017, 11, 721–727. [Google Scholar] [CrossRef]
- Mancuso, M.E.; Linari, S.; Santagostino, E.; Bartolozzi, D.; D’Ambrosio, R.; Borghi, M.; Lampertico, P.; Peyvandi, F.; Castaman, G.; Aghemo, A. High rate of sustained virological response with direct-acting antivirals in haemophiliacs with HCV infection: A multicenter study. Liver Int. 2020, 40, 1062–1068. [Google Scholar] [CrossRef]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Janjua, N.Z.; Chong, M.; Kuo, M.; Woods, R.; Wong, J.; Yoshida, E.M.; Sherman, M.; Butt, Z.A.; Samji, H.; Cook, D.; et al. Long-term effect of sustained virological response on hepatocellular carcinoma in patients with hepatitis C in Canada. J. Hepatol. 2017, 66, 504–513. [Google Scholar] [CrossRef]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Rustgi, V.K.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.-H.; Albrecht, J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958–965. [Google Scholar] [CrossRef]
- Kumada, H.; Suzuki, Y.; Ikeda, K.; Toyota, J.; Karino, Y.; Chayama, K.; Kawakami, Y.; Ido, A.; Yamamoto, K.; Takaguchi, K.; et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology 2014, 59, 2083–2091. [Google Scholar] [CrossRef]
- Suda, G.; Ogawa, K.; Yamamoto, Y.; Katagiri, M.; Furuya, K.; Kumagai, K.; Konno, J.; Kimura, M.; Kawagishi, N.; Ohara, M.; et al. Retreatment with sofosbuvir, ledipasvir, and add-on ribavirin for patients who failed daclatasvir and asunaprevir combination therapy. J. Gastroenterol. 2017, 52, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Itakura, J.; Kurosaki, M.; Hasebe, C.; Osaki, Y.; Joko, K.; Yagisawa, H.; Sakita, S.; Okushin, H.; Satou, T.; Hisai, H.; et al. Complex Pattern of Resistance-Associated Substitutions of Hepatitis C Virus after Daclatasvir/Asunaprevir Treatment Failure. PLoS ONE 2016, 11, e0165339. [Google Scholar] [CrossRef] [PubMed]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Kim, I.H.; Jeong, S.H.; Cho, Y.K.; Lee, J.H.; Jin, Y.J.; Lee, D.; Suh, D.J.; Han, K.H.; Park, N.H.; et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013, 33, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Tsuchiya, K.; Tamaki, N.; Hirayama, I.; Tanaka, T.; Sato, M.; Yasui, Y.; Hosokawa, T.; Ueda, K.; Kuzuya, T.; et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010, 52, 518–527. [Google Scholar] [CrossRef]
- Ogawa, E.; Furusyo, N.; Kajiwara, E.; Takahashi, K.; Nomura, H.; Maruyama, T.; Tanabe, Y.; Satoh, T.; Nakamuta, M.; Kotoh, K.; et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: A prospective, multicenter study. J. Hepatol. 2013, 58, 495–501. [Google Scholar] [CrossRef]
- Kumada, T.; Toyoda, H.; Kiriyama, S.; Sone, Y.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Atsumi, H.; Takagi, M.; Nakano, S.; et al. Incidence of hepatocellular carcinoma in hepatitis C carriers with normal alanine aminotransferase levels. J. Hepatol. 2009, 50, 729–735. [Google Scholar] [CrossRef]
- Triemstra, M.; Rosendaal, F.R.; Smit, C.; Van der Ploeg, H.M.; Briet, E. Mortality in Patients with Hemophilia: Changes in a Dutch Population from 1986 to 1992 and 1973 to 1986. Ann. Intern. Med. 1995, 123, 823–827. [Google Scholar] [CrossRef]

| Variables | Genotype 1 (n = 142) | p Value | Genotype 2 (n = 60) | p Value | ||
|---|---|---|---|---|---|---|
| PEG-IFN (n = 98) | DAA (n = 44) | PEG-IFN (n = 41) | DAA (n = 19) | |||
| Male (%) | 98 (100) | 44 (100) | 41 (100) | 19 (100) | ||
| Mean age, year (range) | 48 (33–83) | 50 (33–79) | 0.307 | 47 (34–83) | 51 (34–83) | 0.119 |
| BMI, kg/m2 (range) | 22.9 (20.6–41.6) | 23.1 (18.7–31.6) | 0.750 | 23.4 (16.4–34.5) | 22.0 (16.9–29.7) | 0.233 |
| ALT, IU/L (range) | 65 (38–255) | 66 (37–238) | 0.905 | 78 (30–377) | 68 (28–377) | 0.648 |
| Platelet, ×103/mm3 (range) | 242 (98–428) | 223 (98–404) | 0.110 | 252 (125–473) | 256 (140–385) | 0.372 |
| HCV RNA, log10 IU/mL (range) | 6.5 (4.1–8.2) | 6.3 (3.7–7.1) | 0.174 | 5.9 (3.7–7.6) | 5.6 (3.7–7.6) | 0.824 |
| Liver cirrhosis (%) | 6 (6.1) | 5 (11.4) | 0.280 | 3 (7.3) | 2 (10.5) | 0.648 |
| HIV co-infection (%) | 1 (1.0) | 0 (2.3) | 1.000 | 0 | 0 | - |
| HBV co-infection (%) | 4 (4.1) | 3 (6.8) | 0.677 | 2 (4.9) | 0 (0.0) | 1.000 |
| Follow-up period, month (range) | 141 (20–174) | 47 (19–68) | <0.001 | 152 (35–174) | 45 (17–59) | <0.001 |
| Patient | Drug | Age, Years | BMI, kg/m2 | Genotype | HCV RNA, log10 IU/mL | ALT, IU/L | Cirrhosis | Duration * | SVR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PegIFN/RBV | 66 | 22.9 | 1a | 7.4 | 69 | 1 | 72 | 1 |
| 2 | PegIFN/RBV | 83 | 17.2 | 1b | 7.1 | 88 | 0 | 108 | 0 |
| 3 | PegIFN/RBV | 74 | 22.6 | 1b | 6.5 | 104 | 0 | 76 | 0 |
| 4 | PegIFN/RBV | 83 | 22.0 | 2a, 2c | 5.0 | 248 | 1 | 132 | 1 |
| 5 | PegIFN/RBV | 79 | 22.5 | 1b | 6.3 | 32 | 1 | 60 | 0 |
| 6 | DAA † | 79 | 23.1 | 1b | 6.2 | 35 | 1 | 24 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.J.; Yoo, S.H.; Kim, S.; Cho, Y.Y.; Yoo, K.Y.; Kim, H.J.; Lee, H.W. Low Incidence of Hepatocellular Carcinoma after Antiviral Therapy in Patients with Chronic Hepatitis C and Hemophilia. J. Clin. Med. 2022, 11, 1451. https://doi.org/10.3390/jcm11051451
Kim IJ, Yoo SH, Kim S, Cho YY, Yoo KY, Kim HJ, Lee HW. Low Incidence of Hepatocellular Carcinoma after Antiviral Therapy in Patients with Chronic Hepatitis C and Hemophilia. Journal of Clinical Medicine. 2022; 11(5):1451. https://doi.org/10.3390/jcm11051451
Chicago/Turabian StyleKim, In Jung, Sung Hwan Yoo, Sora Kim, Young Youn Cho, Ki Young Yoo, Hyung Joon Kim, and Hyun Woong Lee. 2022. "Low Incidence of Hepatocellular Carcinoma after Antiviral Therapy in Patients with Chronic Hepatitis C and Hemophilia" Journal of Clinical Medicine 11, no. 5: 1451. https://doi.org/10.3390/jcm11051451
APA StyleKim, I. J., Yoo, S. H., Kim, S., Cho, Y. Y., Yoo, K. Y., Kim, H. J., & Lee, H. W. (2022). Low Incidence of Hepatocellular Carcinoma after Antiviral Therapy in Patients with Chronic Hepatitis C and Hemophilia. Journal of Clinical Medicine, 11(5), 1451. https://doi.org/10.3390/jcm11051451






