A Retrospective Study of Postictal Suppression during Electroconvulsive Therapy
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Electroconvulsive Therapy
2.3. Postictal Suppression Determination
2.4. Assessments
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. ECT Parameters
3.3. Relationships between Postictal Suppression during the First ECT Session and Clinical Response
3.4. Relationships between Postictal Suppression at Each ECT Session and Clinical Response
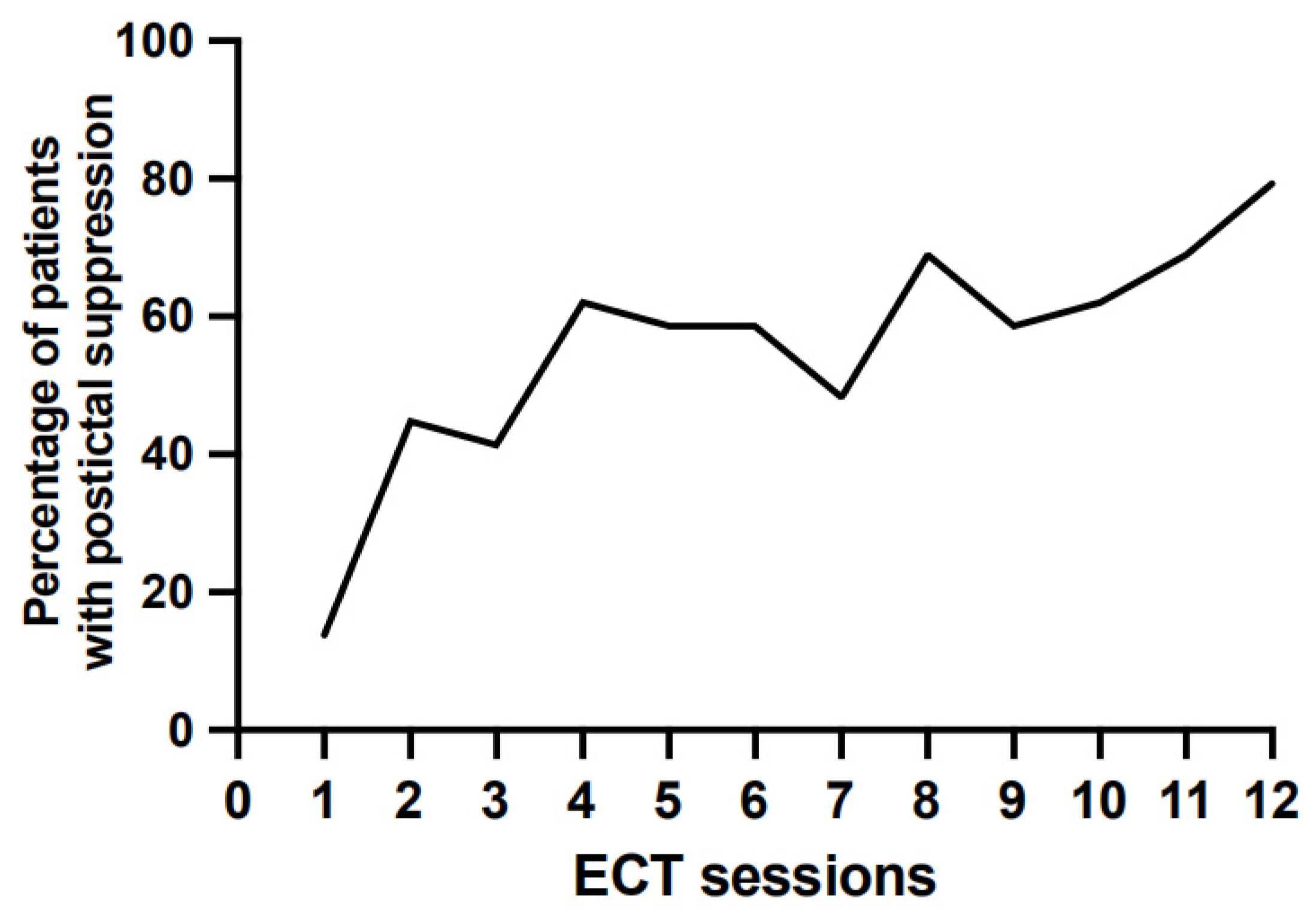
3.5. Postictal Suppression throughout Treatment
3.6. Relationships between Postictal Suppression and Cognitive Side Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haq, A.U.; Sitzmann, A.F.; Goldman, M.L.; Maixner, D.F.; Mickey, B.J. Response of Depression to Electroconvulsive Therapy: A Meta-Analysis of Clinical Predictors. J. Clin. Psychiatry 2015, 76, 1374–1384. [Google Scholar] [CrossRef]
- Weiss, A.; Hussain, S.; Ng, B.; Sarma, S.; Tiller, J.; Waite, S.; Loo, C. Royal Australian and New Zealand College of Psychiatrists Professional Practice Guidelines for the Administration of Electroconvulsive Therapy. Aust. N. Z. J. Psychiatry 2019, 53, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Francis-Taylor, R.; Ophel, G.; Martin, D.; Loo, C. The Ictal EEG in ECT: A Systematic Review of the Relationships between Ictal Features, ECT Technique, Seizure Threshold and Outcomes. Brain Stimul. 2020, 13, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Sackeim, H.A. Convulsant and Anticonvulsant Properties of Electroconvulsive Therapy: Towards a Focal Form of Brain Stimulation. Clin. Neurosci. Res. 2004, 4, 39–57. [Google Scholar] [CrossRef]
- Krystal, A.D.; Weiner, R.D.; Coffey, C.E. The Ictal EEG as a Marker of Adequate Stimulus Intensity with Unilateral ECT. J. Neuropsychiatry Clin. Neurosci. 1995, 7, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Krystal, A.D.; Coffey, C.E.; Weiner, R.D.; Holsinger, T. Changes in Seizure Threshold Over the Course of Electroconvulsive Therapy Affect Therapeutic Response and Are Detected by Ictal EEG Ratings. JNP 1998, 10, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kimball, J.N.; Rosenquist, P.B.; Dunn, A.; McCall, V. Prediction of Antidepressant Response in Both 2.25×threshold RUL and Fixed High Dose RUL ECT. J. Affect. Disord. 2009, 112, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Yamada, A.; Shinagawa, Y.; Nakano, Y.; Watanabe, N.; Akechi, T.; Furukawa, T.A. Ictal Physiological Characteristics of Remitters during Bilateral Electroconvulsive Therapy. Psychiatry Res. 2011, 185, 462–464. [Google Scholar] [CrossRef]
- Nobler, M.S.; Luber, B.; Moeller, J.R.; Katzman, G.P.; Prudic, J.; Devanand, D.P.; Dichter, G.S.; Sackeim, H.A. Quantitative EEG During Seizures Induced by Electroconvulsive Therapy: Relations to Treatment Modality and Clinical Features. I. Global Analyses. J. ECT 2000, 16, 211–228. [Google Scholar] [CrossRef]
- Jagadisha, B.N.; Gangadhar, B.; Janakiramiah, N.; Girish, K.; Ramakrishnan, A. Post-Seizure EEG Fractal Dimension and Spectral Power Predict Antidepressant Response to Unilateral ECT. Indian J. Psychiatry 2003, 45, 16–20. [Google Scholar]
- Suppes, T.; Webb, A.; Carmody, T.; Gordon, E.; Gutierrez-Esteinou, R.; Hudson, J.I.; Pope, H.G. Is Postictal Electrical Silence a Predictor of Response to Electroconvulsive Therapy? J. Affect. Disord. 1996, 41, 55–58. [Google Scholar] [CrossRef]
- Gangadhar, B.N.; Subbakrishna, D.K.; Janakiramaiah, N.; Motreja, S.; Dutt, D.N.; Paramehwara, G. Post-Seizure EEG Fractal Dimension of First ECT Predicts Antidepressant Response at Two Weeks. J. Affect. Disord. 1999, 52, 235–238. [Google Scholar] [CrossRef]
- Nobler, M.S.; Sackeim, H.A.; Solomou, M.; Luber, B.; Devanand, D.P.; Prudic, J. EEG Manifestations during ECT: Effects of Electrode Placement and Stimulus Intensity. Biol. Psychiatry 1993, 34, 321–330. [Google Scholar] [CrossRef]
- Perera, T.D.; Luber, B.; Nobler, M.S.; Prudic, J.; Anderson, C.; Sackeim, H.A. Seizure Expression During Electroconvulsive Therapy: Relationships with Clinical Outcome and Cognitive Side Effects. Neuropsychopharmacology 2004, 29, 813–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, H.; Fujita, A.; Sato, K.; Arahata, K.; Otsuki, K.; Hori, M.; Mochida, Y.; Uchida, M.; Yamada, T.; Akechi, T.; et al. Postictal Suppression Correlates with Therapeutic Efficacy for Depression in Bilateral Sine and Pulse Wave Electroconvulsive Therapy. Psychiatry Clin. Neurosci. 2007, 61, 168–173. [Google Scholar] [CrossRef]
- Thase, M.E.; Rush, A.J. When at First You Don’t Succeed: Sequential Strategies for Antidepressant Nonresponders. J. Clin. Psychiatry 1997, 58 (Suppl. S13), 23–29. [Google Scholar] [PubMed]
- Hamilton, M. Rating Depressive Patients. J. Clin. Psychiatry 1980, 41, 21–24. [Google Scholar]
- Sackeim, H.A.; Long, J.; Luber, B.; Moeller, J.R.; Prohovnik, I.; Devanand, D.P.; Nobler, M.S. Physical Properties and Quantification of the ECT Stimulus: I. Basic Principles. Convuls. Ther. 1994, 10, 93–123. [Google Scholar]
- Kellner, C.H.; Knapp, R.; Husain, M.M.; Rasmussen, K.; Sampson, S.; Cullum, M.; McClintock, S.M.; Tobias, K.G.; Martino, C.; Mueller, M.; et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: Randomized trial. Br. J. Psychiatry J. Ment. Sci. 2010, 196, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Grober, E.; Buschke, H.; Crystal, H.; Bang, S.; Dresner, R. Screening for Dementia by Memory Testing. Neurology 1988, 38, 900. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Wilson, B.A.; Kopelman, M.D. Handbook of Memory Disorders; John Wiley and Sons Ltd.: London, UK, 1995. [Google Scholar]
- Brickenkamp, R. Test D2. Test D’attention Concentrée; Editest: Brussels, Belgium, 1996. [Google Scholar]
- Osterrieth, P.A. Le Test de Copie d’une Figure Complexe: Contribution à l’étude de La Perception et de La Mémoire. Arch. Psychol. 1944, 30, 286–356. [Google Scholar]
- Squire, L.R.; Wetzel, C.D.; Slater, P.C. Memory Complaint after Electroconvulsive Therapy: Assessment with a New Self-Rating Instrument. Biol. Psychiatry 1979, 14, 791–801. [Google Scholar] [PubMed]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and Its Correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Seymour, J. Commentary and Update on the Contribution of the GABA Hypothesis to Understanding the Mechanism of Action of Electroconvulsive Therapy. J. ECT 2021, 37, 4–9. [Google Scholar] [CrossRef]
- Farzan, F.; Boutros, N.N.; Blumberger, D.M.; Daskalakis, Z.J. What does the electroencephalogram tell us about the mechanisms of action of ECT in major depressive disorders? J. ECT 2014, 30, 98–106. [Google Scholar] [CrossRef]
- Su, L.; Jia, Y.; Liang, S.; Shi, S.; Mellor, D.; Xu, Y. Multicenter Randomized Controlled Trial of Bifrontal, Bitemporal, and Right Unilateral Electroconvulsive Therapy in Major Depressive Disorder. Psychiatry Clin. Neurosci. 2019, 73, 636–641. [Google Scholar] [CrossRef]
| Patients Treated for at Least 5 ECT Sessions | Patients Treated for at Least 12 ECT Sessions | |
|---|---|---|
| Sample Size | 42 patients | 29 patients |
| Age (years): mean (SD) | 47.64 (14.64) | 47.86 (13.77) |
| Female sex | 59.5% | 55.2% |
| Graduate studies | 57.1% | 65.5% |
| Married | 50.0% | 48.3% |
| Employed | 40.5% | 44.8% |
| Duration of current episode (months) | 26.69 (27.18) | 23.94 (25.36) |
| Duration of mood disorder (years) | 17.10 (12.15) | 15.93 (10.82) |
| Bipolar disorder | 35.7% | 37.9% |
| Suicide Risk | 88.1% | 86.2% |
| Baseline HDRS | 26.14 (5.43) | 25.86 (5.67) |
| HDRS after 5 ECT sessions | 17.49 (7.37) | 18.10 (7.54) |
| HDRS after 12 ECT sessions | - | 10.26 (5.17) |
| Total number of ECT sessions during treatment: mean (SD), range | 17.12 (4.92), [5;27] | 18.97 (2.13); [13;20] |
| Patients with Postictal Suppression (n = 10) | Patients without Postictal Suppression (n = 30) | p Value | |
|---|---|---|---|
| Age (years) | 51.90 (17.49) | 47.23 (13.16) | 0.246 1 |
| Female sex | 40.0% | 66.7% | 0.159 2 |
| Graduate studies | 70.0% | 53.3% | 0.471 2 |
| Married | 60.0% | 50.0% | 0.721 2 |
| Employed | 20.0% | 46.7% | 0.263 2 |
| Duration of current episode (months) | 34.14 (34.41) | 25.45 (25.15) | 0.432 1 |
| Duration of mood disorder (years) | 18.50 (14.55) | 16.87 (11.56) | 0.866 1 |
| Bipolar disorder | 40.0% | 30.0% | 0.700 2 |
| Suicide Risk | 80.0% | 90.0% | 0.584 2 |
| Baseline HDRS | 26.40 (6.74) | 25.73 (4.96) | 0.548 1 |
| Total number of ECT sessions during treatment | 16.70 (6.21) | 17.40 (4.47) | 0.678 1 |
| Baseline | After 5 ECT Sessions | r | p | |
|---|---|---|---|---|
| MMSE (n = 41) | 26.45 (3.04) | 26.63 (2.73) | −0.186 | 0.245 |
| Doors test (n = 38) | ||||
| part A, scaled score | 6.51 (3.84) | 6.95 (3.97) | −0.322 | 0.049 # |
| part B, scaled score | 6.90 (3.29) | 6.90 (4.16) | −0.286 | 0.082 |
| D2 test of attention (n = 38) | ||||
| TN: Total number of characters processed | 84.80 (8.56) | 88.34 (9.65) | 0.054 | 0.749 |
| CP: Concentration performance | 91.54 (6.34) | 95.74 (6.67) | 0.094 | 0.586 |
| Rey figure (Z score) (n = 37) | −1.32 (1.98) | −0.97 (2.02) | −0.128 | 0.451 |
| RL/RI−16 test (n = 38) | ||||
| Free Recall 1 (z score) | −0.79 (0.98) | −0.95 (0.98) | 0.083 | 0.620 |
| Free Recall 2 (z score) | −0.79 (0.90) | −1.19 (1.08) | 0.150 | 0.369 |
| Free Recall 3 (z score) | −0.88 (1.05) | −1.27 (1.28) | 0.135 | 0.420 |
| Delayed Free Recall (z score) | −0.90 (1.05) | −1.98 (1.27) | 0.011 | 0.946 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulier, V.; Guehl, J.; Evêque-Mourroux, E.; Quesada, P.; Rothärmel, M. A Retrospective Study of Postictal Suppression during Electroconvulsive Therapy. J. Clin. Med. 2022, 11, 1440. https://doi.org/10.3390/jcm11051440
Moulier V, Guehl J, Evêque-Mourroux E, Quesada P, Rothärmel M. A Retrospective Study of Postictal Suppression during Electroconvulsive Therapy. Journal of Clinical Medicine. 2022; 11(5):1440. https://doi.org/10.3390/jcm11051440
Chicago/Turabian StyleMoulier, Virginie, Julien Guehl, Emilie Evêque-Mourroux, Pierre Quesada, and Maud Rothärmel. 2022. "A Retrospective Study of Postictal Suppression during Electroconvulsive Therapy" Journal of Clinical Medicine 11, no. 5: 1440. https://doi.org/10.3390/jcm11051440
APA StyleMoulier, V., Guehl, J., Evêque-Mourroux, E., Quesada, P., & Rothärmel, M. (2022). A Retrospective Study of Postictal Suppression during Electroconvulsive Therapy. Journal of Clinical Medicine, 11(5), 1440. https://doi.org/10.3390/jcm11051440





