Predicting the Need for Renal Replacement Therapy Using a Vascular Occlusion Test and Tissue Oxygen Saturation in Patients in the Early Phase of Multiorgan Dysfunction Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Study Endpoint
2.3. Determination and Grading of Acute Kidney Injury
2.4. Renal Replacement Therapy
2.5. Unprovoked StO2 Measurement and Vascular Occlusion Test
2.6. Statistical Analysis
3. Results
3.1. Patients
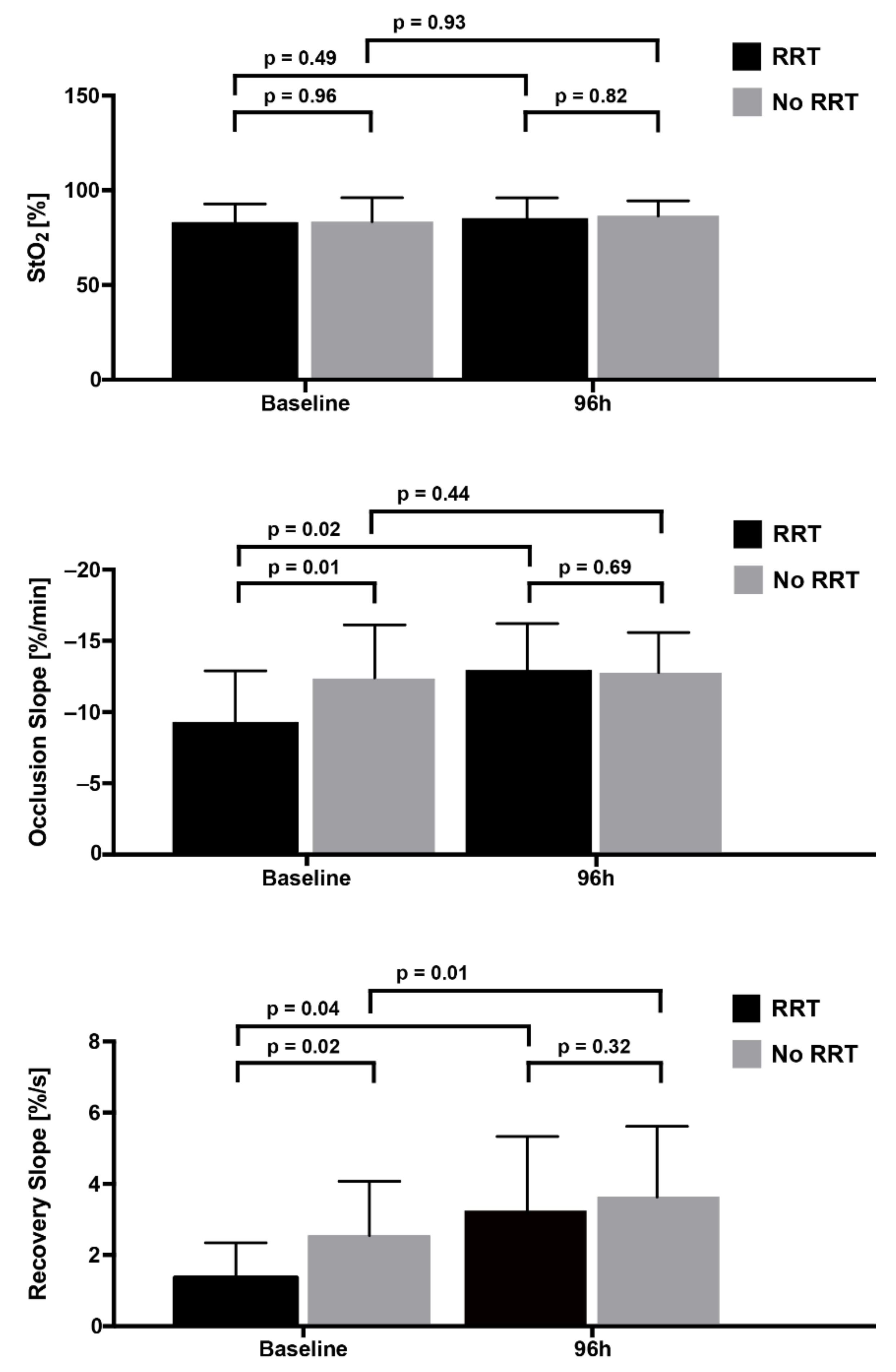
3.2. Changes in Kidney Function
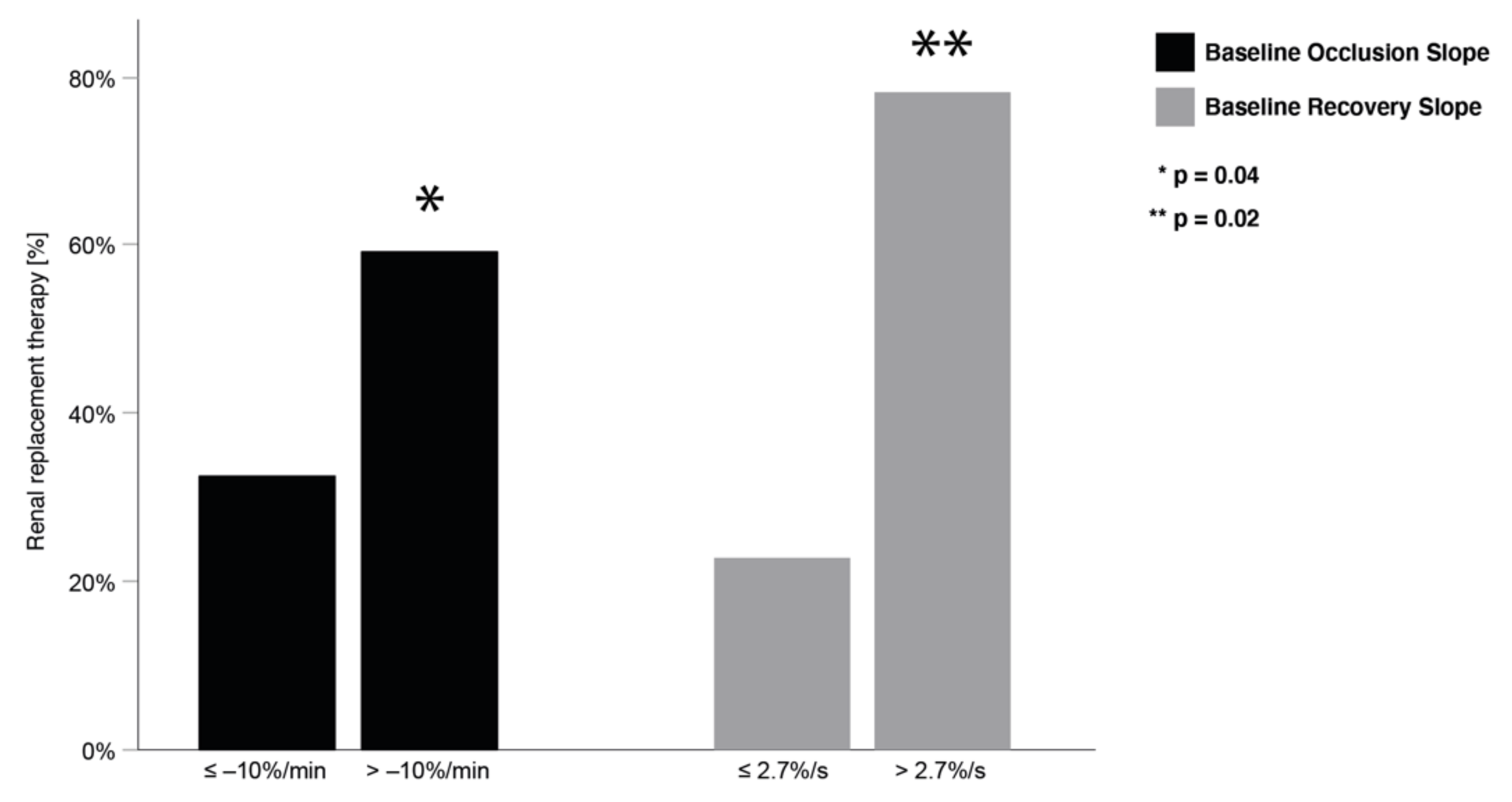
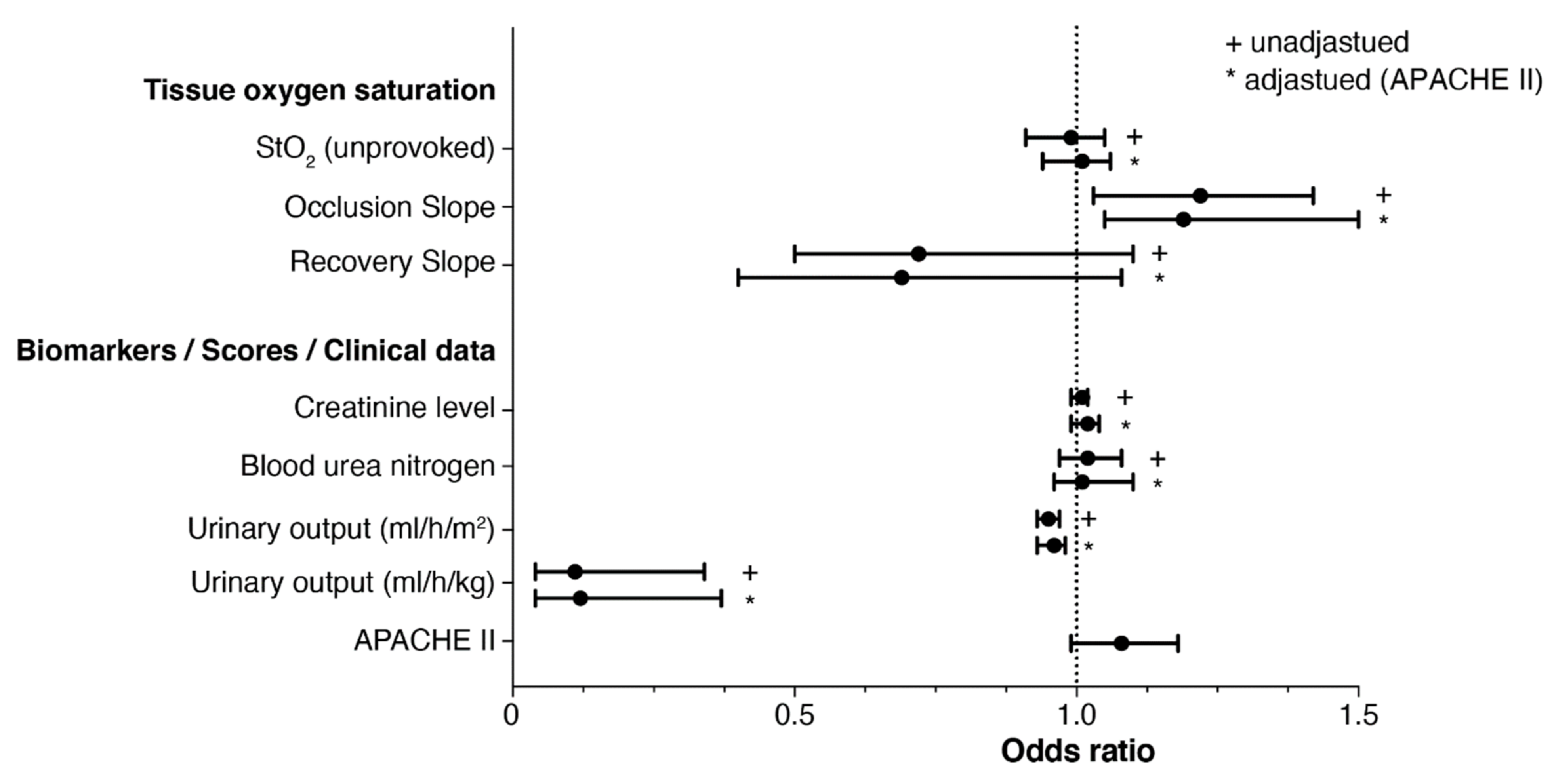
3.3. Association between Tissue Oxygen Saturation and Renal Replacement Therapy
4. Discussion
4.1. Prognostic Relevance of Unprovoked StO2 Regarding Renal Replacement Therapy
4.2. Prognostic Relevance of StO2 under VOT Regarding Renal Replacement Therapy
4.3. Limitations of This Study, StO2 Measurement, and VOT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brivet, F.G.; Kleinknecht, D.J.; Loirat, P.; Landais, P.J. Acute renal failure in intensive care units—Causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit. Care Med. 1996, 24, 192–198. [Google Scholar] [CrossRef] [PubMed]
- D’Avila, D.O.; Cendoroglo Neto, M.; dos Santos, O.F.; Schor, N.; Poli de Figueiredo, C.E. Acute renal failure needing dialysis in the intensive care unit and prognostic scores. Ren. Fail. 2004, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.; Girard, R.; Selli, J.M.; Ayzac, L. Intermittent versus continuous renal replacement therapy for acute renal failure in intensive care units: Results from a multicenter prospective epidemiological survey. Intensive Care Med. 2002, 28, 1411–1418. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Karsou, S.A.; Jaber, B.L.; Pereira, B.J. Impact of intermittent hemodialysis variables on clinical outcomes in acute renal failure. Am. J. Kidney Dis. 2000, 35, 980–991. [Google Scholar] [CrossRef]
- Liano, F.; Junco, E.; Pascual, J.; Madero, R.; Verde, E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int. Suppl. 1998, 66, S16–S24. [Google Scholar]
- McCarthy, J.T. Prognosis of patients with acute renal failure in the intensive-care unit: A tale of two eras. Mayo Clin. Proc. 1996, 71, 117–126. [Google Scholar] [CrossRef]
- Jones, J.; Holmen, J.; De Graauw, J.; Jovanovich, A.; Thornton, S.; Chonchol, M. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am. J. Kidney Dis. 2012, 60, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Gustot, T. Multiple organ failure in sepsis: Prognosis and role of systemic inflammatory response. Curr. Opin. Crit. Care 2011, 17, 153–159. [Google Scholar] [CrossRef]
- Gomez, H.; Mesquida, J.; Simon, P.; Kim, H.K.; Puyana, J.C.; Ince, C.; Pinsky, M.R. Characterization of tissue oxygen saturation and the vascular occlusion test: Influence of measurement sites, probe sizes and deflation thresholds. Crit. Care 2009, 13 (Suppl. 5), S3. [Google Scholar] [CrossRef] [Green Version]
- Lipcsey, M.; Woinarski, N.C.; Bellomo, R. Near infrared spectroscopy (NIRS) of the thenar eminence in anesthesia and intensive care. Ann. Intensive Care 2012, 2, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creteur, J. Muscle StO2 in critically ill patients. Curr. Opin. Crit. Care 2008, 14, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, K.; Kitamura, T.; Kohira, S.; Torii, S.; Mishima, T.; Hanayama, N.; Kobayashi, K.; Ohkubo, H.; Miyaji, K. Regional thigh tissue oxygen saturation during cardiopulmonary bypass predicts acute kidney injury after cardiac surgery. J. Artif. Organs 2020, 23, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Makris, K.; Spanou, L. Acute Kidney Injury: Diagnostic Approaches and Controversies. Clin. Biochem. Rev. 2016, 37, 153–175. [Google Scholar] [PubMed]
- Nuding, S.; Ebelt, H.; Hoke, R.S.; Krummenerl, A.; Wienke, A.; Muller-Werdan, U.; Werdan, K. Reducing elevated heart rate in patients with multiple organ dysfunction syndrome by the I (f) (funny channel current) inhibitor ivabradine: MODI (f)Y trial. Clin. Res. Cardiol. 2011, 100, 915–923. [Google Scholar] [CrossRef]
- Mizuno, T.; Sato, W.; Ishikawa, K.; Shinjo, H.; Miyagawa, Y.; Noda, Y.; Imai, E.; Yamada, K. KDIGO (Kidney Disease: Improving Global Outcomes) criteria could be a useful outcome predictor of cisplatin-induced acute kidney injury. Oncology 2012, 82, 354–359. [Google Scholar] [CrossRef]
- Burton, R.F. Estimating body surface area from mass and height: Theory and the formula of Du Bois and Du Bois. Ann. Hum. Biol. 2008, 35, 170–184. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312-303. [Google Scholar]
- Murkin, J.M.; Arango, M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br. J. Anaesth. 2009, 103 (Suppl. 1), i3–i13. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlile, C.; Wade, C.E.; Baraniuk, M.S.; Holcomb, J.B.; Moore, L.J. Evaluation of StO2 tissue perfusion monitoring as a tool to predict the need for lifesaving interventions in trauma patients. Am. J. Surg. 2015, 210, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.M.; Nathens, A.B.; Moore, F.A.; Rhee, P.; Puyana, J.C.; Moore, E.E.; Beilman, G.J. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J. Trauma 2007, 62, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Crookes, B.A.; Cohn, S.M.; Bloch, S.; Amortegui, J.; Manning, R.; Li, P.; Proctor, M.S.; Hallal, A.; Blackbourne, L.H.; Benjamin, R.; et al. Can near-infrared spectroscopy identify the severity of shock in trauma patients? J. Trauma 2005, 58, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Lipcsey, M.; Eastwood, G.M.; Woinarski, N.C.; Bellomo, R. Near-infrared spectroscopy of the thenar eminence: Comparison of dynamic testing protocols. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2012, 14, 142–147. [Google Scholar]
- Gomez, H.; Torres, A.; Polanco, P.; Kim, H.K.; Zenker, S.; Puyana, J.C.; Pinsky, M.R. Use of non-invasive NIRS during a vascular occlusion test to assess dynamic tissue O2 saturation response. Intensive Care Med. 2008, 34, 1600–1607. [Google Scholar] [CrossRef]
- Luengo, C.; Resche-Rigon, M.; Damoisel, C.; Kerever, S.; Creteur, J.; Payen, D. Comparison of two different generations of “NIRS” devices and transducers in healthy volunteers and ICU patients. J. Clin. Monit. Comput. 2013, 27, 71–79. [Google Scholar] [CrossRef]
- Mayeur, C.; Campard, S.; Richard, C.; Teboul, J.L. Comparison of four different vascular occlusion tests for assessing reactive hyperemia using near-infrared spectroscopy. Crit. Care Med. 2011, 39, 695–701. [Google Scholar] [CrossRef]
- Choi, D.K.; Kim, W.J.; Chin, J.H.; Lee, E.H.; Don Hahm, K.; Yeon Sim, J.; Cheol Choi, I. Intraoperative renal regional oxygen desaturation can be a predictor for acute kidney injury after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 564–571. [Google Scholar] [CrossRef]
- Owens, G.E.; King, K.; Gurney, J.G.; Charpie, J.R. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatric Cardiol. 2011, 32, 183–188. [Google Scholar] [CrossRef]
- Harer, M.W.; Chock, V.Y. Renal Tissue Oxygenation Monitoring-An Opportunity to Improve Kidney Outcomes in the Vulnerable Neonatal Population. Front. Pediatr. 2020, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Bullen, A.; Liu, Z.Z.; Hepokoski, M.; Li, Y.; Singh, P. Renal Oxygenation and Hemodynamics in Kidney Injury. Nephron 2017, 137, 260–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, A. Sepsis-induced acute kidney injury. Indian J. Crit. Care Med. 2010, 14, 14–21. [Google Scholar] [CrossRef]
- Fink, M. Cytopathic hypoxia in sepsis. Acta Anaesthesiol. Scandinavica. Suppl. 1997, 110, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit. Care Clin. 2001, 17, 219–237. [Google Scholar] [CrossRef]
- Gruartmoner, G.; Mesquida, J.; Masip, J.; Martinez, M.L.; Villagra, A.; Baigorri, F.; Pinsky, M.R.; Artigas, A. Thenar oxygen saturation during weaning from mechanical ventilation: An observational study. Eur. Respir. J. 2014, 43, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Ricksten, S.E.; Bragadottir, G.; Redfors, B.; Nordquist, L. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin. Exp. Pharmacol. Physiol. 2013, 40, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Chaves, R.C.F.; Tafner, P.; Chen, F.K.; Meneghini, L.B.; Correa, T.D.; Rabello Filho, R.; Cendoroglo Neto, M.; Santos, O.; Serpa Neto, A. Near-infrared spectroscopy parameters in patients undergoing continuous venovenous hemodiafiltration. Einstein (Sao Paulo) 2019, 17, eAO4439. [Google Scholar] [CrossRef] [Green Version]
- Bezemer, R.; Lima, A.; Myers, D.; Klijn, E.; Heger, M.; Goedhart, P.T.; Bakker, J.; Ince, C. Assessment of tissue oxygen saturation during a vascular occlusion test using near-infrared spectroscopy: The role of probe spacing and measurement site studied in healthy volunteers. Crit. Care 2009, 13 (Suppl. 5), S4. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.B.; Sapra, A. Nephrotoxic Medications; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tafner, P.; Chen, F.K.; Rabello, R.F.; Correa, T.D.; Chaves, R.C.F.; Serpa, A.N. Recent advances in bedside microcirculation assessment in critically ill patients. Rev. Bras. Ter. Intensiva 2017, 29, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Koeze, J.; Keus, F.; Dieperink, W.; van der Horst, I.C.; Zijlstra, J.G.; van Meurs, M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017, 18, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhle, F.; Lichtenstern, C.; Weigand, M.A. Pathophysiologie. In Sepsis und MODS; Schuster, H.P., Müller Werdan, U., Werdan, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 39–62. [Google Scholar]
- Pipili, C.; Vasileiadis, I.; Grapsa, E.; Tripodaki, E.S.; Ioannidou, S.; Papastylianou, A.; Kokkoris, S.; Routsi, C.; Politou, M.; Nanas, S. Microcirculatory alterations during continuous renal replacement therapy in ICU: A novel view on the ‘dialysis trauma’ concept. Microvasc. Res. 2016, 103, 14–18. [Google Scholar] [CrossRef]
- Creteur, J.; Carollo, T.; Soldati, G.; Buchele, G.; De Backer, D.; Vincent, J.L. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007, 33, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Payen, D.; Luengo, C.; Heyer, L.; Resche-Rigon, M.; Kerever, S.; Damoisel, C.; Losser, M.R. Is thenar tissue hemoglobin oxygen saturation in septic shock related to macrohemodynamic variables and outcome? Crit. Care 2009, 13 (Suppl. 5), S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
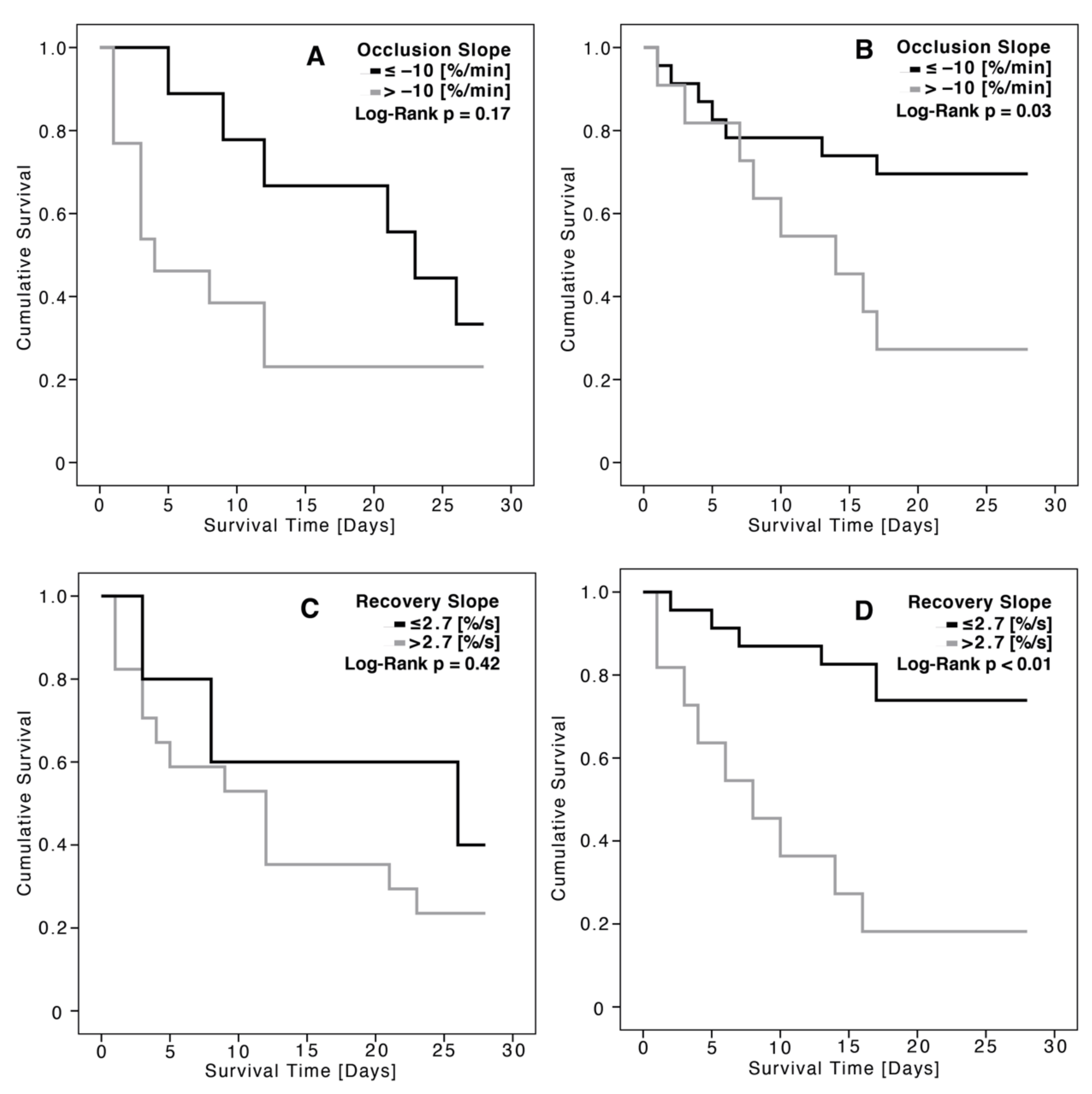
| Study Population (N = 56) | |||
|---|---|---|---|
| No RRT | RRT | p-Value | |
| (N = 34) | (N = 22) | ||
| Demographics | |||
| Age (years, mean ± SD) | 64.5 ± 15.0 | 59.4 ± 13.2 | n.s. |
| Male (N, %) | 25 (73.5) | 15 (68.2) | n.s. |
| Female (N, %) | 9 (26.5) | 7 (31.8) | n.s. |
| BMI (kg/m2, mean ± SD) | 27.6 ± 8.9 | 24.9 ± 4.6 | n.s. |
| BSA (m2, mean ± SD) | 1.96 ± 0.29 | 1.91 ± 0.21 | n.s. |
| <70 years (N, %) | 18 (52.9) | 16 (47.1) | n.s. |
| ≥70 years (N, %) | 16 (47.1) | 6 (27.3) | n.s. |
| Tissue oxygen saturation parameters | |||
| StO2 unprovoked (%, mean ± SD) | 82 ± 10.8 | 82.1 ± 10.9 | n.s. |
| Occlusion slope (%/min, mean ± SD) | −11.7 ± 4.1 | −9.1 ± 3.7 | 0.02 |
| Recovery slope (%/s, mean ± SD) | 2.3 ± 1.6 | 1.7 ± 0.9 | 0.01 |
| Clinical features | |||
| APACHE II score (mean ± SD) | 33.4 ± 6.2 | 36.3 ± 6.4 | n.s. |
| Creatinine level (µmol/L) | 157.9 ± 77.0 | 209. 9 ± 121.3 | n.s. |
| BUN (mmol/L, mean ± SD) | 13.8 ± 10.2 | 16.4 ± 8.6 | n.s. |
| Urinary output (mL/h/m2 BSA, mean ± SD) | 53.4 ± 14.9 | 21.6 ± 47.1 | 0.001 |
| Urinary output (ml/h/kg, mean ± SD) | 1.3 ± 0.2 | 0.5 ± 1.0 | <0.001 |
| Body temperature (°C, mean ± SD) | 37.0 ± 1.4 | 36.9 ± 1.3 | n.s. |
| CRP (mg/L, mean ± SD) | 186.0 ± 144.1 | 258.2 ± 173.4 | n.s. |
| Invasive mechanical ventilation (N, %) | 32 (94.1) | 20 (90.1) | n.s. |
| SpO2 (%, mean ± SD) | 96.2 ± 5.8 | 95.1 ± 6.5 | n.s. |
| FiO2 (%, mean ± SD) | 73.2 ± 21.2 | 67.9 ± 19.3 | n.s. |
| pO2 (kPa, mean ± SD) | 15.7 ± 7.4 | 14.6 ± 4.9 | n.s. |
| Time of MODS diagnosis relative to ICU admission (h, mean ± SD) | 35.4 ± 31.6 | 18.2 ± 13.1 | 0.03 |
| Haemoglobin (mmol/L, mean ± SD) | 6.9 ± 1.2 | 6.4 ± 1.3 | n.s. |
| Relative norepinephrine dose (μg/kg/min, mean ± SD) | 0.46 ± 0.06 | 0.59 ± 0.12 | n.s. |
| Relative doputamine dose (μg/kg/min, mean ± SD) | 3.1 ± 0.5 | 4.3 ± 0.9 | n.s. |
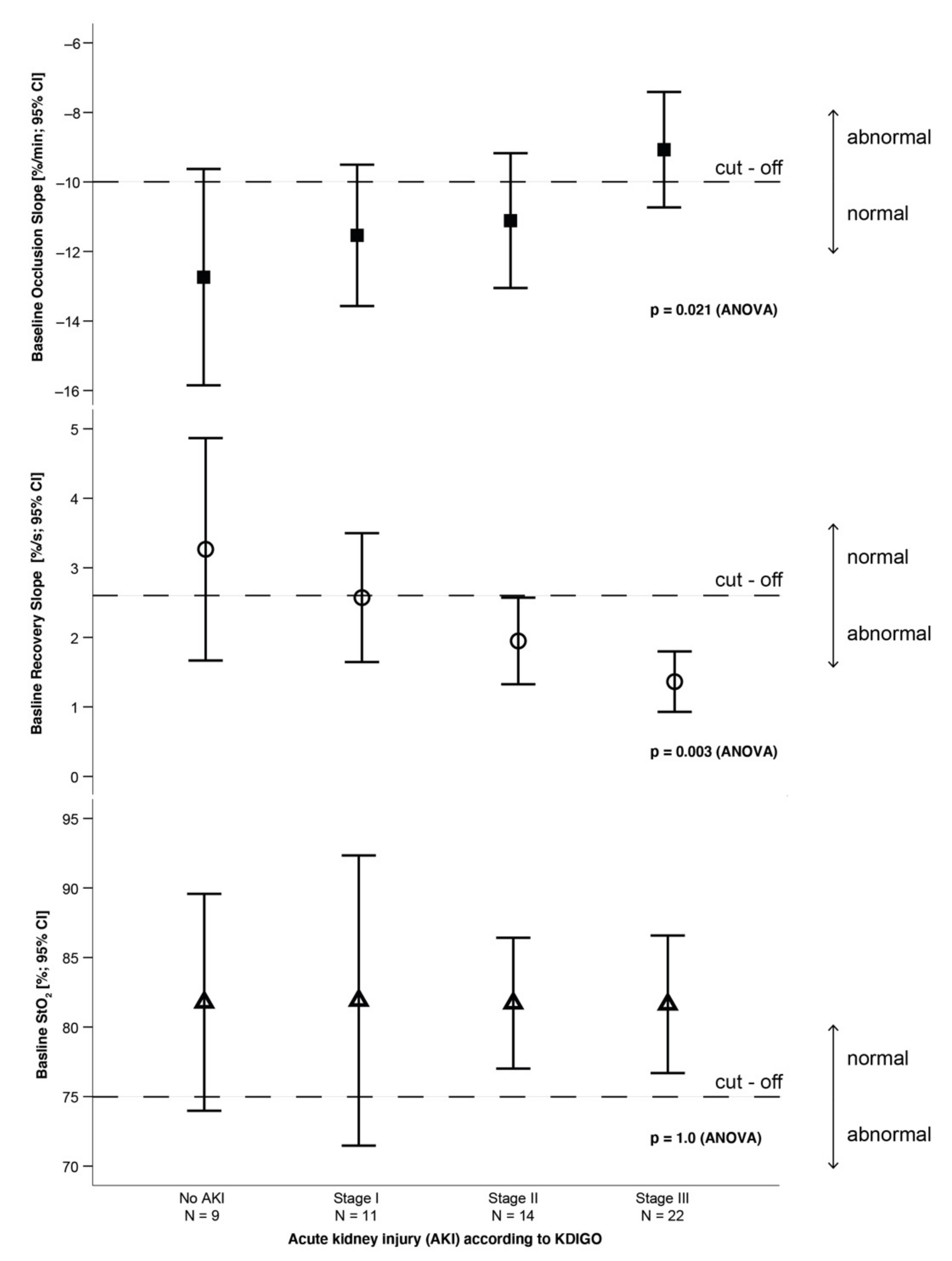
| AKI according to KDIGO | |||
| No AKI (N, %) | 9 (26.5) | 0 (0) | <0.001 |
| Stage I (N, %) | 11 (32.4) | 0 (0) | <0.001 |
| Stage II (N, %) | 14 (41.1) | 0 (0) | <0.001 |
| Stage III (N, %) | 0 (0) | 22 (100) | <0.001 |
| Type of MODS | |||
| Cardiogenic MODS (N, %) | 9 (26.5) | 6 (27.3) | n.s. |
| Septic MODS (N, %) | 25 (73.5) | 16 (72.7) | n.s. |
| Comorbidities | |||
| Hypertension (N, %) | 17 (50) | 7 (31.8) | n.s. |
| Diabetes (N, %) | 12 (35.3) | 5 (22.7) | n.s. |
| CKD (N, %) | 3 (8.8) | 3 (13.6) | n.s. |
| Past myocardial infarction (N, %) | 7 (20.6) | 5 (22.7) | n.s. |
| Past stroke (N, %) | 1 (2.9) | 1 (4.5) | n.s. |
| Active malignancy (N, %) | 7 (20.6) | 4 (18.2) | n.s. |
| AUC | CI (95%) | p-Value | |
|---|---|---|---|
| Tissue oxygen saturation parameters | |||
| StO2, unprovoked | 0.52 | 0.36–0.67 | n.s. |
| Occlusion slope | 0.70 | 0.54–0.84 | 0.04 |
| Recovery slope | 0.59 | 0.44–0.74 | n.s. |
| Clinical parameters | |||
| Creatinine level | 0.64 | 0.47–0.83 | n.s. |
| Blood urea nitrogen | 0.61 | 0.48–0.74 | n.s. |
| Urinary output | 0.94 | 0.85–1.0 | <0.001 |
| APACHE II score | 0.63 | 0.47–0.78 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haertel, F.; Reisberg, D.; Peters, M.; Nuding, S.; Schulze, P.C.; Werdan, K.; Ebelt, H. Predicting the Need for Renal Replacement Therapy Using a Vascular Occlusion Test and Tissue Oxygen Saturation in Patients in the Early Phase of Multiorgan Dysfunction Syndrome. J. Clin. Med. 2022, 11, 1420. https://doi.org/10.3390/jcm11051420
Haertel F, Reisberg D, Peters M, Nuding S, Schulze PC, Werdan K, Ebelt H. Predicting the Need for Renal Replacement Therapy Using a Vascular Occlusion Test and Tissue Oxygen Saturation in Patients in the Early Phase of Multiorgan Dysfunction Syndrome. Journal of Clinical Medicine. 2022; 11(5):1420. https://doi.org/10.3390/jcm11051420
Chicago/Turabian StyleHaertel, Franz, Diana Reisberg, Martin Peters, Sebastian Nuding, P. Christian Schulze, Karl Werdan, and Henning Ebelt. 2022. "Predicting the Need for Renal Replacement Therapy Using a Vascular Occlusion Test and Tissue Oxygen Saturation in Patients in the Early Phase of Multiorgan Dysfunction Syndrome" Journal of Clinical Medicine 11, no. 5: 1420. https://doi.org/10.3390/jcm11051420
APA StyleHaertel, F., Reisberg, D., Peters, M., Nuding, S., Schulze, P. C., Werdan, K., & Ebelt, H. (2022). Predicting the Need for Renal Replacement Therapy Using a Vascular Occlusion Test and Tissue Oxygen Saturation in Patients in the Early Phase of Multiorgan Dysfunction Syndrome. Journal of Clinical Medicine, 11(5), 1420. https://doi.org/10.3390/jcm11051420






