Comparative Impact of Pharmacological Therapies on Cluster Headache Management: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
2.4.1. Statistical Analysis
2.4.2. Pairwise Meta-Analysis
2.4.3. Network Meta-Analysis
2.4.4. Assessment of Consistency and Heterogeneity
3. Results
3.1. Study Selection
3.2. Study Description
3.3. Efficacy Outcomes
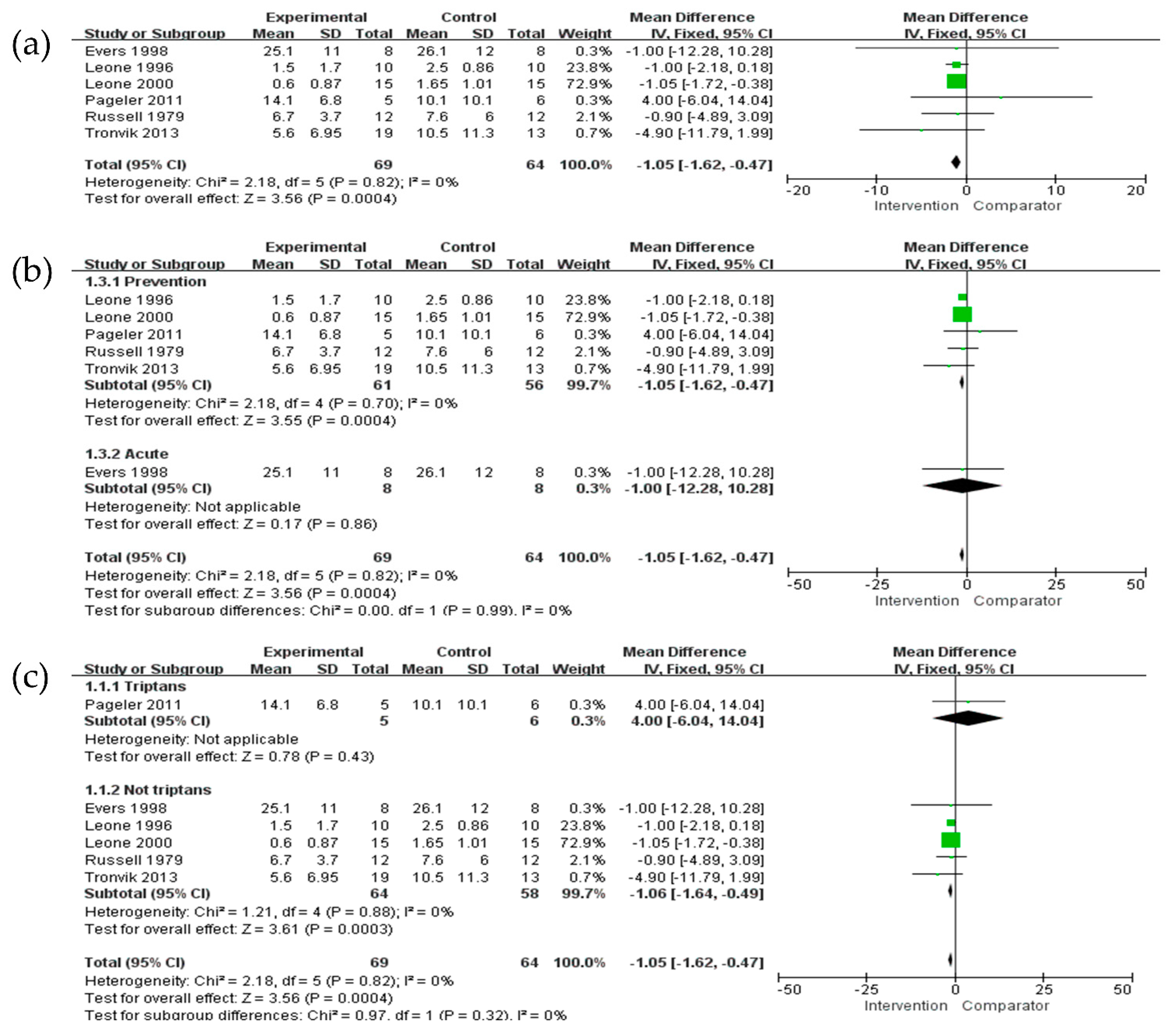
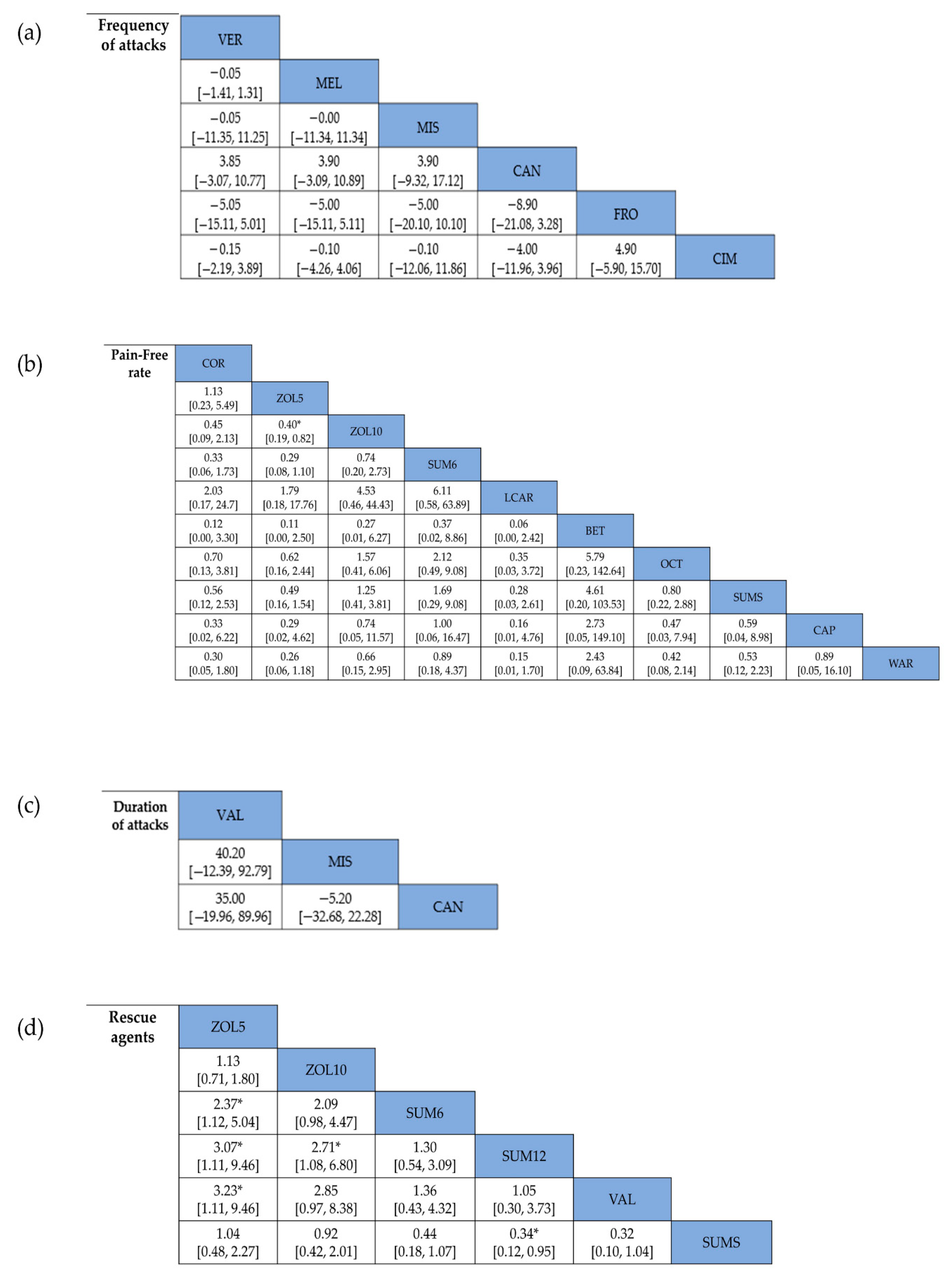
3.3.1. Frequency of Attacks
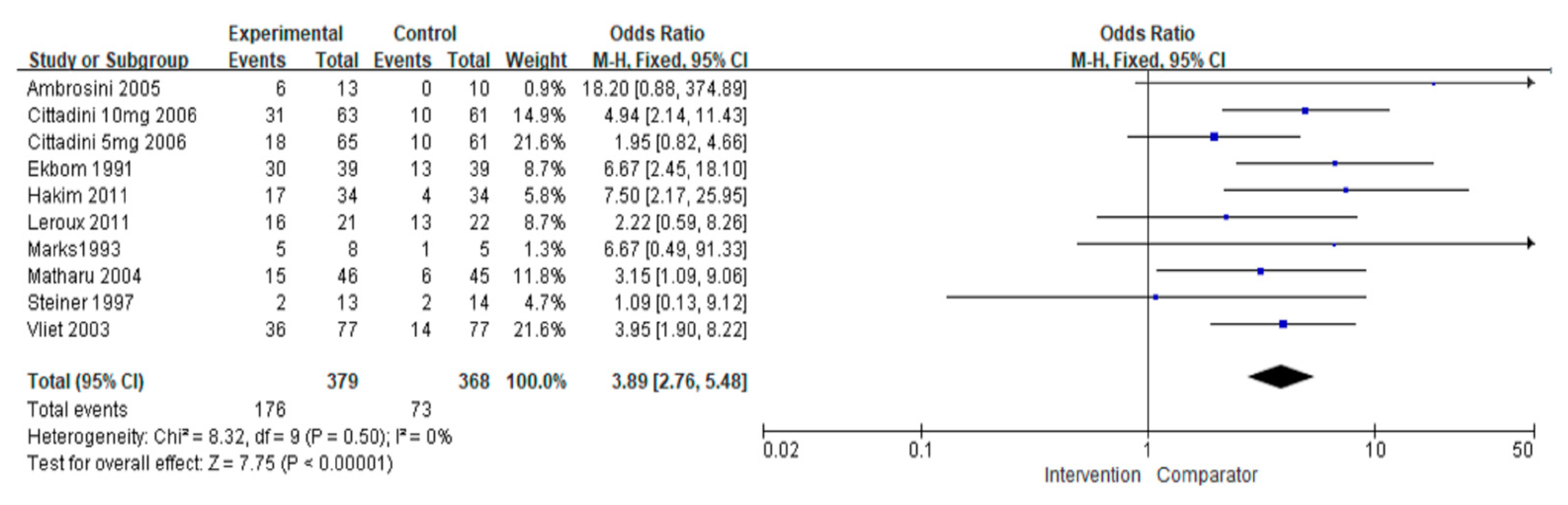
3.3.2. Pain-Free Rate
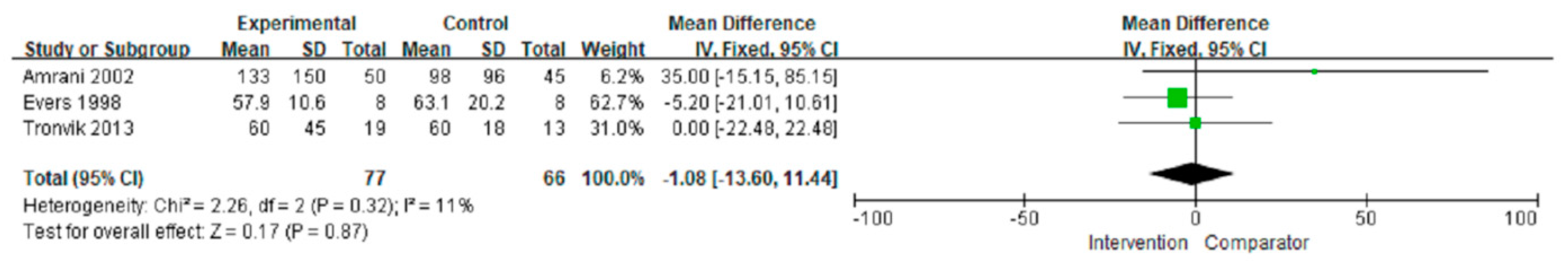
3.3.3. Duration of Attacks
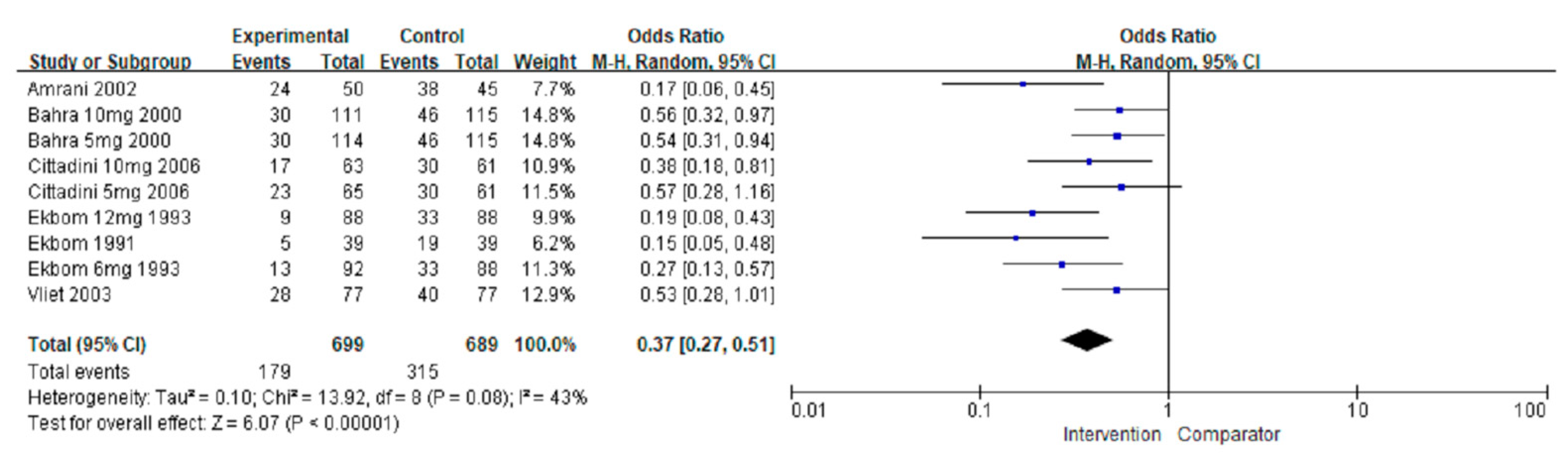
3.3.4. Number of Patients Using Rescue Agents
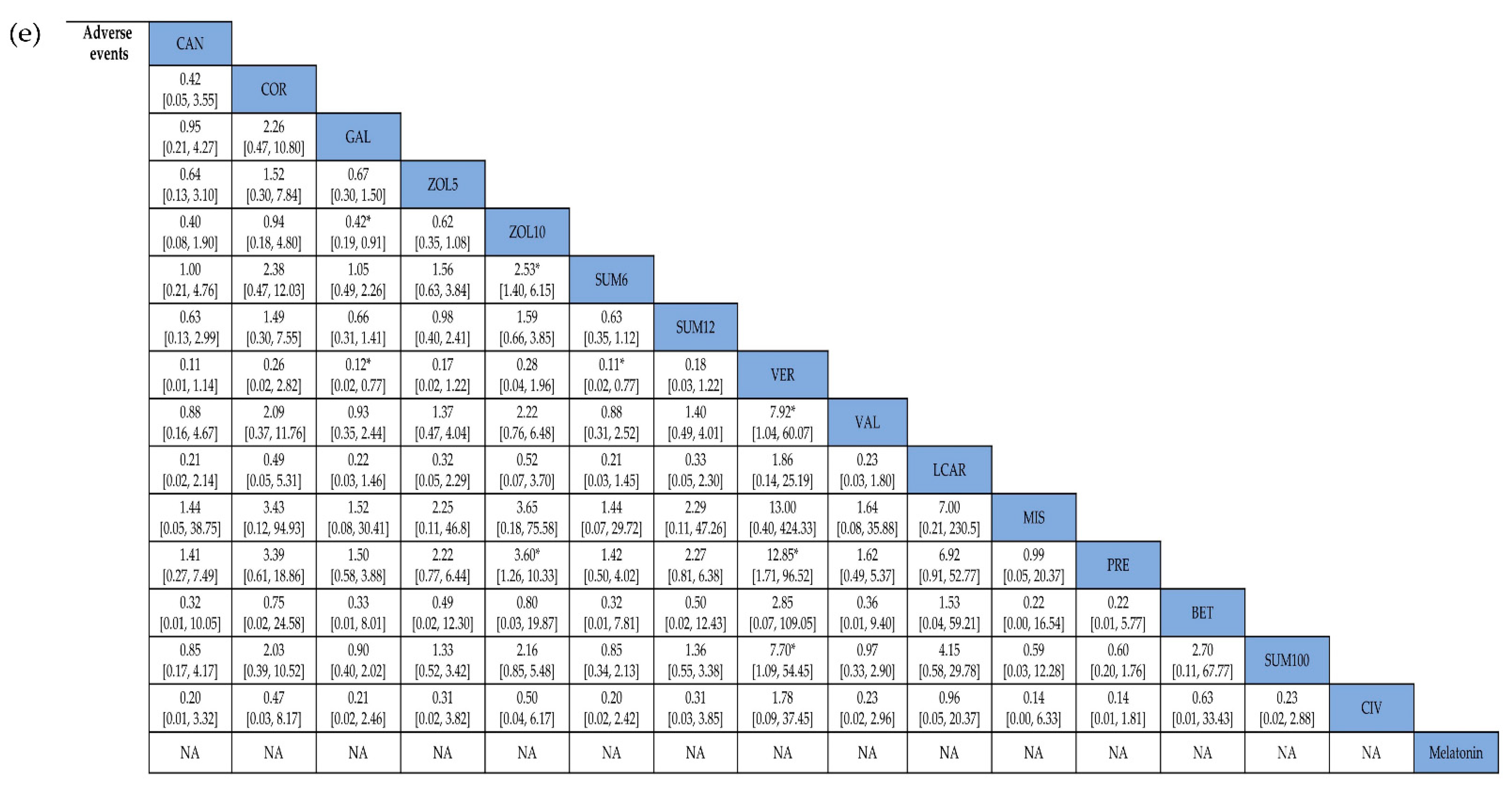
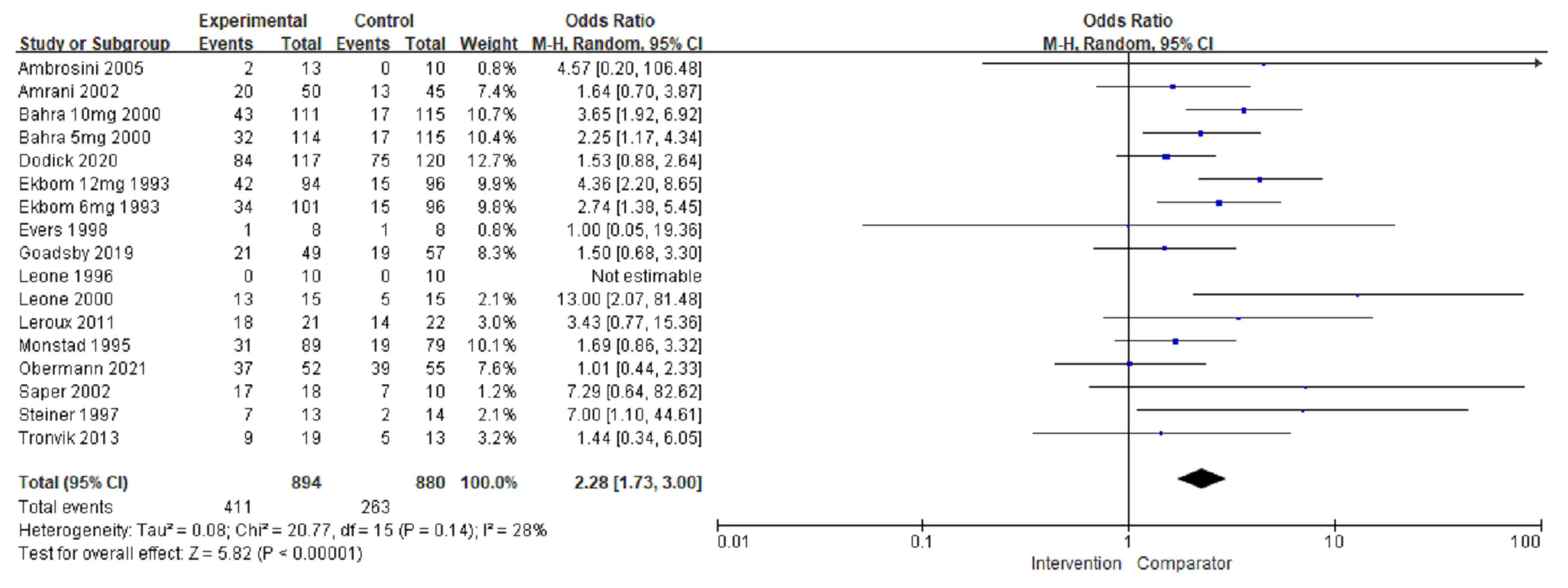
3.4. Adverse Events
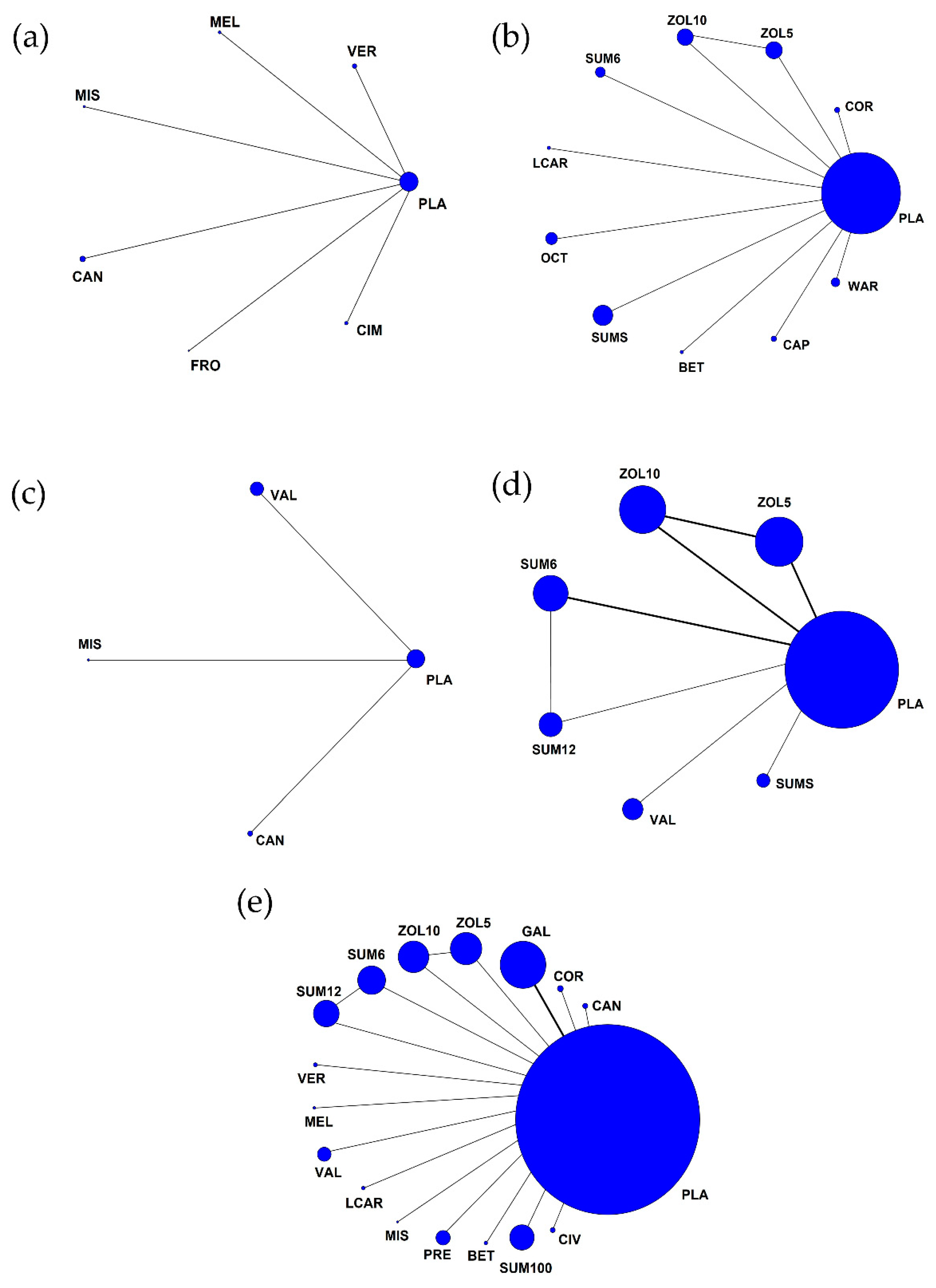
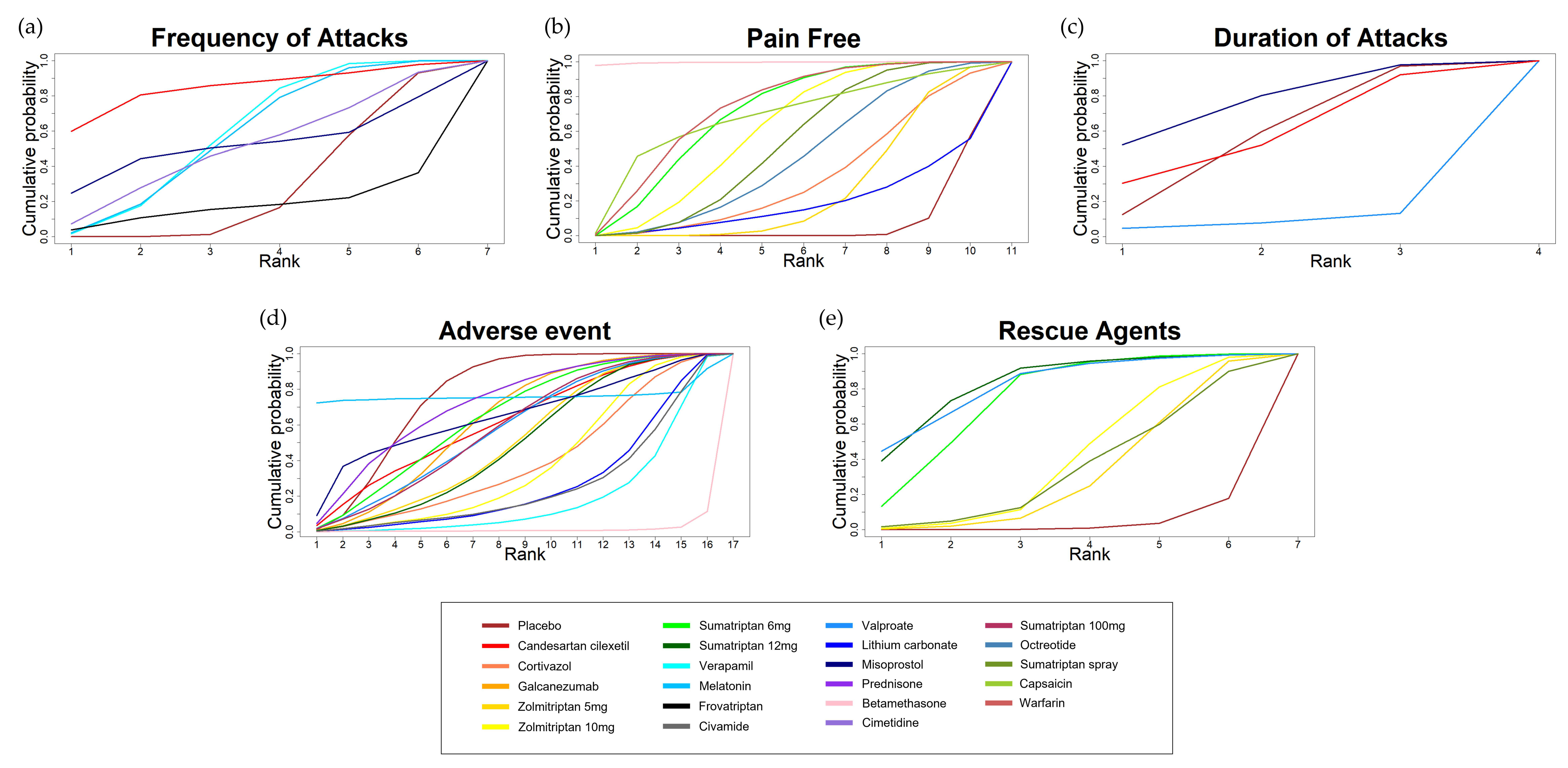
3.5. Rank Probability and SUCRA
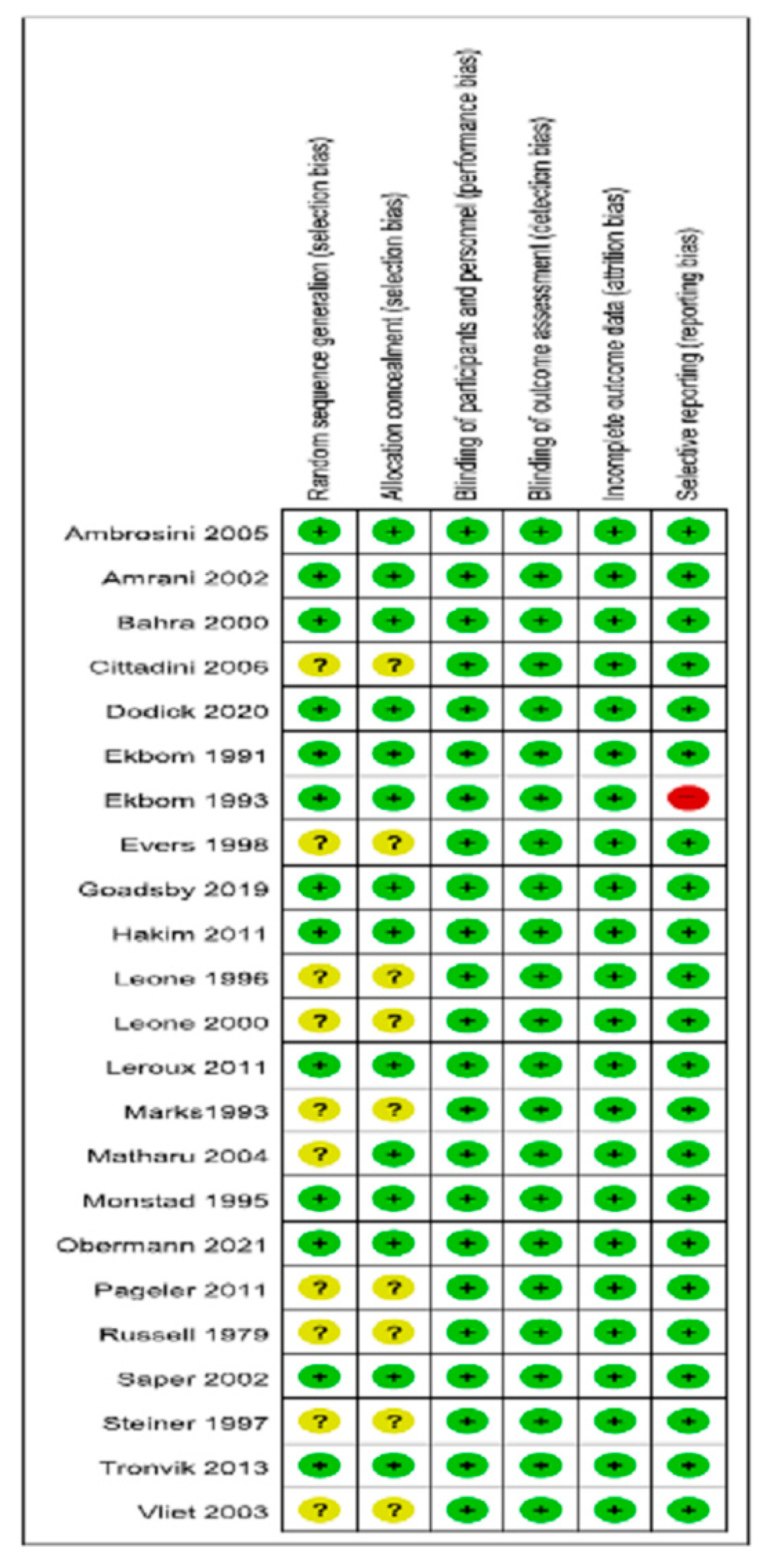
3.6. Risk of Bias and Quality of Evidence
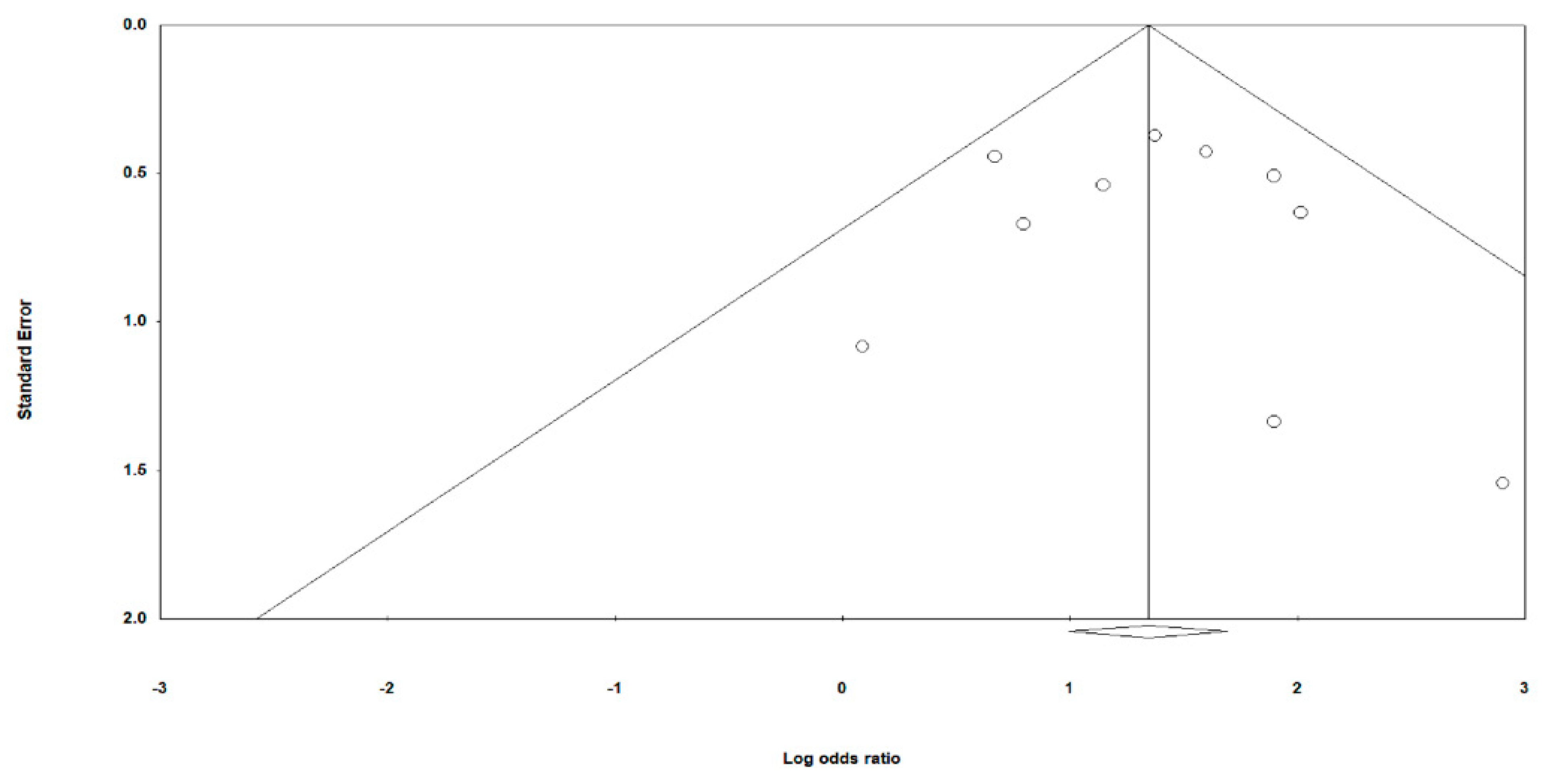
3.7. Meta-Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Robbins, M.S.; Starling, A.J. Treatment of Cluster Headache: The American Headache Society Evidence-Based Guidelines. Headache 2016, 56, 1093–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, J.; May, A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol. 2018, 17, 75–83. [Google Scholar] [CrossRef]
- Rozen, T.D.; Fishman, R.S. Cluster headache in the United States of America: Demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache 2012, 52, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.M.; Lyngberg, A.; Jensen, R.H. Burden of cluster headache. Cephalalgia 2007, 27, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Schoenen, J. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia 2006, 26, 1168–1170. [Google Scholar] [CrossRef]
- Pedersen, J.L.; Barloese, M.; Jensen, R.H. Neurostimulation in cluster headache: A review of current progress. Cephalalgia 2013, 33, 1179–1193. [Google Scholar] [CrossRef]
- Ljubisavljevic, S.; Zidverc-Trajkovic, J. Cluster headache: Pathophysiology, diagnosis and treatment. J. Neurol. 2019, 266, 1059–1066. [Google Scholar] [CrossRef]
- Nilsson Remahl, A.I.; Laudon Meyer, E. Placebo response in cluster headache trials: A review. Cephalalgia 2003, 23, 504–510. [Google Scholar] [CrossRef]
- Francis, G.J.; Becker, W.J.; Pringsheim, T.M. Acute and preventive pharmacologic treatment of cluster headache. Neurology 2010, 75, 463–473. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Vikelis, M. Recently available and emerging therapeutic strategies for the acute and prophylactic management of cluster headache: A systematic review and expert opinion. Expert Rev. Neurother. 2021, 21, 235–248. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z. Different doses of galcanezumab versus placebo in patients with migraine and cluster headache: A meta-analysis of randomized controlled trials. J. Headache Pain 2020, 21, 14. [Google Scholar] [CrossRef] [Green Version]
- Brandt, R.B.; Doesborg, P.G.G. Pharmacotherapy for Cluster Headache. CNS Drugs 2020, 34, 171–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodick, D.W.; Rozen, T.D. Cluster headache. Cephalalgia 2000, 20, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, T.; Nikolakopoulou, A. In network meta-analysis, most of the information comes from indirect evidence: Empirical study. J. Clin. Epidemiol. 2020, 124, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.J.; Naci, H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 2013, 11, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 33, b2700. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Balshem, H.; Helfand, M. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Russell, D. Cluster headache: Trial of a combined histamine H1 and H2 antagonist treatment. J. Neurol. Neurosurg. Psychiatry 1979, 42, 668–669. [Google Scholar] [CrossRef]
- Sumatriptan Cluster Headache Study Group. Treatment of acute cluster headache with sumatriptan. N. Engl. J. Med. 1991, 325, 322–326. [Google Scholar] [CrossRef]
- Ekbom, K.; Monstad, I. Subcutaneous sumatriptan in the acute treatment of cluster headache: A dose comparison study. Acta Neurol. Scand. 1993, 88, 63–69. [Google Scholar] [CrossRef]
- Marks, D.R.; Rapoport, A. A double-blind placebo-controlled trial of intranasal capsaicin for cluster headache. Cephalalgia 1993, 13, 114–116. [Google Scholar] [CrossRef]
- Monstad, I.; Krabbe, A. Preemptive oral treatment with sumatriptan during a cluster period. Headache 1995, 35, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; D’Amico, D. Melatonin versus placebo in the prophylaxis of cluster headache: A double-blind pilot study with parallel groups. Cephalalgia 1996, 16, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Hering, R. Double-blind placebo-controlled trial of lithium in episodic cluster headache. Cephalalgia 1997, 17, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Masur, H. Prostaglandin analog mechanisms are not effective in refractory chronic cluster headache. Headache 1998, 38, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Bahra, A.; Gawel, M.J. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology 2000, 54, 1832–1839. [Google Scholar] [CrossRef]
- Leone, M.; D’Amico, D. Verapamil in the prophylaxis of episodic cluster headache: A double-blind study versus placebo. Neurology 2000, 54, 1382–1385. [Google Scholar] [CrossRef]
- Saper, J.R.; Klapper, J. Intranasal civamide for the treatment of episodic cluster headaches. Arch. Neurol. 2002, 59, 990–994. [Google Scholar] [CrossRef] [Green Version]
- El Amrani, M.; Massiou, H.; Bousser, M.G. A negative trial of sodium valproate in cluster headache: Methodological issues. Cephalalgia 2002, 22, 205–208. [Google Scholar] [CrossRef]
- Van Vliet, J.A.; Bahra, A. Intranasal sumatriptan in cluster headache: Randomized placebo-controlled double-blind study. Neurology 2003, 60, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Matharu, M.S.; Levy, M.J. Subcutaneous octreotide in cluster headache: Randomized placebo-controlled double-blind crossover study. Ann. Neurol. 2004, 56, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; Vandenheede, M. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: A double-blind placebo-controlled study. Pain 2005, 118, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, E.; May, A. Effectiveness of intranasal zolmitriptan in acute cluster headache: A randomized, placebo-controlled, double-blind crossover study. Arch. Neurol. 2006, 63, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Pageler, L.; Katsarava, Z. Frovatriptan for prophylactic treatment of cluster headache: Lessons for future trial design. Headache 2011, 51, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Leroux, E.; Valade, D. Suboccipital steroid injections for transitional treatment of patients with more than two cluster headache attacks per day: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011, 10, 891–897. [Google Scholar] [CrossRef]
- Hakim, S.M. Warfarin for refractory chronic cluster headache: A randomized pilot study. Headache 2011, 51, 713–725. [Google Scholar] [CrossRef]
- Tronvik, E.; Wenecke, T. Randomised trial on episodic cluster headache with an angiotensin II receptor blocker. Cephalalgia 2013, 33, 1026–1034. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Dodick, D.W. Trial of Galcanezumab in Prevention of Episodic Cluster Headache. N. Engl. J. Med. 2019, 381, 132–141. [Google Scholar] [CrossRef]
- Dodick, D.W.; Goadsby, P.J. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: Results from 3-month double-blind treatment. Cephalalgia 2020, 40, 935–948. [Google Scholar] [CrossRef]
- Obermann, M.; Nagel, S. Safety and efficacy of prednisone versus placebo in short-term prevention of episodic cluster headache: A multicentre, double-blind, randomised controlled trial. Lancet Neurol. 2021, 20, 29–37. [Google Scholar] [CrossRef]
- Palacios-Ceña, M.; Fernández-Muñoz, J.J. The association of headache frequency with pain interference and the burden of disease is mediated by depression and sleep quality, but not anxiety, in chronic tension type headache. J. Headache Pain 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, J.H.; Park, J.W. Clinical factors influencing the impact of cluster headache from a prospective multicenter study. Sci. Rep. 2020, 10, 2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, A.; Schwedt, T.J. Cluster headache. Nat. Rev. Dis. Primers 2018, 4, 18006. [Google Scholar] [CrossRef] [PubMed]
- Probyn, K.; Bowers, H. Non-pharmacological self-management for people living with migraine or tension-type headache: A systematic review including analysis of intervention components. BMJ Open 2017, 7, e016670. [Google Scholar] [CrossRef] [Green Version]
- Etminan, M.; Levine, M.A. Efficacy of Angiotensin II Receptor Antagonists in Preventing Headache: A Systematic Overview and Meta-analysis. Am. J. Med. 2002, 112, 642–646. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Guidance for Industry: Analgesic Indications: Developing Drug and Biological Products. Available online: https://www.fdanews.com/ext/resources/files/02/02-05-14-Analgesic.pdf (accessed on 6 February 2014).
- Kivitz, A.J.; Conaghan, P.G. Rescue Analgesic Medication Use by Patients Treated with Triamcinolone Acetonide Extended-Release for Knee Osteoarthritis Pain: Pooled Analysis of Three Phase 2/3 Randomized Clinical Trials. Pain Ther. 2019, 8, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.C.; Schwedt, T.J. Treatment of medication-overuse headache: A systematic review. Cephalalgia 2016, 36, 371–386. [Google Scholar] [CrossRef]
- Dodick, D.; Freitag, F. Evidence-based understanding of medication-overuse headache: Clinical implications. Headache 2006, 46, S202–S211. [Google Scholar] [CrossRef]
- Diener, H.C.; Holle, D. Chronic Headache Due to Overuse of Analgesics and Anti-Migraine Agents. Dtsch. Arztebl. Int. 2018, 115, 365–370. [Google Scholar] [CrossRef]
- Law, S.; Derry, S.; Moore, R.A. Triptans for acute cluster headache. Cochrane Database Syst. Rev 2013, 2013, CD008042. [Google Scholar] [CrossRef] [Green Version]
- Pomeroy, J.L.; Marmura, M.J. Pharmacotherapy Options for the Management of Cluster Headache. Clin. Med. Insights Ther. 2013, 5, 53–74. [Google Scholar] [CrossRef] [Green Version]
- Lund, N.; Barloese, M. Chronobiology differs between men and women with cluster headache, clinical phenotype does not. Neurology 2017, 88, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Bahra, A.A.; May, A.; Goadsby, P.J. Cluster headache: A prospective clinical study with diagnostic implications. Neurology 2002, 58, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.M.; Burish, M.J. Effectiveness of Oxygen and Other Acute Treatments for Cluster Headache: Results From the Cluster Headache Questionnaire, an International Survey. Headache 2019, 59, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Drescher, J.; Khouri, A. Effectiveness of medication in cluster headache. BMC Neurol 2021, 21, 174. [Google Scholar] [CrossRef]
- Porta, M.; Granella, F. Treatment of cluster headache attacks with hyperbaric oxygen. Cephalalgia 1991, 11, 236–237. [Google Scholar] [CrossRef]
- Non-Pharmacological therapy of migraine. J. Headache Pain 2001, 2, 162–167. [CrossRef]

| Study Name | Publication Year | No. of Patients | Aims of Therapy | Types of Medications | Routes of Administration | Study Design | |
|---|---|---|---|---|---|---|---|
| Intervention | Comparator | ||||||
| Russell et al. [19] | 1979 | 12 | prevention | cimetidine, chlorpheniramine | placebo | PO | crossover |
| Ekbom et al. [20] | 1991 | 39 | acute | sumatriptan | placebo | SC | crossover |
| Ekbom et al. [21] | 1993 | 134 | acute | sumatriptan | placebo | SC | crossover |
| Marks et al. [22] | 1993 | 13 | acute | capsaicin | placebo | nasal cream | parallel |
| Monstad et al. [23] | 1995 | 168 | prevention | sumatriptan | placebo | PO | parallel |
| Leone et al. [24] | 1996 | 20 | prevention | melatonin | placebo | PO | parallel |
| Steiner et al. [25] | 1997 | 27 | acute | lithium carbonate | placebo | PO | parallel |
| Evers et al. [26] | 1998 | 8 | acute | misoprostol | placebo | PO | crossover |
| Bahra et al. [27] | 2000 | 124 | acute | zolmitriptan | placebo | PO | crossover |
| Leone et al. [28] | 2000 | 30 | prevention | verapamil | placebo | PO | parallel |
| Saper et al. [29] | 2002 | 28 | prevention | civamide | placebo | nasal | parallel |
| Amrani et al. [30] | 2002 | 95 | prevention | sodium valproate | placebo | PO | parallel |
| Vliet et al. [31] | 2003 | 118 | acute | sumatriptan | placebo | nasal | crossover |
| Matharu et al. [32] | 2004 | 57 | acute | octreotide | placebo | SC | crossover |
| Ambrosini et al. [33] | 2005 | 23 | prevention | betamethasone | placebo | SC | parallel |
| Cittadini et al. [34] | 2006 | 92 | acute | zolmitriptan | placebo | nasal | crossover |
| Pageler et al. [35] | 2011 | 10 | prevention | frovatriptan | placebo | PO | parallel |
| Leroux et al. [36] | 2011 | 43 | acute | cortivazol | placebo | SC | parallel |
| Hakim et al. [37] | 2011 | 34 | prevention | warfarin | placebo | PO | crossover |
| Tronvik et al. [38] | 2013 | 32 | prevention | candesartan cilexetil | placebo | PO | parallel |
| Goadsby et al. [39] | 2019 | 106 | prevention | galcanezumab | placebo | SC | parallel |
| Dodick et al. [40] | 2020 | 237 | prevention | galcanezumab | placebo | SC | parallel |
| Obermann et al. [41] | 2021 | 109 | prevention | prednisone | placebo | PO | parallel |
| Study Name | Publication Year | Mean Age (Year) | Male Proportion (%) | |
|---|---|---|---|---|
| Intervention Group | Control Group | |||
| Russell et al. [19] | 1979 | 49 | - | - |
| Ekbom et al. [20] | 1991 | 42 ± 10 | - | 79.5 |
| Ekbom et al. [21] | 1993 | 41 ± 9 | - | 86.6 |
| Marks et al. [22] | 1993 | - | - | 23.1 |
| Monstad et al. [23] | 1995 | 40 ± 10 | 40 ± 10 | 88.7 |
| Leone et al. [24] | 1996 | 38 | 34 | 75 |
| Steiner et al. [25] | 1997 | 34.5 ± 19 | 35.7 ± 20.8 | 100 |
| Evers et al. [26] | 1998 | 44.5 | - | 100 |
| Bahra et al. [27] | 2000 | 43.8 ± 10.9 | - | 86.3 |
| Leone et al. [28] | 2000 | 44 ± 8 | 43 ± 10 | 90 |
| Saper et al. [29] | 2002 | 45.1 ± 10.5 | 43.9 ± 16.3 | 89.3 |
| Amrani et al. [30] | 2002 | 47 ± 11.3 | 43.6 ± 11.5 | 88.4 |
| Vliet et al. [31] | 2003 | 43 ± 11 | 43 ± 11 | 82.2 |
| Matharu et al. [32] | 2004 | 40 ± 10 | - | 78.9 |
| Ambrosini et al. [33] | 2005 | 42 | 37.7 | 86.9 |
| Cittadini et al. [34] | 2006 | 40 ± 10 | - | 86.9 |
| Pageler et al. [35] | 2011 | - | - | - |
| Leroux et al. [36] | 2011 | CCH: 41.3 ± 13.3 ECH: 40.0 ± 7.8 | CCH: 42.8 ± 11.9 ECH: 41.9 ± 10.4 | 88.4 |
| Hakim et al. [37] | 2011 | 44.1 ± 5.1 | 45.2 ± 4.5 | 76.5 |
| Tronvik et al. [38] | 2013 | 42 ± 10.1 | 41 ± 12.1 | 84.4 |
| Goadsby et al. [39] | 2019 | 47 ± 11 | 45 ± 11 | 83 |
| Dodick et al. [40] | 2020 | 45.6 ± 11 | 44.4 ± 10.8 | 72.6 |
| Obermann et al. [41] | 2021 | 42.4 ± 11.4 | 40.3 ± 10.5 | 83.5 |
| Outcomes | No. of Participants (Studies) | Limitation | Inconsistency | Indirection | Imprecision | Publication Bias | Pooled Estimates | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|
| Efficacy outcomes | ||||||||
| Frequency of attacks | ||||||||
| Overall | 133 (6) | No serious | No serious | No serious | No serious | No serious | −1.05 (−1.62, −0.47) | ⊕⊕⊕⊕ High |
| Acute | 117 (5) | No serious | No serious | No serious | No serious | No serious | −1.05 (−1.62, −0.47) | ⊕⊕⊕⊕ High |
| Transitional | 133 (1) | No serious | No serious | No serious | No serious | No serious | −1.00 (−12.28, 10.28) | ⊕⊕⊕⊕ High |
| Triptans | 11 (1) | No serious | No serious | No serious | No serious | No serious | 4.00 (−6.04, 14.04) | ⊕⊕⊕⊕ High |
| Non-triptans | 122 (5) | No serious | No serious | No serious | No serious | No serious | −1.06 (−1.64, −0.49) | ⊕⊕⊕⊕ High |
| Duration of attacks | ||||||||
| Overall | 143 (3) | No serious | No serious | No serious | No serious | No serious | −1.08 (−13.60, 11.44) | ⊕⊕⊕⊕ High |
| Pain-free rate | ||||||||
| Overall | 747 (9) | No serious | No serious | No serious | No serious | No serious | 3.89 (2.76, 5.48) | ⊕⊕⊕⊕ High |
| Preventive | 91 (2) | No serious | No serious | No serious | No serious | No serious | 8.90 (2.85, 27.79) | ⊕⊕⊕⊕ High |
| Acute | 656 (7) | No serious | No serious | No serious | No serious | No serious | 3.53 (2.46, 5.07) | ⊕⊕⊕⊕ High |
| Triptans | 482 (3) | No serious | No serious | No serious | No serious | No serious | 3.88 (2.55, 5.90) | ⊕⊕⊕⊕ High |
| Non-triptans | 747 (6) | No serious | No serious | No serious | No serious | No serious | 3.90 (2.14, 7.11) | ⊕⊕⊕⊕ High |
| Number of people needing rescue agents | ||||||||
| Overall | 1388 (6) | No serious | No serious | No serious | No serious | No serious | 0.37 (0.27, 0.51) | ⊕⊕⊕⊕ High |
| Preventive | 95 (1) | No serious | No serious | No serious | No serious | No serious | 0.17 (0.06, 0.45) | ⊕⊕⊕⊕ High |
| Acute | 1293 (5) | No serious | No serious | No serious | No serious | No serious | 0.41 (0.32, 0.52) | ⊕⊕⊕⊕ High |
| Triptans | 1293 (5) | No serious | No serious | No serious | No serious | No serious | 0.40 (0.29, 0.54) | ⊕⊕⊕⊕ High |
| Non-triptans | 95 (1) | No serious | No serious | No serious | No serious | No serious | 0.17 (0.06, 0.45) | ⊕⊕⊕⊕ High |
| Safety outcomes | ||||||||
| Adverse events | ||||||||
| Overall | 1822 (16) | No serious | No serious | No serious | No serious | No serious | 2.16 (1.59, 2.94) | ⊕⊕⊕⊕ High |
| Preventive | 846 (10) | No serious | No serious | No serious | No serious | No serious | 1.66 (1.23, 2.23) | ⊕⊕⊕⊕ High |
| Acute | 928 (5) | No serious | No serious | No serious | No serious | No serious | 3.19 (2.33, 4.39) | ⊕⊕⊕⊕ High |
| Triptans | 1010 (3) | No serious | No serious | No serious | No serious | No serious | 2.79 (2.07, 3.76) | ⊕⊕⊕⊕ High |
| Non-triptans | 812 (13) | No serious | No serious | No serious | No serious | No serious | 1.64 (1.21, 2.23) | ⊕⊕⊕⊕ High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.-H.; Han, J.-Y.; Choi, J.-W.; Park, H.-R.; Lee, H. Comparative Impact of Pharmacological Therapies on Cluster Headache Management: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2022, 11, 1411. https://doi.org/10.3390/jcm11051411
Kwon J-H, Han J-Y, Choi J-W, Park H-R, Lee H. Comparative Impact of Pharmacological Therapies on Cluster Headache Management: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2022; 11(5):1411. https://doi.org/10.3390/jcm11051411
Chicago/Turabian StyleKwon, Jae-Hee, Ja-Young Han, Ji-Woong Choi, Hye-Rim Park, and Heeyoung Lee. 2022. "Comparative Impact of Pharmacological Therapies on Cluster Headache Management: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 11, no. 5: 1411. https://doi.org/10.3390/jcm11051411
APA StyleKwon, J.-H., Han, J.-Y., Choi, J.-W., Park, H.-R., & Lee, H. (2022). Comparative Impact of Pharmacological Therapies on Cluster Headache Management: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 11(5), 1411. https://doi.org/10.3390/jcm11051411






