Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting
Abstract
:1. Introduction
2. Materials and Methods
2.1. FFR and Angiogram Acquisition
2.2. ‘Virtual Stenting’ (Residual) vFFR Analysis
2.3. Statistical Analysis
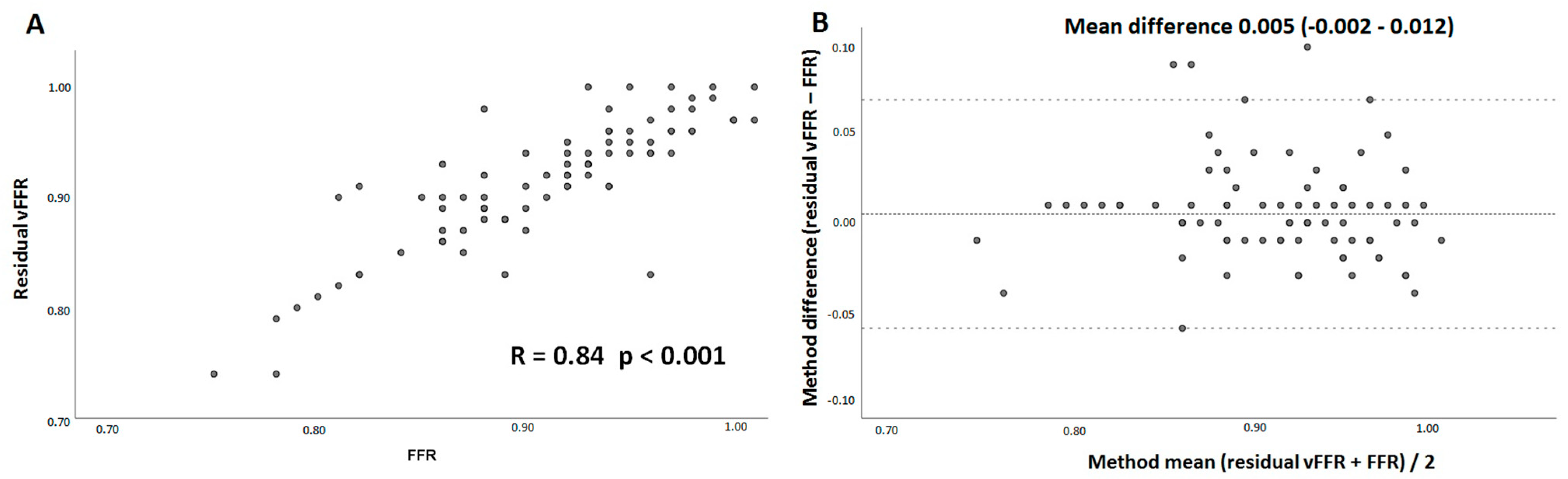
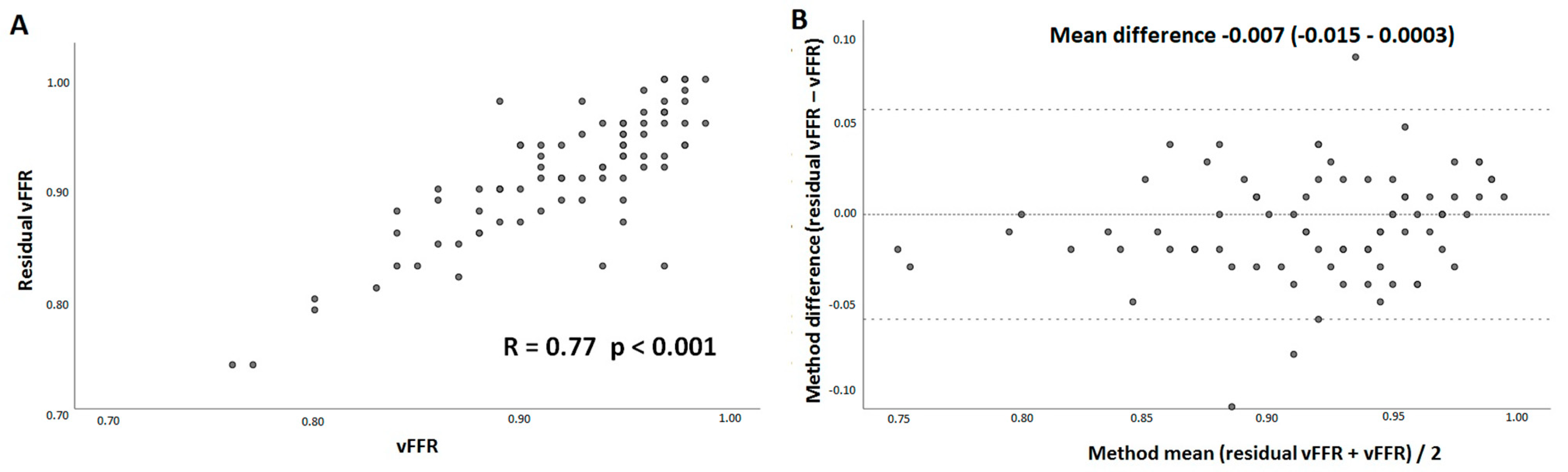
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.J.; Fearon, W.F.; Barbato, E.; Tonino, P.A.L.; Engstrom, T.; Kaab, S.; Dambrink, J.H.; Rioufol, G.; et al. Five-year outcomes with PCI Guided by fractional flow reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; vant Veer, M.; Klauss, V.; Manoharan, G.; Engstrom, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, F.M.; Omerovic, E.; Fournier, S.; Kelbaek, H.; Johnson, N.P.; Rothenbuhler, M.; Xaplanteris, P.; Abdel-Wahab, M.; Barbato, E.; Hofsten, D.E.; et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: Meta-analysis of individual patient data. Eur. Heart J. 2019, 40, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Patel, M.R.; Calhoon, J.H.; Dehmer, G.J.; Grantham, J.A.; Maddox, T.M.; Maron, D.J.; Smith, P.K. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: A report of the American college of cardiology appropriate use criteria task force, American association for thoracic surgery, American heart association, American society of echocardiography, American society of nuclear cardiology, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, and society of thoracic surgeons. J. Am. Coll. Cardiol. 2017, 69, 2212–2241. [Google Scholar] [CrossRef]
- Jeremias, A.; Davies, J.E.; Maehara, A.; Matsumura, M.; Schneider, J.; Tang, K.; Talwar, S.; Marques, K.; Shammas, N.W.; Gruberg, L.; et al. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: The DEFINE PCI study. JACC Cardiovasc. Interv. 2019, 12, 1991–2001. [Google Scholar] [CrossRef]
- Rimac, G.; Fearon, W.F.; De Bruyne, B.; Ikeno, F.; Matsuo, H.; Piroth, Z.; Costerousse, O.; Bertrand, O.F. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: A systematic review and meta-analysis. Am. Heart J. 2017, 183, 1–9. [Google Scholar] [CrossRef]
- Kasula, S.; Agarwal, S.K.; Hacioglu, Y.; Pothineni, N.K.; Bhatti, S.; Ahmed, Z.; Uretsky, B.; Hakeem, A. Clinical and prognostic value of poststenting fractional flow reserve in acute coronary syndromes. Heart 2016, 102, 1988–1994. [Google Scholar] [CrossRef]
- Wolfrum, M.; Fahrni, G.; de Maria, G.L.; Knapp, G.; Curzen, N.; Kharbanda, R.K.; Frohlich, G.M.; Banning, A.P. Impact of impaired fractional flow reserve after coronary interventions on outcomes: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2016, 16, 177. [Google Scholar] [CrossRef] [Green Version]
- Piroth, Z.; Toth, G.G.; Tonino, P.A.L.; Barbato, E.; Aghlmandi, S.; Curzen, N.; Rioufol, G.; Pijls, N.H.J.; Fearon, W.F.; Juni, P.; et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ. Cardiovasc. Interv. 2017, 10, e005233. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Tonino, P.A.; De Bruyne, B.; Siebert, U.; Pijls, N.H.; Investigators, F.S. Rationale and design of the fractional flow reserve versus angiography for multivessel evaluation (FAME) study. Am. Heart J. 2007, 154, 632–636. [Google Scholar] [CrossRef]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Mobius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Hwang, D.; Choi, K.H.; Rhee, T.M.; Park, J.; Kim, H.Y.; Jung, H.W.; Hwang, J.W.; Lee, H.J.; Jang, H.J.; et al. Prognostic implications of relative increase and final fractional flow reserve in patients with stent implantation. JACC Cardiovasc. Interv. 2018, 11, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lee, J.M.; Lee, H.J.; Kim, S.H.; Nam, C.W.; Hahn, J.Y.; Shin, E.S.; Matsuo, A.; Tanaka, N.; Matsuo, H.; et al. Influence of target vessel on prognostic relevance of fractional flow reserve after coronary stenting. EuroIntervention 2019, 15, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, A.; Llewellyn, A.; Walker, R.; Schmitt, L.; Wright, K.; Walker, S.; Rothery, C.; Simmonds, M. Non-invasive imaging software to assess the functional significance of coronary stenoses: A systematic review and economic evaluation. Health Technol. Assess. 2021, 25, 1–230. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Onuma, Y.; Sonck, J.; Asano, T.; Vandeloo, B.; Kornowski, R.; Tu, S.; Westra, J.; Holm, N.R.; Xu, B.; et al. Diagnostic performance of angiography-derived fractional flow reserve: A systematic review and Bayesian meta-analysis. Eur. Heart J. 2018, 39, 3314–3321. [Google Scholar] [CrossRef] [Green Version]
- Masdjedi, K.; van Zandvoort, L.J.C.; Balbi, M.M.; Gijsen, F.J.H.; Ligthart, J.M.R.; Rutten, M.C.M.; Lemmert, M.E.; Wilschut, J.; Diletti, R.; De Jaegere, P.; et al. Validation of 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve: Fast assessment of stenosis severity (FAST)-study. EuroIntervention 2019, 16, 591–599. [Google Scholar] [CrossRef]
- Westra, J.; Tu, S.; Winther, S.; Nissen, L.; Vestergaard, M.B.; Andersen, B.K.; Holck, E.N.; Maule, C.F.; Johansen, J.K.; Andreasen, L.N.; et al. Evaluation of Coronary artery stenosis by quantitative flow ratio during invasive coronary angiography: The WIFI II study (wire-free functional imaging II). Circ. Cardiovasc. Imaging 2018, 11, e007107. [Google Scholar] [CrossRef]
- Westra, J.; Andersen, B.K.; Campo, G.; Matsuo, H.; Koltowski, L.; Eftekhari, A.; Liu, T.; Di Serafino, L.; Di Girolamo, D.; Escaned, J.; et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve: The FAVOR II Europe-Japan study. J. Am. Heart Assoc. 2018, 7, e009603. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J. Am. Coll. Cardiol. 2017, 70, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, M.; Lavi, I.; De Bruyne, B.; Vaknin-Assa, H.; Assali, A.; Valtzer, O.; Lotringer, Y.; Weisz, G.; Almagor, Y.; Xaplanteris, P.; et al. Validation study of image-based fractional flow reserve during coronary angiography. Circ. Cardiovasc. Interv. 2017, 10, e005259. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Achenbach, S.; Engstrom, T.; Assali, A.; Shlofmitz, R.; Jeremias, A.; Fournier, S.; Kirtane, A.J.; Kornowski, R.; Greenberg, G.; et al. Accuracy of fractional flow reserve derived from coronary angiography. Circulation 2019, 139, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Masdjedi, K.; van Zandvoort, L.J.; Balbi, M.M.; Nuis, R.J.; Wilschut, J.; Diletti, R.; de Jaegere, P.P.T.; Zijlstra, F.; Van Mieghem, N.M.; Daemen, J. Validation of novel 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve post stenting. Catheter. Cardiovasc. Interv. 2020, 98, 671–677. [Google Scholar] [CrossRef]
- Rubimbura, V.; Guillon, B.; Fournier, S.; Amabile, N.; Pan, C.C.; Combaret, N.; Eeckhout, E.; Kibler, M.; Silvain, J.; Wijns, W.; et al. Quantitative flow ratio virtual stenting and post stenting correlations to post stenting fractional flow reserve measurements from the DOCTORS (Does optical coherence tomography optimize results of stenting) study population. Catheter. Cardiovasc. Interv. 2020, 96, 1145–1153. [Google Scholar] [CrossRef]
- Neleman, T.; Masdjedi, K.; Van Zandvoort, L.J.C.; Tomaniak, M.; Ligthart, J.M.R.; Witberg, K.T.; Vermaire, A.A.; Boersma, E.; Van Mieghem, N.M.; Daemen, J. Extended validation of novel 3D quantitative coronary angiography-based software to calculate vFFR: The FAST EXTEND study. JACC Cardiovasc. Imaging 2021, 14, 504–506. [Google Scholar] [CrossRef]
- Feldmann, K.; Cami, E.; Safian, R.D. Planning percutaneous coronary interventions using computed tomography angiography and fractional flow reserve-derived from computed tomography: A state-of-the-art review. Catheter. Cardiovasc. Interv. 2019, 93, 298–304. [Google Scholar] [CrossRef]
- Gosling, R.C.; Morris, P.D.; Soto, D.A.S.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Virtual coronary intervention: A treatment planning tool based upon the angiogram. JACC Cardiovasc. Imaging 2019, 12, 865–872. [Google Scholar] [CrossRef]
- Haley, H.A.; Ghobrial, M.; Morris, P.D.; Gosling, R.; Williams, G.; Mills, M.T.; Newman, T.; Rammohan, V.; Pederzani, G.; Lawford, P.V.; et al. Virtual (computed) fractional flow reserve: Future role in acute coronary syndromes. Front. Cardiovasc. Med. 2021, 8, 735008. [Google Scholar] [CrossRef]
- Gosling, R.C.; Adam, Z.; Barmby, D.S.; Iqbal, J.; Morgan, K.P.; Richardson, J.D.; Rothman, A.M.K.; Lawford, P.V.; Hose, D.R.; Gunn, J.P.; et al. The impact of virtual fractional flow reserve and virtual coronary intervention on treatment decisions in the cardiac catheter laboratory. Can. J. Cardiol. 2021, 37, 1530–1538. [Google Scholar] [CrossRef]
- Diletti, R.; Masdjedi, K.; Daemen, J.; van Zandvoort, L.J.C.; Neleman, T.; Wilschut, J.; Den Dekker, W.K.; van Bommel, R.J.; Lemmert, M.; Kardys, I.; et al. Impact of poststenting fractional flow reserve on long-term clinical outcomes: The FFR-SEARCH study. Circ. Cardiovasc. Interv. 2021, 14, e009681. [Google Scholar] [CrossRef] [PubMed]
- van Bommel, R.J.; Masdjedi, K.; Diletti, R.; Lemmert, M.E.; van Zandvoort, L.; Wilschut, J.; Zijlstra, F.; de Jaegere, P.; Daemen, J.; van Mieghem, N.M. Routine fractional flow reserve measurement after percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2019, 12, e007428. [Google Scholar] [CrossRef]
- Jin, C.; Ramasamy, A.; Safi, H.; Kilic, Y.; Tufaro, V.; Bajaj, R.; Fu, G.; Mathur, A.; Bourantas, C.V.; Baumbach, A. Diagnostic accuracy of quantitative flow ratio (QFR) and vessel fractional flow reserve (vFFR) estimated retrospectively by conventional radiation saving X-ray angiography. Int. J. Cardiovasc. Imaging 2021, 37, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Gosling, R.; Rammohan, V.; Pederzani, G.; Garg, P.; Heppenstall, J.; Hose, D.R.; Lawford, P.V.; Narracott, A.J.; Fenner, J.; et al. The importance of three dimensional coronary artery reconstruction accuracy when computing virtual fractional flow reserve from invasive angiography. Sci. Rep. 2021, 11, 19694. [Google Scholar] [CrossRef] [PubMed]
- van Zandvoort, L.J.C.; Masdjedi, K.; Witberg, K.; Ligthart, J.; Forero, M.N.T.; Diletti, R.; Lemmert, M.E.; Wilschut, J.; de Jaegere, P.P.T.; Boersma, E.; et al. Explanation of postprocedural fractional flow reserve below 0.85. Circ. Cardiovasc. Interv. 2019, 12, e007030. [Google Scholar] [CrossRef]
- Beygui, F.; Lemaitre, A.; Bignon, M.; Wain-Hobson, J.; Briet, C.; Ardouin, P.; Sabatier, R.; Parienti, J.J.; Blanchart, K.; Roule, V. A head-to-head comparison of three coronary fractional flow reserve measurement technologies: The fractional flow reserve-device study. Catheter. Cardiovasc. Interv. 2020, 95, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Jaffe, W.; Watson, T.; Webster, M. Assessment of coronary fractional flow reserve using a monorail pressure catheter: The first-in-human ACCESS-NZ trial. EuroIntervention 2015, 11, 257–263. [Google Scholar] [CrossRef]


| n = 81 | |
|---|---|
| Age | 64.0 ± 11.0 |
| Male | 48 (59.3%) |
| BMI | 27.1 ± 4.6 |
| Diabetes | 20 (24.7%) |
| Hypertension | 47 (58.0%) |
| Dyslipidaemia | 42 (51.9%) |
| Prior PCI | 25 (30.9%) |
| Prior MI | 15 (18.5%) |
| Prior stroke | 15 (18.5%) |
| Peripheral artery disease | 11 (13.5%) |
| Current smoker | 25 (30.9%) |
| Creatinine (mmol/dl) | 90.8 ± 31.0 |
| n = 81 | |
|---|---|
| Measured artery | |
| Left main coronary artery | 2 (2.4) |
| Left anterior descending | 40 (49.4) |
| Left circumflex | 20 (24.7) |
| Right coronary artery | 19 (23.5) |
| American College of Cardiology (ACC)/American Heart Associations (AHA) lesion type | |
| A | 10 (12.3) |
| B1 | 21 (25.9) |
| B2 | 22 (27.2) |
| C | 28 (34.6) |
| Bifurcation | 10 (12.3%) |
| Calcification | 64.0 ± 11.0 |
| Three-dimensional-quantitative coronary angiography (QCA) analyses | |
| Diameter stenosis (%) | 53 ± 15 |
| Minimal lumen diameter, mm | 1.39 ± 0.96 |
| Lesion length, mm | 17 ± 9 |
| Reference diameter, mm | 2.90 ± 0.65 |
| Percutaneous coronary intervention (PCI) Procedure | |
| Predilatation | 52 (64.2%) |
| Number of stents implanted | 1.44 ± 0.67 |
| Postdilatation | 62 (76.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaniak, M.; Neleman, T.; Ziedses des Plantes, A.; Masdjedi, K.; van Zandvoort, L.J.C.; Kochman, J.; den Dekker, W.K.; Wilschut, J.M.; Diletti, R.; Kardys, I.; et al. Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting. J. Clin. Med. 2022, 11, 1397. https://doi.org/10.3390/jcm11051397
Tomaniak M, Neleman T, Ziedses des Plantes A, Masdjedi K, van Zandvoort LJC, Kochman J, den Dekker WK, Wilschut JM, Diletti R, Kardys I, et al. Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting. Journal of Clinical Medicine. 2022; 11(5):1397. https://doi.org/10.3390/jcm11051397
Chicago/Turabian StyleTomaniak, Mariusz, Tara Neleman, Anniek Ziedses des Plantes, Kaneshka Masdjedi, Laurens J. C. van Zandvoort, Janusz Kochman, Wijnand K. den Dekker, Jeroen M. Wilschut, Roberto Diletti, Isabella Kardys, and et al. 2022. "Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting" Journal of Clinical Medicine 11, no. 5: 1397. https://doi.org/10.3390/jcm11051397
APA StyleTomaniak, M., Neleman, T., Ziedses des Plantes, A., Masdjedi, K., van Zandvoort, L. J. C., Kochman, J., den Dekker, W. K., Wilschut, J. M., Diletti, R., Kardys, I., Zijlstra, F., Van Mieghem, N. M., & Daemen, J. (2022). Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting. Journal of Clinical Medicine, 11(5), 1397. https://doi.org/10.3390/jcm11051397






