Predictors of Abdominal Aortic Aneurysm Shrinkage after Endovascular Repair
Abstract
1. Introduction
2. Materials and Methods
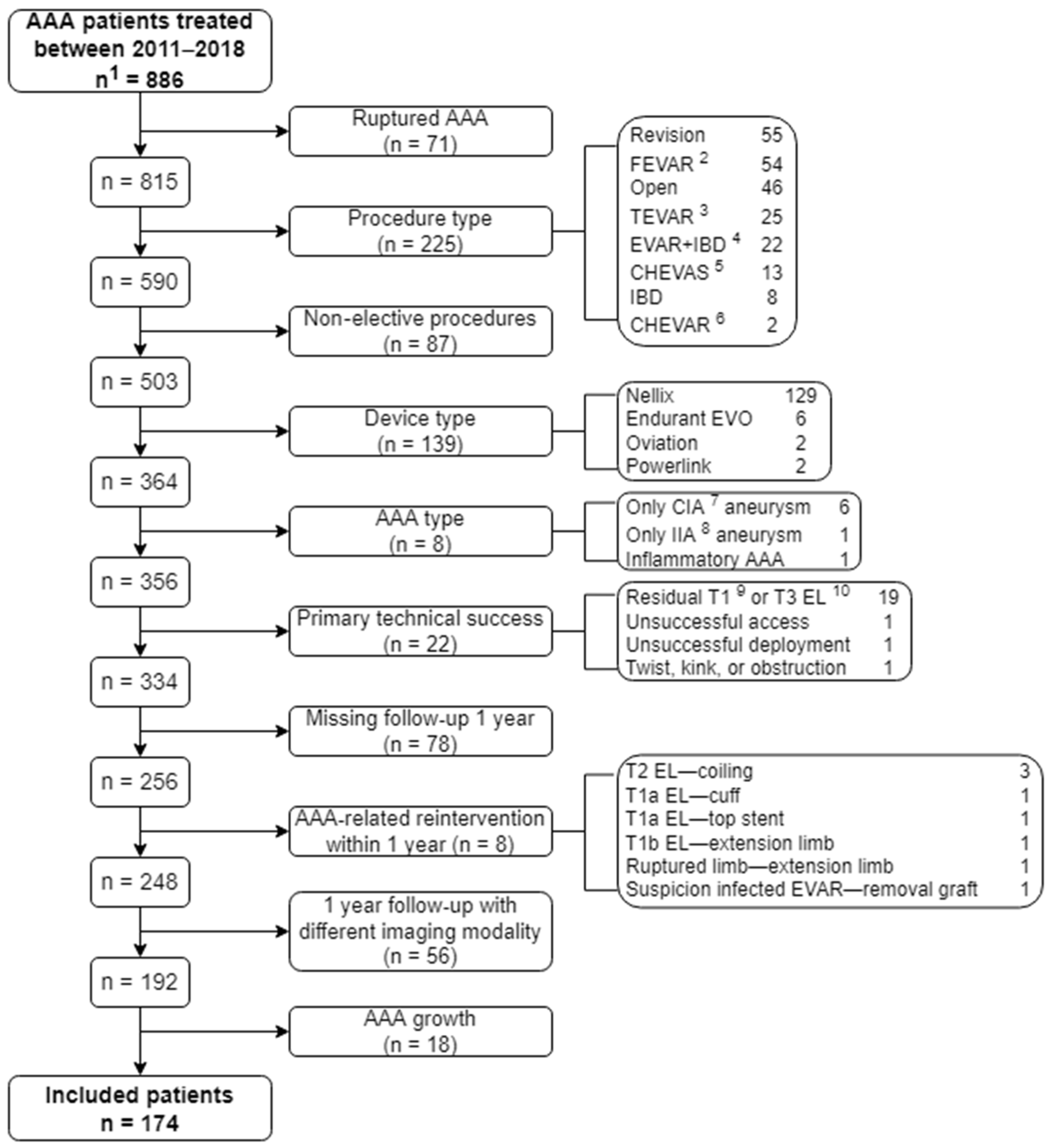
2.1. Study Design and Patient Population
2.2. Definitions and Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albuquerque, F.C.; Tonnessen, B.H.; Noll, R.E.; Cires, G.; Kim, J.K.; Sternbergh, W.C. Paradigm shifts in the treatment of abdominal aortic aneurysm: Trends in 721 patients between 1996 and 2008. J. Vasc. Surg. 2010, 51, 1348–1352. [Google Scholar] [CrossRef][Green Version]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef]
- Schanzer, A.; Greenberg, R.K.; Hevelone, N.; Robinson, W.P.; Eslami, M.H.; Goldberg, R.J.; Messina, L. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011, 123, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Tucker, L.-Y.; Goodney, P.; Candell, L.; Hua, H.; Okuhn, S.; Hill, B.; Chang, R.W. Type II endoleak with or without intervention after endovascular aortic aneurysm repair does not change aneurysm-related outcomes despite sac growth. J. Vasc. Surg. 2015, 62, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.L.; Zeebregts, C.J.; Verhagen, H.J.M.; Teijink, J.A.W.; Power, A.H.; Bockler, D.; Peeters, P.; Riambau, V.; Becquemin, J.P.; Reijnen, M.M.P.J. Incidence, natural course, and outcome of type II endoleaks in infrarenal endovascular aneurysm repair based on the ENGAGE registry data. J. Vasc. Surg. 2020, 71, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Kim, H.-K.; Huh, S. Incidence and Risk Factors for Sac Expansion after Endovascular Aneurysm Repair of Abdominal Aortic Aneurysms. Vasc. Spec. Int. 2021, 37. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, T.F.X.; Deery, S.E.; Boitano, L.T.; Siracuse, J.J.; Schermerhorn, M.L.; Scali, S.T.; Schanzer, A.; Lancaster, R.T.; Patel, V.I. Aneurysm sac failure to regress after endovascular aneurysm repair is associated with lower long-term survival. J. Vasc. Surg. 2019, 69, 414–422. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Alfahad, A.; Antoniou, S.A.; Torella, F. Prognostic significance of aneurysm sac shrinkage after endovascular aneurysm repair. J. Endovasc. Ther. 2020, 27, 857–868. [Google Scholar] [CrossRef]
- Houbballah, R.; Majewski, M.; Becquemin, J.P. Significant sac retraction after endovascular aneurysm repair is a robust indicator of durable treatment success. J. Vasc. Surg. 2010, 52, 878–883. [Google Scholar] [CrossRef]
- Bastos Gonçalves, F.; Baderkhan, H.; Verhagen, H.J.M.; Wanhainen, A.; Björck, M.; Stolker, R.J.; Hoeks, S.E.; Mani, K. Early sac shrinkage predicts a low risk of late complications after endovascular aortic aneurysm repair. Br. J. Surg. 2014, 101, 802–810. [Google Scholar] [CrossRef]
- Plá Sánchez, F.; Martínez López, I.; Hernández Mateo, M.M.; Marqués de Marino, P.; Ucles Cabeza, O.; Baturone Blanco, A.; Serrano Hernando, F.J. Prognostic Value of Initial Aneurysmal Sac Regression after EVAR. Ann. Vasc. Surg. 2020, 64, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lalys, F.; Daoudal, A.; Gindre, J.; Göksu, C.; Lucas, A.; Kaladji, A. Influencing factors of sac shrinkage after endovascular aneurysm repair. J. Vasc. Surg. 2017, 65, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- van Rijswijk, R.E.; Groot Jebbink, E.; Zeebregts, C.J.A.M.; Reijnen, M.M.P.J. A systematic review on anatomical predictors of abdominal aortic aneurysm remodeling after endovascular repair. J. Vasc. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Fillinger, M.F.; Matsumura, J.S.; Rutherford, R.B.; White, G.H.; Blankensteijn, J.D.; Bernhard, V.M.; Harris, P.L.; Kent, K.C.; May, J.; et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1061–1066. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Karthikesalingam, A.; Holt, P.J.; Vidal-Diez, A.; Choke, E.C.; Patterson, B.O.; Thompson, L.J.; Ghatwary, T.; Bown, M.J.; Sayers, R.D.; Thompson, M.M. Predicting aortic complications after endovascular aneurysm repair. Br. J. Surg. 2013, 100, 1302–1311. [Google Scholar] [CrossRef]
- Karthikesalingam, A.; Vidal-Diez, A.; De Bruin, J.L.; Thompson, M.M.; Hinchliffe, R.J.; Loftus, I.M.; Holt, P.J. International validation of a risk score for complications and reinterventions after endovascular aneurysm repair. Br. J. Surg. 2015, 102, 509–515. [Google Scholar] [CrossRef]
- Wanhainen, A.; Bergqvist, D.; Björck, M. Measuring the Abdominal Aorta with Ultrasonography and Computed Tomography—Difference and Variability. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 428–434. [Google Scholar] [CrossRef]
- Elliott, A.C.; Woodward, W.A. Statistical Analysis Quick Reference Guidebook: With SPSS Examples; Sage: Newcastle upon Tyne, UK, 2007. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Yeung, J.J.; Hernandez-Boussard, T.M.; Song, T.K.; Dalman, R.L.; Lee, J.T.A. Preoperative thrombus volume predicts sac regression after endovascular aneurysm repair. J. Endovasc. Ther. 2009, 16, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, T.; Dardik, A.; Koizumi, J.; Kano, M.; Akiyama, S.; Iwahashi, T.; Kamiya, K.; Fujiyoshi, T.; Ogino, H. Simple renal cyst and its association with sac shrinkage after endovascular aneurysm repair for abdominal aortic aneurysms. J. Vasc. Surg. 2020, 71, 1890–1898.e1. [Google Scholar] [CrossRef] [PubMed]
- Muhs, B.E.; Jordan, W.; Ouriel, K.; Rajaee, S.; de Vries, J.P. Matched cohort comparison of endovascular abdominal aortic aneurysm repair with and without EndoAnchors. J. Vasc. Surg. 2018, 67, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, E.A.; Fillinger, M.; Robinson, W.P.; Hodgson, K.; Jordan, W.D.; Beck, A.W.; Malas, M.; Belkin, M. Lombard Aorfix high angulation device, sac behavior following implantation. J. Vasc. Surg. 2017, 66, 71–78. [Google Scholar] [CrossRef]
- Shingaki, M.; Morishita, K.; Baba, T.; Shibata, T.; Narayama, K. Predictive Factors for Abdominal Aortic Aneurysm Shrinkage One Year after Successful Endovascular Aneurysm Repair. Ann. Vasc. Surg. 2018, 53, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.J.; Bartoli, M.A.; Mancini, J.; Lerussi, G.; Thevenin, B.; Sarlon-Bartoli, G.; Magnan, P.E. Aneurysm sac shrinkage after endovascular repair: Predictive factors and long-term follow-up. Ann. Vasc. Surg. 2015, 29, 770–779. [Google Scholar] [CrossRef]
- Hiraoka, A.; Chikazawa, G.; Ishida, A.; Miyake, K.; Totsugawa, T.; Tamura, K.; Sakaguchi, T.; Yoshitaka, H. Impact of age and intraluminal thrombus volume on abdominal aortic aneurysm sac enlargement after endovascular repair. Ann. Vasc. Surg. 2015, 29, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Boutrous, M.L.; Peterson, B.G.; Smeds, M.R. Predictors of Aneurysm Sac Shrinkage Utilizing a Global Registry. Ann. Vasc. Surg. 2021, 71, 40–47. [Google Scholar] [CrossRef]
- Pejcic, S.; Hassan, S.M.A.; Rival, D.E.; Bisleri, G. Characterizing the mechanical properties of the aortic wall. Vessel Plus 2019, 3, 32. [Google Scholar] [CrossRef]
- Teijink, J.A.W.; Power, A.H.; Böckler, D.; Peeters, P.; van Sterkenburg, S.; Bouwman, L.H.; Verhagen, H.J.; Bosiers, M.; Riambau, V.; Becquemin, J.P.; et al. Editor’s Choice—Five Year Outcomes of the Endurant Stent Graft for Endovascular Abdominal Aortic Aneurysm Repair in the ENGAGE Registry. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, J.; Ferreira, R.S.; Oliveira, N.F.G.; Hoeks, S.; Van Rijn, M.J.; Raa, S.T.; Mansilha, A.; Verhagen, H.J.M.; Gonçalves, F.B. Total Luminal Volume Predicts Risk after Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Ciompi, F.; Wolterink, J.M.; de Vos, B.D.; Leiner, T.; Teuwen, J.; Išgum, I. State-of-the-Art Deep Learning in Cardiovascular Image Analysis. JACC Cardiovasc. Imaging 2019, 12, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2015, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Hao, Y.; Wang, Z.; Xuan, X.; Kong, L.; Xue, H.; Jin, Z. CT texture analysis predicts abdominal aortic aneurysm post-endovascular aortic aneurysm repair progression. Sci. Rep. 2020, 10, 12268. [Google Scholar] [CrossRef]
- Hiraoka, A.; Chikazawa, G.; Ishida, A.; Totsugawa, T.; Tamura, K.; Sakaguchi, T.; Yoshitaka, H. Preoperative Coil Embolization of Side Branches and Postoperative Antifibrinolytic Therapy in Endovascular Aneurysm Repair: A Propensity Score Analysis. J. Vasc. Interv. Radiol. 2017, 28, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Branzan, D.; Geisler, A.; Steiner, S.; Doss, M.; Matschuck, M.; Scheinert, D.; Schmidt, A. Type II endoleak and aortic aneurysm sac shrinkage after preemptive embolization of aneurysm sac side branches. J. Vasc. Surg. 2021, 73, 1973–1979.e1. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Deery, S.E.; Ergul, E.A.; Schermerhorn, M.L.; Siracuse, J.J.; Schanzer, A.; Goodney, P.P.; Cambria, R.P.; Patel, V.I. Aneurysm sac expansion is independently associated with late mortality in patients treated with endovascular aneurysm repair. J. Vasc. Surg. 2018, 67, 157–164. [Google Scholar] [CrossRef]

| Variables | Total Population | Stable | Shrinkage | p-Value |
|---|---|---|---|---|
| Number of patients | 174 | 107 (61.5) | 67 (38.5) | |
| Age (years) | 71.8 ± 7.6 | 72.6 ± 7.3 | 70.4 ± 7.9 | 0.061 |
| Male sex | 149 (85.6) | 88 (82.2) | 61 (91.0) | 0.107 |
| BMI 1 (kg/m2) | 26.7 ± 3.7 | 26.9 ± 4.0 | 26.4 ± 3.1 | 0.361 |
| Systolic blood pressure (mmHg) | 142 ± 21 | 142 ± 23 | 142 ± 20 | 0.969 |
| Diastolic blood pressure (mmHg) | 80 ± 10 | 80 ± 11 | 81 ± 10 | 0.609 |
| ASA 2 classification | 0.734 | |||
| 1 | 2 (1.2) | 1 (0.9) | 1 (1.5) | |
| 2 | 94 (54.3) | 55 (51.4) | 39 (59.1) | |
| 3 | 70 (40.5) | 46 (43.0) | 24 (36.4) | |
| 4 | 7 (4) | 5 (4.7) | 2 (3.0) | |
| SVS/AAVS 3 risk score | 7.5 ± 5.2 | 7.8 ± 5.2 | 7.0 ± 5.2 | 0.383 |
| SVS/AAVS risk score 0–3 | 0.77 ± 0.67 | 0.81 ± 0.68 | 0.71 ± 0.65 | 0.350 |
| SVS/AAVS risk score category | 0.628 | |||
| Absent | 57 (36.1) | 34 (34.0) | 23 (39.7) | |
| Mild | 80 (50.6) | 51 (51.0) | 29 (50.0) | |
| Moderate | 21 (13.3) | 15 (15.0) | 6 (10.3) | |
| SGVI 4 score | 3.1 ± 0.5 | 3.1 ± 0.5 | 3.0 ± 0.5 | 0.735 |
| SGVI score high risk | 11 (7.7) | 7 (7.9) | 4 (7.4) | 0.921 |
| Risk factors | ||||
| Smoking | 60 (36.1) | 34 (33.7) | 26 (40.0) | 0.407 |
| Diabetes mellitus | 31 (17.8) | 17 (15.9) | 14 (20.9) | 0.401 |
| Hypertension | 123 (70.7) | 79 (73.8) | 44 (65.7) | 0.250 |
| Hyperlipidemia | 134 (84.3) | 81 (82.7) | 53 (86.9) | 0.476 |
| Inflammatory diseases | 27 (15.7) | 19 (18.1) | 8 (11.9) | 0.279 |
| Comorbidities | ||||
| Cardiac status | 77 (46.7) | 50 (48.5) | 27 (43.5) | 0.533 |
| Renal status | 48 (27.9) | 31 (29.2) | 17 (25.8) | 0.620 |
| Pulmonary status | 37 (22.0) | 26 (25.0) | 11 (17.2) | 0.235 |
| Coronary artery disease | 15 (8.6) | 10 (9.3) | 5 (7.5) | 0.667 |
| COPD 5 | 29 (16.7) | 20 (18.7) | 9 (13.4) | 0.365 |
| Lab results | ||||
| Hemoglobin (mmol/L) | 9.0 ± 0.9 | 8.9 ± 0.9 | 9.1 ± 0.8 | 0.045 |
| Leukocytes (×109/L) | 8.4 ± 2.5 | 8.5 ± 2.7 | 8.2 ± 2.0 | 0.441 |
| Creatinine (µmol/L) | 93 ± 30 | 94 ± 32 | 91 ± 28 | 0.428 |
| GFR 6 (mL/min/1.73 m2) | 70 ± 17 | 69 ± 18 | 73 ± 16 | 0.094 |
| Medication | ||||
| Anticoagulant therapy | 141 (84.9) | 87 (85.3) | 54 (84.4) | 0.872 |
| Antiplatelet therapy | 146 (83.9) | 86 (80.4) | 60 (89.6) | 0.109 |
| Metformin | 26 (14.9) | 15 (14.0) | 11 (16.4) | 0.666 |
| Statins | 135 (77.6) | 80 (74.8) | 55 (82.1) | 0.260 |
| Variables | Total Population | Stable | Shrinkage | p-Value |
|---|---|---|---|---|
| Number of patients | 174 | 107 (61.5) | 67 (38.5) | |
| Preoperative AAA 1 geometry | ||||
| Infrarenal neck diameter (mm) | 23.7 ± 3.2 | 23.9 ± 3.4 | 23.4 ± 3.0 | 0.395 |
| Infrarenal neck length (mm) | 29.4 ± 13.2 | 30.0 ± 13.6 | 28.3 ± 12.7 | 0.421 |
| Infrarenal β angle (°) | 51.7 ± 16.3 | 53.2 ± 16.8 | 49.2 ± 15.3 | 0.121 |
| Maximum AAA diameter (mm) | 54.3 ± 8.6 | 53.7 ± 9.1 | 55.3 ± 7.6 | 0.221 |
| Maximum CIA diameter (mm) | 18.6 ± 8.0 | 19.1 ± 7.9 | 17.9 ± 8.2 | 0.392 |
| Maximum EIA 2 diameter (mm) | 9.4 ± 2.5 | 9.3 ± 2.0 | 9.6 ± 3.2 | 0.449 |
| Device | 0.982 | |||
| Medtronic Endurant | 102 (58.6) | 64 (59.8) | 38 (56.7) | |
| Gore Excluder | 51 (29.3) | 31 (29.0) | 20 (29.9) | |
| Endologix AFX | 16 (9.2) | 9 (8.4) | 7 (10.4) | |
| Cook Zenith | 3 (1.7) | 2 (1.9) | 1 (1.5) | |
| Vascutek Anaconda | 2 (1.1) | 1 (0.9) | 1 (1.5) | |
| Graft material | 0.701 | |||
| Polyester | 107 (61.5) | 67 (62.6) | 40 (59.7) | |
| PTFE 3 | 67 (38.5) | 40 (37.4) | 27 (40.3) | |
| Blood loss (mL) | 183 ± 313 | 177 ± 293 | 193 ± 346 | 0.746 |
| Procedure time (min) | 98 ± 45 | 100 ± 46 | 95 ± 42 | 0.459 |
| Perioperative residual endoleak | 36 (20.9) | 22 (21.0) | 14 (20.9) | 0.993 |
| Type I endoleak | 0 (0) | 0 (0) | 0 (0) | NA 4 |
| Type II endoleak | 33 (19.0) | 19 (17.8) | 14 (20.9) | 0.607 |
| Type III endoleak | 0 (0) | 0 (0) | 0 (0) | NA |
| Type IV endoleak | 1 (0.6) | 1 (0.9) | 1 (0.9) | 0.427 |
| Days at hospital | 3.4 ± 1.9 | 3.6 ± 2.0 | 3.2 ± 1.7 | 0.139 |
| Days at ICU 5 | 0.01 ± 0.08 | 0.01 ± 0.1 | 0.00 ± 0.00 | 0.417 |
| Complications during hospitalization | 31 (17.9) | 20 (18.9) | 11 (16.4) | 0.682 |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 0.96 (0.92–1.00) | 0.063 | 0.95 (0.91–0.995) | 0.027 |
| Male sex | 2.20 (0.83–5.82) | 0.114 | NA | |
| Hemoglobin (mmol/L) | 1.47 (1.00–2.16) | 0.051 | NA | |
| GFR (mL/min/1.73 m2) | 1.02 (1.00–1.04) | 0.096 | NA | |
| Infrarenal β angle (°) | 0.99 (0.97–1.00) | 0.122 | 0.98 (0.91–0.995) | 0.045 |
| Maximum AAA diameter (mm) | 1.02 (0.99–1.06) | 0.223 | 1.05 (1.004–1.09) | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rijswijk, R.E.; Groot Jebbink, E.; Holewijn, S.; Stoop, N.; van Sterkenburg, S.M.; Reijnen, M.M.P.J. Predictors of Abdominal Aortic Aneurysm Shrinkage after Endovascular Repair. J. Clin. Med. 2022, 11, 1394. https://doi.org/10.3390/jcm11051394
van Rijswijk RE, Groot Jebbink E, Holewijn S, Stoop N, van Sterkenburg SM, Reijnen MMPJ. Predictors of Abdominal Aortic Aneurysm Shrinkage after Endovascular Repair. Journal of Clinical Medicine. 2022; 11(5):1394. https://doi.org/10.3390/jcm11051394
Chicago/Turabian Stylevan Rijswijk, Rianne E., Erik Groot Jebbink, Suzanne Holewijn, Nicky Stoop, Steven M. van Sterkenburg, and Michel M. P. J. Reijnen. 2022. "Predictors of Abdominal Aortic Aneurysm Shrinkage after Endovascular Repair" Journal of Clinical Medicine 11, no. 5: 1394. https://doi.org/10.3390/jcm11051394
APA Stylevan Rijswijk, R. E., Groot Jebbink, E., Holewijn, S., Stoop, N., van Sterkenburg, S. M., & Reijnen, M. M. P. J. (2022). Predictors of Abdominal Aortic Aneurysm Shrinkage after Endovascular Repair. Journal of Clinical Medicine, 11(5), 1394. https://doi.org/10.3390/jcm11051394






