Clinical Outcomes in Patients Aged 80 Years or Older Receiving Non-Invasive Respiratory Support for Hypoxemic Acute Respiratory Failure Consequent to COVID-19
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Interventions
2.3. Outcome Measures and Statistical Analysis
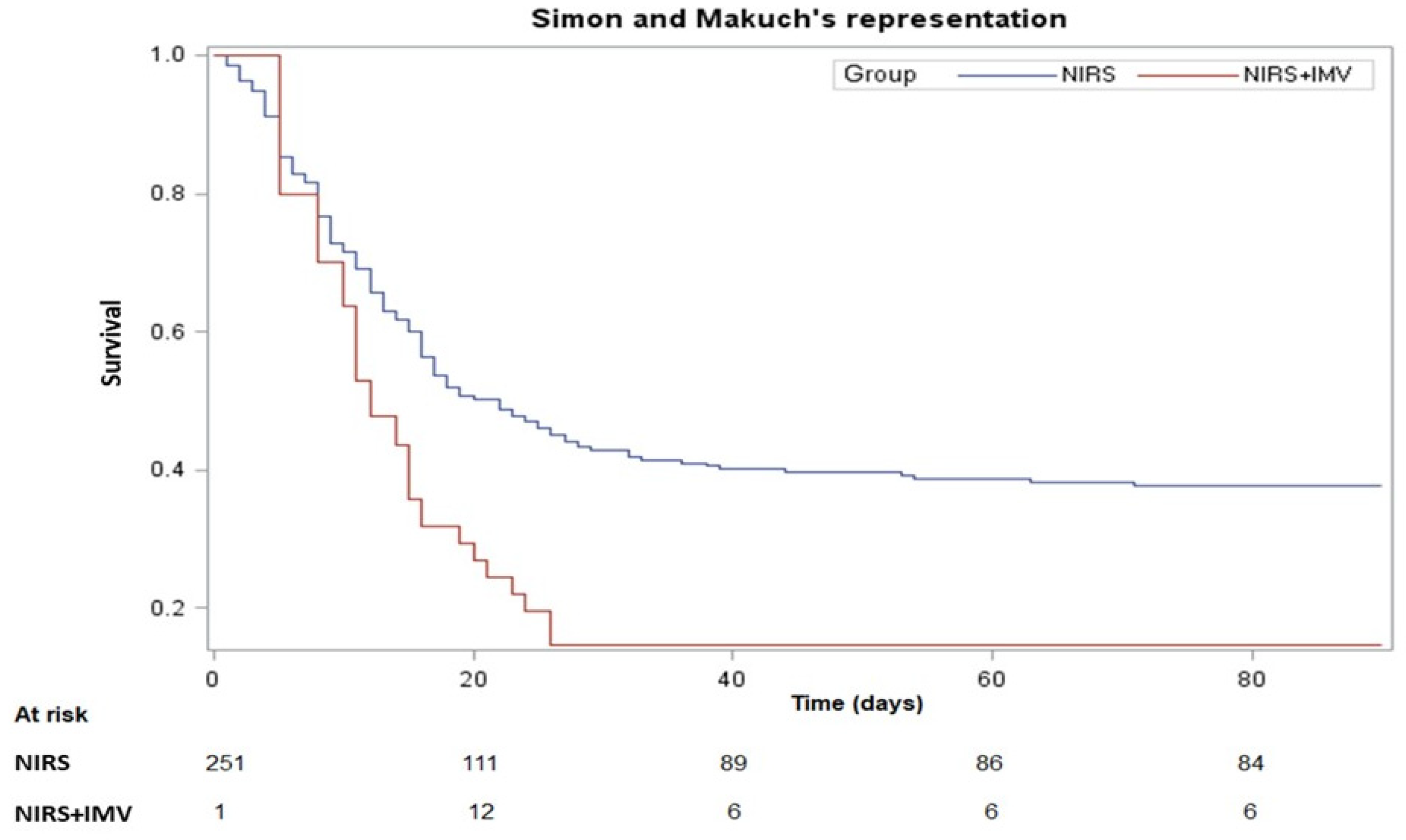
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Livingston, E.; Bucher, K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 2020, 323, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Capdevila-Reniu, A.; Pellice, M.; Prieto-Gonzalez, S.; Ventosa, H.; Ladino, A.; Naval, J.; Rodriguez-Nuñez, O.; César Milisenda, J.; Moreno-Lozano, P.J.; Soriano, A.; et al. Clinical characteristics and outcome of patients aged over 80 years with COVID-19. Medicine 2021, 100, e24750. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Flaatten, H.; Fjølner, J.; Bruno, R.R.; Wernly, B.; Artigas, A.; Pinto, B.B.; Schefold, J.C.; Wolff, G.; Kelm, M.; et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: The COVIP study. Crit. Care 2021, 25, 149. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Aziz, S.; Arabi, Y.M.; Alhazzani, W.; Evans, L.; Citerio, G.; Fischkoff, K.; Salluh, J.; Meyfroidt, G.; Alshamsi, F.; Oczkowski, S.; et al. Managing ICU surge during the COVID-19 crisis: Rapid guidelines. Intensiv. Care Med. 2020, 46, 1303–1325. [Google Scholar] [CrossRef]
- Franco, C.; Facciolongo, N.; Tonelli, R.; Dongilli, R.; Vianello, A.; Pisani, L.; Scala, R.; Malerba, M.; Carlucci, A.; Negri, E.A.; et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur. Respir. J. 2020, 56, 2002130. [Google Scholar] [CrossRef]
- Aliberti, S.; Radovanovic, D.; Billi, F.; Sotgiu, G.; Costanzo, M.; Pilocane, T.; Saderi, L.; Gramegna, A.; Rovellini, A.; Perotto, L.; et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: A multicentre cohort study. Eur. Respir. J. 2020, 56, 2001935. [Google Scholar] [CrossRef]
- Vaschetto, R.; Barone-Adesi, F.; Racca, F.; Pissaia, C.; Maestrone, C.; Colombo, D.; Olivieri, C.; Vita, N.D.; Santangelo, E.; Scotti, L.; et al. Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Res. 2021, 7, 00541–2020. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Dolly, S.; Ng, L.; Prior-Ong, M.; Sabapathy, K. The role of CPAP as a potential bridge to invasive ventilation and as a ceiling-of-care for patients hospitalized with COVID-19-An observational study. PLoS ONE 2020, 15, e0244857. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance; World Health Organization: Geneva, Switzerland, 13 March 2020. [Google Scholar]
- Gungor, G.; Tatar, D.; Salturk, C.; Çimen, P.; Karakurt, Z.; Kirakli, C.; Adıgüzel, N.; Ediboğlu, Ö.; Yılmaz, H.; Moçin, Ö.Y.; et al. Why do patients with interstitial lung diseases fail in the ICU? A 2-center cohort study. Respir. Care 2013, 58, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Blaylock, A.; Cason, C.L. Discharge planning predicting patients’ needs. J. Gerontol. Nurs. 1992, 18, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; De Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Strini, V.; Piazzetta, N.; Gallo, A.; Schiavolin, R. Barthel Index: Creation and validation of two cut-offs using the BRASS Index. Acta Biomed. 2020, 91, 19–26. [Google Scholar]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef]
- Lim, Z.J.; Subramaniam, A.; Ponnapa, R.M.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66. [Google Scholar] [CrossRef]
- Guidet, B.; De Lange, D.W.; Boumendil, A.; Leaver, S.; Watson, X.; Boulanger, C.; Szczeklik, W.; Artigas, A.; Morandi, A.; Andersen, F.; et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: The VIP2 study. Intensive Care Med. 2020, 46, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Flaatten, H.; De Lange, D.W.; Morandi, A.; Andersen, F.H.; Artigas, A.; Bertolini, G.; Boumendil, A.; Cecconi, M.; Christensen, S.; Faraldi, L.; et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/=80 years). Intensive Care Med. 2017, 43, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Coppadoro, A.; Benini, A.; Fruscio, R.; Verga, L.; Mazzola, P.; Bellelli, G.; Carbone, M.; Mulinacci, G.; Soria, A.; Noè, B.; et al. Helmet CPAP to treat hypoxic pneumonia outside the ICU: An observational study during the COVID-19 outbreak. Crit. Care 2021, 25, 80. [Google Scholar] [CrossRef] [PubMed]
- Avdeev, S.N.; Yaroshetskiy, A.I.; Tsareva, N.A.; Merzhoeva, Z.M.; Trushenko, N.V.; Nekludova, G.V.; Chikina, S.Y. Noninvasive ventilation for acute hypoxemic respiratory failure in patients with COVID-19. Am. J. Emerg. Med. 2021, 39, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Grasselli, G.; Cecconi, M.; Antolini, L.; Borelli, M.; De Giacomi, F.; Bosio, G.; Latronico, N.; Filippini, M.; Gemma, M.; et al. Noninvasive ventilatory support of COVID-19 patients outside the intensive care units (WARd-COVID). Ann. Am. Thorac. Soc. 2021, 18, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Schortgen, F.; Follin, A.; Piccari, L.; Roche-Campo, F.; Carteaux, G.; Taillandier-Heriche, E.; Krypciak, S.; Thille, A.W.; Paillaud, E.; Brochard, L. Results of noninvasive ventilation in very old patients. Ann Intensive Care 2012, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Montoneri, G.; Noto, P.; Trovato, F.M.; Mangano, G.; Malatino, L.; Carpinteri, G. Outcomes of non-invasive ventilation in ’very old’ patients with acute respiratory failure: A retrospective study. Emerg. Med. J. 2019, 36, 303–305. [Google Scholar] [CrossRef]
- Liu, S.; Yao, N.; Qiu, Y.; He, C. Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease. Am. J. Emerg. Med. 2020, 38, 2074–2080. [Google Scholar] [CrossRef]
- Sharifpour, M.; Rangaraju, S.; Liu, M.; Alabyad, D.; Nahab, F.B.; Creel-Bulos, C.M.; Jabaley, C.S.; on behalf of the Emory COVID-19 Quality; Clinical Research Collaborative. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PLoS ONE 2020, 15, e0242400. [Google Scholar] [CrossRef]
- Imam, Z.; Odish, F.; Gill, I.; O’Connor, D.; Armstrong, J.; Vanood, A.; Ibironke, O.; Hanna, A.; Ranski, A.; Halalau, A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J. Intern. Med. 2020, 288, 469–476. [Google Scholar] [CrossRef]
- Heras, E.; Garibaldi, P.; Boix, M.; Valero, O.; Castillo, J.; Curbelo, Y.; Gonzalez, E.; Mendoza, O.; Anglada, M.; Miralles, J.C.; et al. COVID-19 mortality risk factors in older people in a long-term care center. Eur. Geriatr. Med. 2021, 12, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Lerolle, N.; Trinquart, L.; Bornstain, C.; Tadié, J.M.; Imbert, A.; Diehl, J.L.; Fagon, J.-Y.; Guérot, E. Increased intensity of treatment and decreased mortality in elderly patients in an intensive care unit over a decade. Crit. Care Med. 2010, 38, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Overall (n = 252) | NIRS (n = 228) | NIRS+IMV (n = 24) | p-Value | |
|---|---|---|---|---|
| Baseline demographic and clinical data | ||||
| Age, years | 84 (82–87) | 84 (82–87) | 82 (81–84) | 0.0183 |
| Female, n | 92 (37) | 86 (38) | 6 (25) | 0.2183 |
| Non-smokers, n | 158 (73) | 148 (73) | 10 (63) | 0.3867 ^ |
| Level of dependence | 0.3167 | |||
| -low | 74 (42) | 68 (42) | 6 (35) | |
| -moderate | 27 (15) | 26 (16) | 1 (6) | |
| -high | 77 (43) | 67 (42) | 10 (59) | |
| Body mass index, kg/m2 | 26 (23–29) | 26 (23–29) | 24 (22–29) | 0.1536 |
| ACCI | 6 (4–7) | 6 (4–7) | 6 (4–8) | 0.7976 |
| Clinical, laboratory and blood gas data on IRCU admission | ||||
| Time since symptom onset, days | 5 (3–8) | 5 (3–8) | 6 (3–10) | 0.6606 |
| Heart rate, beats/min | 83 (74–95) | 82 (74–95) | 85 (75–94) | 0.7098 |
| Respiratory rate, breaths/min | 26 (22–30) | 26 (22–30) | 25 (18–27) | 0.0726 |
| Temperature, Celsius | 36.5 (36–37) | 36.5 (36–37) | 36.8 (36.7–37) | 0.1179 |
| White blood cell count, ×103/μL | 8.5 (6.1–12.1) | 8.5 (6.1–12.1) | 8.6 (6.9–12.1) | 0.5512 |
| D-dimer, μgFEU/L | 628 (238–1900) | 620 (241–1691) | 1317 (207–3180) | 0.2915 |
| Serum C-reactive protein, mg/dL | 11 (6–16) | 11 (6–15) | 15 (10–25) | 0.0280 |
| PaO2, mmHg | 56 (45–69) | 56 (46–69) | 53 (41–73) | 0.4886 |
| PaCO2 mmHg | 34 (30–38) | 34 (31–38) | 32 (28–37) | 0.0449 |
| Arterial pH | 7.45 (7.42–7.49) | 7.45 (7.42–7.48) | 7.46 (7.42–7.49) | 0.5132 |
| SaO2, % | 89 (82–93) | 89 (83–93) | 86 (76–95) | 0.3328 |
| PaO2/FiO2, mmHg | 110 (80–172) | 111 (81–171) | 100 (61–190) | 0.4361 |
| SOFA score | 4 (3–5) | 3 (3–4) | 5 (3–6) | 0.0338 |
| Clinical outcomes | ||||
| Days on mechanical ventilation | 6 (3–10) | 6 (2–10) | 8 (6–13) | 0.0121 |
| Days on non-invasive ventilation | 5 (2–10) | 5 (2–10) | 5 (1–9) | 0.1870 |
| Days on invasive ventilation * | NA | NA | 4 (0–7) | NA |
| Length of IRCU stay, days | 8 (4–13) | 8 (4–13) | 5 (1–11) | 0.1126 |
| Length of hospital stay, days | 14 (8–23) | 14 (8–23) | 18 (11–26) | 0.1968 |
| Pts discharged alive, n (%) | 96 (38) | 89 (39) | 7 (29) | 0.7636 |
| Pts alive at 90-day follow-up, n (%) | 89 (35) | 83 (36) | 6 (25) | 0.3854 |
| Deceased at 90-Day Follow-Up | ||||
|---|---|---|---|---|
| No (n = 89) | Yes (n = 163) | All (n = 252) | HR (95%CI) | |
| Age, years | 83 (81–85) | 85 (82–87) | 84 (82–87) | 1.064 (1.022–1.107) |
| Female (M/F), % | 33 (37) | 59 (36) | 92 (37) | 1.097 (0.797–1.510) |
| Non-smokers, n | 58 (70) | 100 (74) | 158 (73) | 1 |
| Level of dependence, n | ||||
| -low | 39 (57) | 35 (32) | 74 (42) | 1 |
| -moderate | 14 (21) | 13 (12) | 27 (15) | 1.020 (0.540–1.927) |
| -high | 15 (22) | 62 (56) | 77 (43) | 2.217 (1.462–3.362) |
| Body mass index, kg/m2 | 26 (23–29) | 26 (24–29) | 26 (23–29) | 0.980 (0.930–1.033) |
| ACCI | 5 (4–7) | 6 (4–7) | 6 (4–7) | 1.053 (0.995–1.114) |
| Time since symptom onset, days | 5 (3–10) | 5 (3–8) | 5 (3–8) | 0.979 (0.945–1.014) |
| Heart rate, beats/min | 80 (74–94) | 83 (74–98) | 83 (74–95) | 1.008 (0.998–1.018) |
| Respiratory rate, breaths/min | 26 (22–30) | 26 (22–30) | 26 (22–30) | 1.015 (0.988–1.043) |
| Temperature, Celsius | 36.5 (36–37) | 36.5 (36–37) | 36.5 (36–37) | 1.097 (0.833–1.446) |
| White blood cell count, ×103/μL | 8.24 (6.13–11) | 8.79 (6.17–12.64) | 8.5 (6.13–12.1) | 1.006 (0.979–1.035) |
| D-dimer, μgFEU/L | 524 (198–1544) | 711 (241–2356) | 628 (238–1900) | 1.000 (1.000–1.000) |
| Serum C-reactive protein, mg/dL | 9.59 (5.3–14.76) | 11.44 (7.01–16.9) | 11 (6.36–15.9) | 1.037 (1.016–1.059) |
| PaO2, mmHg | 59 (47–75) | 53 (44–67) | 56 (45–69) | 0.992 (0.984–1.000) |
| PaCO2 mmHg | 35 (31–38) | 33 (30–37) | 34 (30–38) | 0.992 (0.973–1.011) |
| Arterial pH | 7.46 (7.42–7.49) | 7.45 (7.41–7.48) | 7.45 (7.42–7.49) | 0.463 (0.053–4.045) |
| SaO2, % | 90 (84–94) | 88 (82–93) | 89 (82–93) | 0.975 (0.959–0.992) |
| PaO2/FiO2, mmHg | 120 (88–179) | 104 (76–170) | 110 (79–172) | 0.998 (0.995–1.001) |
| SOFA score | 3 (2–4) | 4 (3–5) | 4 (3–5) | 1.195 (1.111–1.285) |
| Days on mechanical ventilation | 7 (3–12) | 5 (2–8.5) | 6 (3–10) | 0.946 (0.919–0.974) |
| Univariable Model HR (95% CI) | Multivariable Model HR (95% CI) | |
|---|---|---|
| NIRS+IMV vs. NIRS | 2.17 (1.33–3.56) | 1.76 (0.86–3.60) |
| Age | 1.11 (1.05–1.17) | |
| ACCI | 1.11 (1.03–1.18) | |
| CRP | 1.05 (1.02–1.07) | |
| SOFA score | 1.16 (1.06–1.26) | |
| SaO2 | 0.97 (0.95–0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vianello, A.; De Vita, N.; Scotti, L.; Guarnieri, G.; Confalonieri, M.; Bonato, V.; Molena, B.; Maestrone, C.; Airoldi, G.; Olivieri, C.; et al. Clinical Outcomes in Patients Aged 80 Years or Older Receiving Non-Invasive Respiratory Support for Hypoxemic Acute Respiratory Failure Consequent to COVID-19. J. Clin. Med. 2022, 11, 1372. https://doi.org/10.3390/jcm11051372
Vianello A, De Vita N, Scotti L, Guarnieri G, Confalonieri M, Bonato V, Molena B, Maestrone C, Airoldi G, Olivieri C, et al. Clinical Outcomes in Patients Aged 80 Years or Older Receiving Non-Invasive Respiratory Support for Hypoxemic Acute Respiratory Failure Consequent to COVID-19. Journal of Clinical Medicine. 2022; 11(5):1372. https://doi.org/10.3390/jcm11051372
Chicago/Turabian StyleVianello, Andrea, Nello De Vita, Lorenza Scotti, Gabriella Guarnieri, Marco Confalonieri, Valeria Bonato, Beatrice Molena, Carlo Maestrone, Gianluca Airoldi, Carlo Olivieri, and et al. 2022. "Clinical Outcomes in Patients Aged 80 Years or Older Receiving Non-Invasive Respiratory Support for Hypoxemic Acute Respiratory Failure Consequent to COVID-19" Journal of Clinical Medicine 11, no. 5: 1372. https://doi.org/10.3390/jcm11051372
APA StyleVianello, A., De Vita, N., Scotti, L., Guarnieri, G., Confalonieri, M., Bonato, V., Molena, B., Maestrone, C., Airoldi, G., Olivieri, C., Sainaghi, P. P., Lionello, F., Arcaro, G., Della Corte, F., Navalesi, P., & Vaschetto, R. (2022). Clinical Outcomes in Patients Aged 80 Years or Older Receiving Non-Invasive Respiratory Support for Hypoxemic Acute Respiratory Failure Consequent to COVID-19. Journal of Clinical Medicine, 11(5), 1372. https://doi.org/10.3390/jcm11051372








