Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. Technical Recommendations
2.3. EFF Scenarios
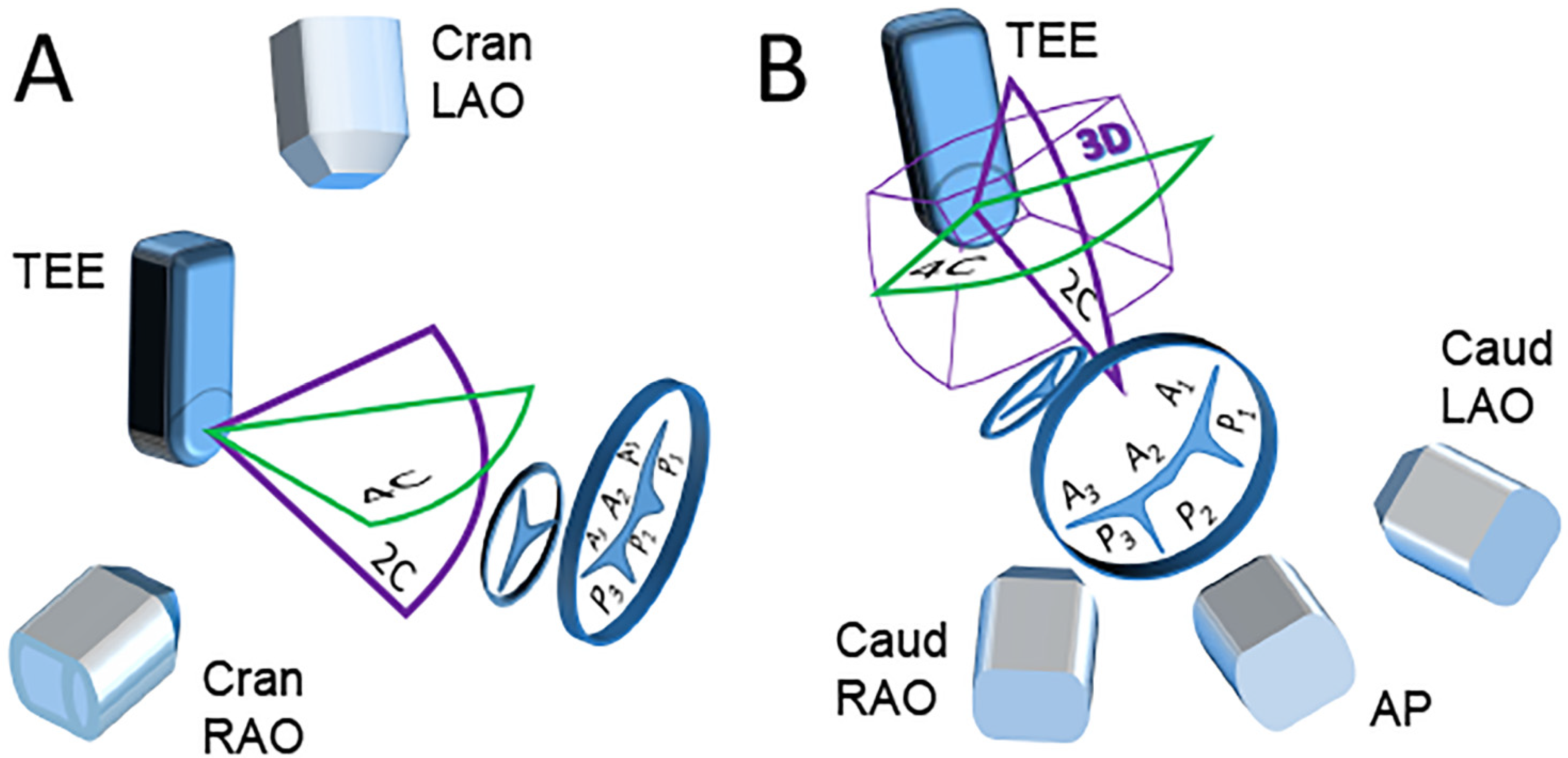
2.3.1. Scenario A “Side View of the TEE Probe”
2.3.2. Scenario B “Front View of the TEE Probe”
2.3.3. Scenario C “Free View of the TEE Probe”
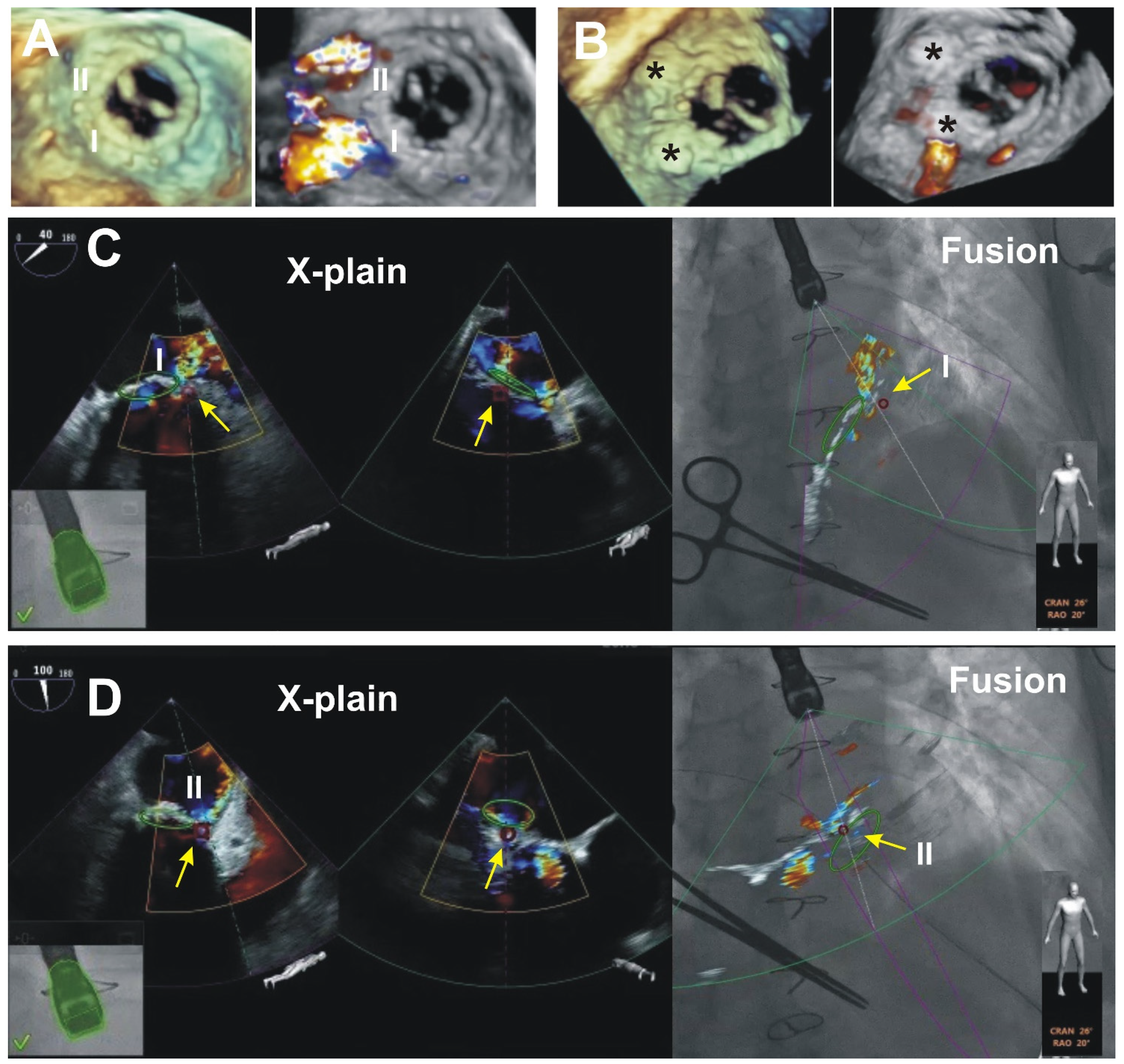
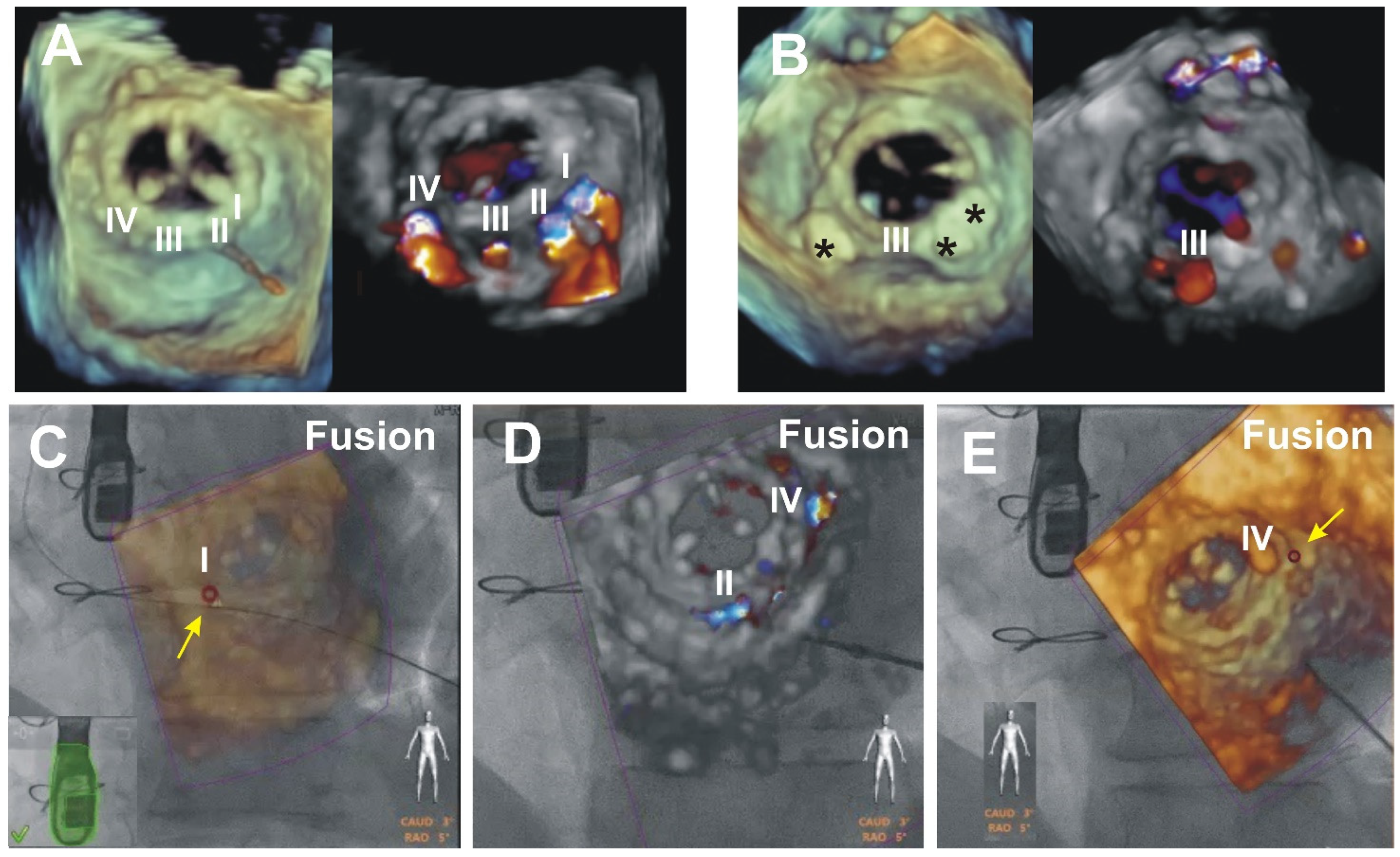
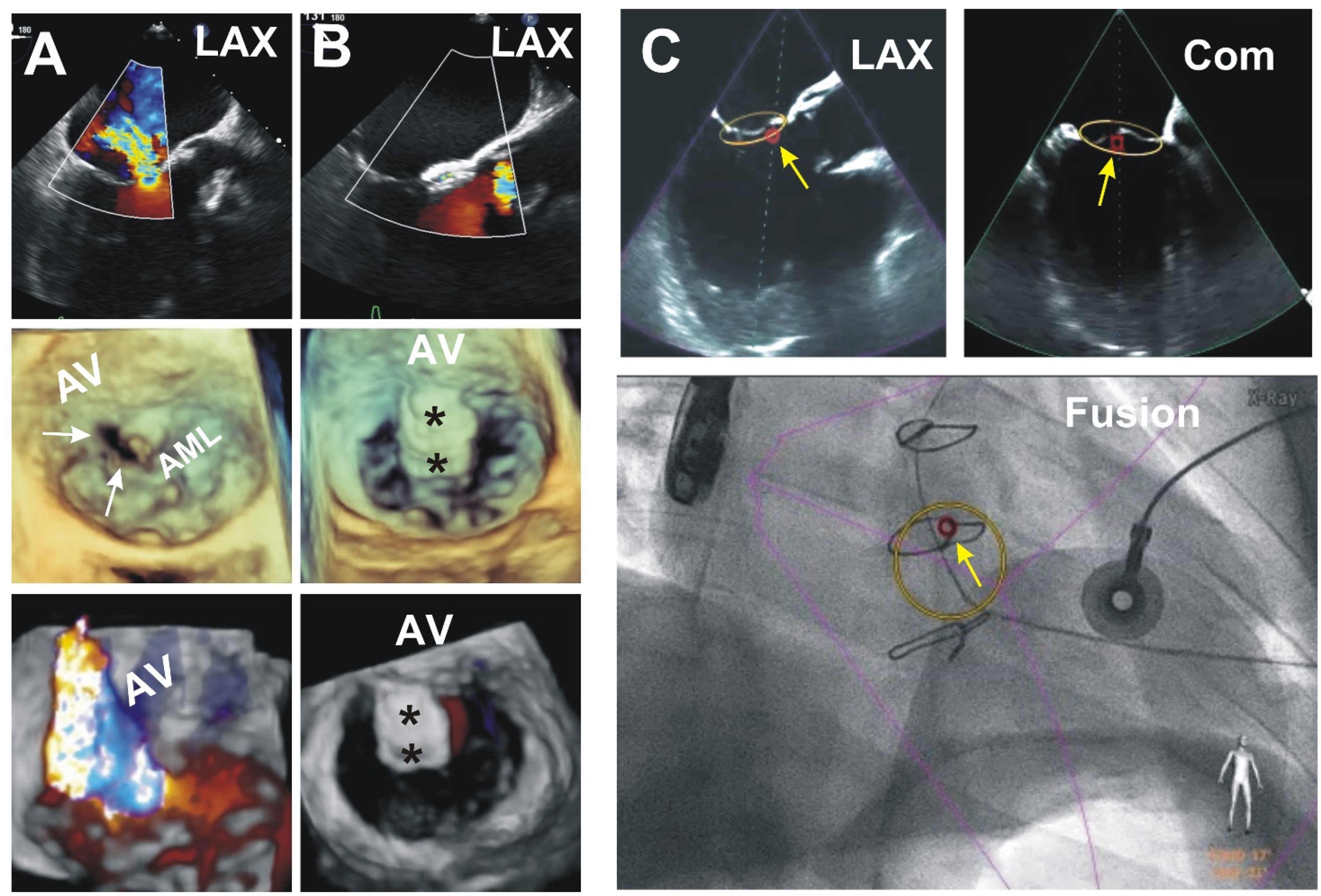
3. Results (Outcomes of the Patients Presented)
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Onorato, E.M.; Muratori, M.; Smolka, G.; Zorinas, A.; Zakarkaite, D.; Mussayev, A.; Christos, C.P.; Bauer, F.; Gandet, T.; Martinelli, G.L.; et al. Midterm procedural and clinical outcomes of percutaneous paravalvular leak closure with the Occlutech Paravalvular Leak Device. Eurointervention 2020, 15, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.E.; Jelnin, V.; Kronzon, I.; Dudiy, Y.; Del Valle-Fernandez, R.; Einhorn, B.N.; Chiam, P.T.L.; Martinez, C.; Eiros, R.; Roubin, G.; et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J. Am. Coll. Cardiol. 2011, 58, 2210–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorinas, A.; Janušauskas, V.; Davidavičius, G.; Šimakauskas, R.; Puodžiukaitė, L.; Zakarkaitė, D.; Bilkis, V.; Čypienė, R.J.; Samalavičius, R.S.; Onorato, E.M.; et al. Retrospective analysis of single-center early and midterm results of transapical catheter-based mitral paravalvular leak closure with a purpose-specific device. Postep. Kardiol. Interwencyjnej 2018, 14, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Patrianakos, A.P.; Zacharaki, A.A.; Skalidis, E.I.; Hamilos, M.I.; Parthenakis, F.I.; Vardas, P.E. The growing role of echocardiography in interventional cardiology: The present and the future. Hell. J. Cardiol. 2017, 58, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Pozzoli, A.; Agricola, E.; Guidotti, A.; Biasco, L.; Leo, L.A.; Taramasso, M.; Pasotti, E.; Kuwata, S.; Moccetti, M.; et al. Echocardiographic-fluoroscopic fusion imaging for transcatheter mitral valve repair guidance. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Clegg, S.D.; Chen, S.J.; Nijhof, N.; Kim, M.S.; Salcedo, E.E.; Quaife, R.A.; Messenger, J.C.; Bracken, J.; Carroll, J.D. Integrated 3D echo-x-ray to optimize image guidance for structural heart interventions. J. Am. Coll. Cardiol. Img. 2015, 8, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzer, J.; Zeus, T.; Hellhammer, K.; Veulemans, V.; Eschenhagen, S.; Kehmeier, E.; Meyer, C.; Rassaf, T.; Kelm, M. Initial clinical experience using the EchoNavigator©-system during structural heart disease. World J. Cardiol. 2015, 7, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Penney, G.; Ma, Y.; Gogin, N.; Cathier, P.; Arujuna, A.; Morton, G.; Caulfield, D.; Gill, J.; Rinaldi, C.A.; et al. Registration of 3D trans-esophageal echocardiography to X-ray fluoroscopy using image-based probe tracking. Med. Image Anal. 2012, 16, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Balzer, J.; Zeus, T.; Veulemans, V.; Kelm, M. Hybrid Imaging in the Catheter Laboratory: Real-time Fusion of Echocardiography and Fluoroscopy During Percutaneous Structural Heart Disease Interventions. Interv. Cardiol. 2016, 11, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Buzaev, I.V.; Khalikova, G.; Plechev, V.V.; Onorato, E.M. EchoNavigator® technology facilitates transapical mitral paravalvular leak closure: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab190. [Google Scholar] [CrossRef] [PubMed]
- Zorinas, A.; Janusauskas, V.; Davidavicius, G.; Puodziukaite, L.; Zakarkaite, D.; Kramena, R.; Čypienė, R.; Bilkis, V.; Rucinskas, K.; Aidietis, A.; et al. Fusion of real-time 3D transesophageal echocardiography and cardiac fluoroscopy imaging in transapical catheter-based mitral paravalvular leak closure. Postep. Kardiol. Interwencyjnej 2017, 13, 263–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentier, A.F.; Lessana, A.; Relland, J.Y.; Belli, E.; Mihaileanu, S.; Berrebi, A.J.; Palsky, E.; Loulmet, D.F. The ‘physio-ring’: An advanced concept in mitral valve annuloplasty. Ann. Thorac. Surg. 1995, 60, 1177–1185. [Google Scholar] [CrossRef]
- Ruiz, C.E.; Hahn, R.T.; Berrebi, A.; Borer, J.S.; Cutlip, D.E.; Fontana, G.; Gerosa, G.; Ibrahim, R.; Jelnin, V.; Jilaihawi, H.; et al. Paravalvular Leak Academic Research Consortium. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis. Eur. Heart J. 2018, 39, 1224–1245. [Google Scholar] [CrossRef] [PubMed]
- Galrinho, A.; Branco, L.M.; Fiarresga, A.; Cacela, D.; Sousa, L.; Ramos, R.; Ferreira, R.C. Paravalvular leak closure: Still a challenge with unpredictable results. Rev. Port. Cardiol. 2021, 40, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Sannino, A.; Cohen, D.J.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; Rinaldi, M.J.; Kapadia, S.R.; Rajagopal, V.; et al. Predictors of clinical response to transcatheter reduction of secondary mitral regurgitation: The COAPT trial. J. Am. Coll. Cardiol. 2020, 76, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Rihal, C.S.; Zack, C.J.; Eleid, M.F.; Maor, E.; Sarraf, M.; Cabalka, A.K.; Reeder, G.S.; Hagler, D.J.; Maalouf, J.F.; et al. Transcatheter and surgical management of mitral paravalvular leak: Long-term outcomes. JACC Cardiovasc. Interv. 2017, 10, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Julien, T.; Gallet, R.; Nguyen, A.; Deux, J.F.; Fiore, A.; Teiger, E.; Dubois-Randé, J.L.; Riant, E.; Lim, P. Usefulness of echocardiographic-fluoroscopic fusion imaging in adult structural heart disease. Arch. Cardiovasc. Dis. 2018, 111, 441–448. [Google Scholar]
- Jone, P.N.; Haak, A.; Ross, M.; Wiktor, D.M.; Gill, E.; Quaife, R.A.; Messenger, J.C.; Salcedo, E.E.; Carroll, J.D. Congenital and Structural Heart Disease Interventions Using Echocardiography-Fluoroscopy Fusion Imaging. J. Am. Soc. Echocardiogr. 2019, 32, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Ito, H. New horizon of fusion imaging using echocardiography: Its progress in the diagnosis and treatment of cardiovascular disease. J. Echocardiogr. 2020, 18, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Stangenberg, L.; Shuja, F.; Carelsen, B.; Elenbaas, T.; Wyers, M.C.; Schermerhorn, M.L. A novel tool for three-dimensional roadmapping reduces radiation exposure and contrast agent dose in complex endovascular interventions. J. Vasc. Surg. 2015, 62, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorinas, A.; Zakarkaitė, D.; Janušauskas, V.; Austys, D.; Puodžiukaitė, L.; Zuozienė, G.; Samalavičius, R.S.; Jovaišienė, I.; Davidavičius, G.; Ručinskas, K.; et al. Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures. J. Clin. Med. 2022, 11, 1328. https://doi.org/10.3390/jcm11051328
Zorinas A, Zakarkaitė D, Janušauskas V, Austys D, Puodžiukaitė L, Zuozienė G, Samalavičius RS, Jovaišienė I, Davidavičius G, Ručinskas K, et al. Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures. Journal of Clinical Medicine. 2022; 11(5):1328. https://doi.org/10.3390/jcm11051328
Chicago/Turabian StyleZorinas, Aleksejus, Diana Zakarkaitė, Vilius Janušauskas, Donatas Austys, Lina Puodžiukaitė, Gitana Zuozienė, Robertas Stasys Samalavičius, Ieva Jovaišienė, Giedrius Davidavičius, Kęstutis Ručinskas, and et al. 2022. "Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures" Journal of Clinical Medicine 11, no. 5: 1328. https://doi.org/10.3390/jcm11051328
APA StyleZorinas, A., Zakarkaitė, D., Janušauskas, V., Austys, D., Puodžiukaitė, L., Zuozienė, G., Samalavičius, R. S., Jovaišienė, I., Davidavičius, G., Ručinskas, K., & Onorato, E. M. (2022). Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures. Journal of Clinical Medicine, 11(5), 1328. https://doi.org/10.3390/jcm11051328







