Impact of Contact Force-Sensing Catheters on Fluoroscopy Time in Interventional Electrophysiology: A European Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Main Objective
2.2. Data Collection and Reporting
2.3. Statistical Analysis
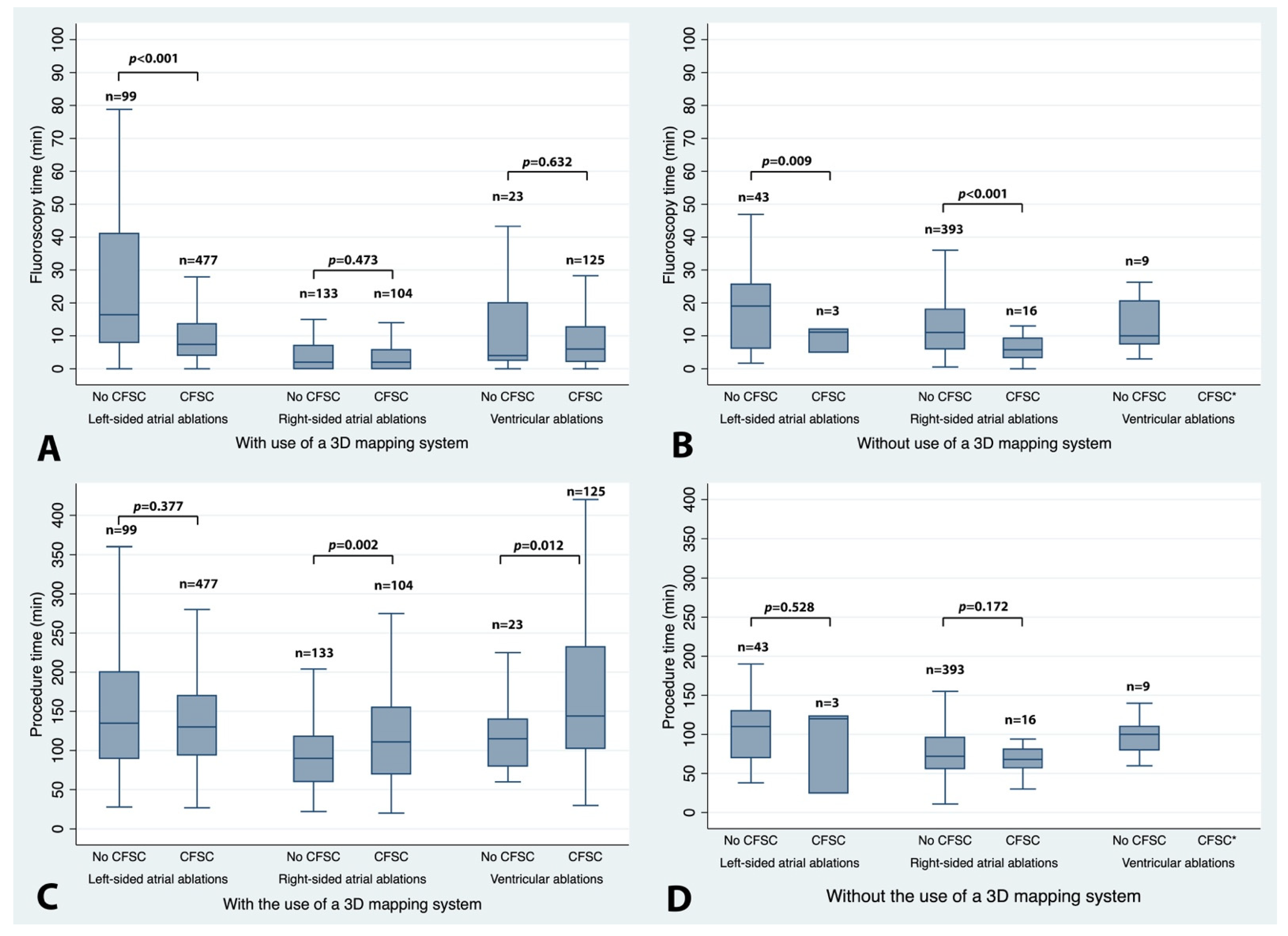
3. Results
3.1. Baseline Characteristics of Participating Centers and Operators
3.2. Types of Procedures and Use of 3D Mapping Systems
3.3. Main Analysis: Impact of Contact Force-Sensing Catheters (CFSCs) on Fluoroscopy and Procedure Time According to EP Procedure Group
3.4. Impact of Career Level, Operator Caseload, Center Volume, and Body Mass Index
3.5. Impact of EP Procedure Type and Use of 3D Mapping Systems
4. Discussion
4.1. Impact of Career Level, Operator Caseload, Center Volume, and BMI
4.2. Right-Sided Atrial Procedures
4.3. Left-Sided Atrial Procedures
4.4. Ventricular Procedures
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ariyarathna, N.; Kumar, S.; Thomas, S.P.; Stevenson, W.G.; Michaud, G.F. Role of Contact Force Sensing in Catheter Ablation of Cardiac Arrhythmias: Evolution or History Repeating Itself? JACC Clin. Electrophysiol. 2018, 4, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Chatta, J.; Samanta, A.; Waheed, S.; Mahmoudi, M.; Vukas, R.; Gunda, S.; Reddy, M.; Dawn, B.; Lakkireddy, D. Use of contact force sensing technology during radiofrequency ablation reduces recurrence of atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm 2015, 12, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Shurrab, M.; Di Biase, L.; Briceno, D.F.; Kaoutskaia, A.; Haj-Yahia, S.; Newman, D.; Lashevsky, I.; Nakagawa, H.; Crystal, E. Impact of Contact Force Technology on Atrial Fibrillation Ablation: A Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, W.; Schilling, R.J.; Wong, T. Contact Force and Atrial Fibrillation Ablation. J. Atr. Fibrillation 2016, 8, 1282. [Google Scholar] [PubMed]
- Heidbuchel, H.; Wittkampf, F.H.; Vano, E.; Ernst, S.; Schilling, R.; Picano, E.; Mont, L.; Jais, P.; De Bono, J.; Piorkowski, C.; et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace 2014, 16, 946–964. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, A.; De Potter, T.; Heidbuchel, H.; Ernst, S.; Kosiuk, J.; Vano, E.; Picano, E.; Arbelo, E.; Tedrow, U.; Potpara, T.; et al. Occupational radiation exposure in the electrophysiology laboratory with a focus on personnel with reproductive potential and during pregnancy: A European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS). Europace 2017, 19, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Venneri, L.; Rossi, F.; Botto, N.; Andreassi, M.G.; Salcone, N.; Emad, A.; Lazzeri, M.; Gori, C.; Vano, E.; Picano, E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: Insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am. Heart J. 2009, 157, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casella, M.; Dello Russo, A.; Russo, E.; Catto, V.; Pizzamiglio, F.; Zucchetti, M.; Majocchi, B.; Riva, S.; Vettor, G.; Dessanai, M.A.; et al. X-Ray Exposure in Cardiac Electrophysiology: A Retrospective Analysis in 8150 Patients Over 7 Years of Activity in a Modern, Large-Volume Laboratory. J. Am. Heart Assoc. 2018, 7, e008233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estner, H.L.; Grazia Bongiorni, M.; Chen, J.; Dagres, N.; Hernandez-Madrid, A.; Blomstrom-Lundqvist, C. Use of fluoroscopy in clinical electrophysiology in Europe: Results of the European Heart Rhythm Association Survey. Europace 2015, 17, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Fagerland, M.W. t-tests, non-parametric tests, and large studies—A paradox of statistical practice? BMC Med. Res. Methodol. 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, J.G.; Monir, G.; Pollak, S.J.; Khairy, P.; Dubuc, M.; Roy, D.; Talajic, M.; Deyell, M.; Rivard, L.; Thibault, B.; et al. Pulmonary vein isolation using “contact force” ablation: The effect on dormant conduction and long-term freedom from recurrent atrial fibrillation—A prospective study. Heart Rhythm 2014, 11, 1919–1924. [Google Scholar] [CrossRef]
- Kuck, K.H.; Reddy, V.Y.; Schmidt, B.; Natale, A.; Neuzil, P.; Saoudi, N.; Kautzner, J.; Herrera, C.; Hindricks, G.; Jaïs, P.; et al. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm 2012, 9, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Giehm-Reese, M.; Kronborg, M.B.; Lukac, P.; Kristiansen, S.B.; Jensen, H.K.; Gerdes, C.; Kristensen, J.; Nielsen, J.M.; Nielsen, J.C. A randomized trial of contact force in atrial flutter ablation. Europace 2020, 22, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Papageorgiou, N.; Lim, W.Y.; Wongwarawipat, T.; Hunter, R.J.; Dhillon, G.; Schilling, R.J.; Creta, A.; El Haddad, M.; Duytschaever, M.; et al. Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: An updated meta-analysis. Europace 2020, 22, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Ambrus, M.; Herczeg, S.; Szegedi, N.; Nagy, K.V.; Sallo, Z.; Osztheimer, I.; Széplaki, G.; Tahin, T.; Merkely, B.; et al. Similar outcomes with manual contact force ablation catheters and traditional catheters in the treatment of outflow tract premature ventricular complexes. Europace 2021, 23, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, T.; Baldinger, S.H.; Pruvot, E.; Bisch, L.; Ammann, P.; Altmann, D.; Berte, B.; Kobza, R.; Haegeli, L.; Schlatzer, C.; et al. Impact of contact force sensing technology on outcome of catheter ablation of idiopathic pre-mature ventricular contractions originating from the outflow tracts. Europace 2021, 23, 603–609. [Google Scholar] [CrossRef] [PubMed]
| Type of Arrhythmia/Procedure | Overall Number of Studies | Number of EP Studies Using a CFSC | Number of EP Studies Using 3D Mapping | Number of EP Studies Using a CFSC + 3D Mapping | Median Fluoroscopy Time (min) * | Median Procedure Time (min) * |
|---|---|---|---|---|---|---|
| AV nodal reentrant tachycardia (AVNRT) | 267 | 27 (10.1%) | 70 (26.2%) | 19 (7.1%) | 8.0 (3.0–13.3) [0.0–48.0] | 78.0 (60.0–110.0) [13.0–259.0] |
| Cavo-tricuspid isthmus ablation | 301 | 63 (20.9%) | 121 (40.2%) | 56 (18.6%) | 6.0 (2.0–15.0) [0.0–48.1] | 70.0 (55.0–103.0) [11.0–275.0] |
| Right atrial tachycardia | 38 | 20 (52.6%) | 27 (71.1%) | 20 (52.6%) | 7.1 (4.0–20.0) [0.0–55.6] | 120.0 (90.0–140.0) [29.0–307.0] |
| Right-sided accessory pathway ablation | 40 | 10 (25.0%) | 19 (47.5%) | 9 (22.5%) | 8.5 (2.5–19.6) [0.0–60.3] | 90.0 (60.0–155.0) [28.0–240.0] |
| Pulmonary vein isolation (RF) | 393 | 352 (89.6%) | 393 (100.0%) | 352 (89.6%) | 8.0 (4.2–14.0) [0.0–70.4] | 124.0 (92.0–167.0) [27.0–407.0] |
| Pulmonary vein isolation with additional lesions (RF) | 122 | 95 (77.9%) | 122 (100.0%) | 95 (77.9%) | 10.0 (5.0–20.0) [0.0–119.6] | 169.5 (130.0–210.0) [36.0–425.0] |
| Left atrial tachycardia | 42 | 23 (54.8%) | 35 (83.3%) | 22 (52.4%) | 9.0 (6.0–20.4) [0.0–128.1] | 114.5 (60.0–180.0) [25.0–543.0] |
| Left-sided accessory pathway ablation | 65 | 10 (15.4%) | 26 (40.0%) | 8 (12.3%) | 8.5 (4.0–21.0) [0.0–84.6] | 94.5 (65.0–121.5) [28.0–270.0] |
| VT ablation | 66 | 56 (84.9%) | 66 (100.0%) | 56 (84.8%) | 9.9 (4.0–23.0) [0.0–73.0] | 180.0 (123.0–260.0) [46.0–420.0] |
| PVC ablation | 91 | 69 (75.8%) | 82 (90.1%) | 69 (75.8%) | 5.0 (2.0–8.0) [0.0–33.1] | 110.5 (80.0–159.0) [30.0–360.0] |
| Overall | 1425 | 725 (50.9%) | 961 (67.4%) | 706 (49.5%) | 8.0 (3.3–15.2) [0.0–128.1] | 105.0 (70.0–150.0) [11.0–543.0] |
| Group | Number of EP Studies (Overall/w/o CFSC/w CFSC) * | Fluoro Time w/o CFSC (min) † | Fluoro Time w CFSC (min) † | p-Value ‡ | Procedure Time w/o CFSC (min) † | Procedure Time w CFSC (min) † | p-Value ‡ |
|---|---|---|---|---|---|---|---|
| Group 1 (right-sided atrial procedures) | 646/526/120 | 9.0 (4.0–16.0) | 2.6 (0.0–6.0) | <0.001 | 75.0 (57.0–105.0) | 101.0 (65.0–150.0) | <0.001 |
| Group 2 (left-sided atrial procedures) | 622/142/480 | 17.0 (7.0–38.0) | 7.4 (4.0–13.2) | <0.001 | 124.0 (85.0–180.0) | 130.0 (93.5–170.0) | 0.721 |
| Group 3 (ventricular ablation procedures) | 157/32/125 | 7.0 (3.0–20.3) | 6.0 (2.2–12.7) | 0.424 | 100.0 (80.0–127.5) | 144.0 (102.5–232) | <0.001 |
| Group | Number of EP Studies (Overall/w/o CFSC/w CFSC) * | Fluoro Time w/o CFSC (min) † | Fluoro Time w CFSC (min) † | p-Value ‡ | Procedure Time w/o CFSC (min) † | Procedure Time w CFSC (min) † | p-Value ‡ |
|---|---|---|---|---|---|---|---|
| 3A: Operator career level | |||||||
| Early career | 421/247/174 | 10.5 (6.0–19.7) | 7.0 (3.0-13.0) | <0.001 | 75.0 (60.0–113.0) | 140.0 (105.0–180.0) | <0.001 |
| Mid-career | 620/306/314 | 7.5 (2.7–18.0) | 6.0 (3.0-9.5) | <0.001 | 83.0 (60.0–120.0) | 130.0 (94.0–180.0) | <0.001 |
| Mentor | 384/147/237 | 12.0 (6.0–19.0) | 7.5 (2.6–15.0) | <0.001 | 100.0 (60.0–140.0) | 119.0 (83.0–155.5) | 0.108 |
| 3B: Operator caseload per month | |||||||
| 1–9/mo | 362/223/139 | 12.0 (6.0–25.0) | 6.0 (1.3–14.0) | <0.001 | 90.0 (64.0–127.0) | 130.0 (90.0–180.0) | <0.001 |
| 10–19/mo | 380/177/203 | 7.0 (0.8–18.0) | 6.0 (3.2–10.1) | <0.001 | 96.0 (70.0–135.0) | 150.0 (105.0–180.0) | <0.001 |
| 20–39/mo | 337/167/170 | 11.0 (6.0–18.0) | 7.5 (3.2–13.0) | <0.001 | 70.0 (40.0–110.0) | 120.0 (85.0–169.0) | <0.001 |
| >40/mo | 296/103/193 | 8.0 (4.0–14.0) | 6.1 (3.0–13.0) | 0.406 | 70.0 (55.0–96.0) | 120.0 (90.0–165.0) | <0.001 |
| 3C: Cases per center per year | |||||||
| <200/yr | 36/17/19 | 4.0 (0.0–22.0) | 5.0 (0.0–7.0) | 0.197 | 120.0 (102.5–145.0) | 190.0 (150.0–215.0) | <0.001 |
| 200–499/yr | 767/418/349 | 9.0 (4.0–15.0) | 7.5 (4.0–14.9) | 0.959 | 83.0 (60.0–115.0) | 150.0 (120.0–185.0) | <0.001 |
| 500–999/yr | 311/191/120 | 17.0 (8.0–35.6) | 9.0 (5.0–14.0) | <0.001 | 90.0 (60.0–140.0) | 120.0 (87.5–182.5) | <0.001 |
| ≥1000/yr | 311/74/237 | 4.0 (2.1–11.0) | 4.2 (2.0–8.0) | 0.286 | 80.0 (60.0–113.0) | 97.0 (76.0–129.0) | 0.018 |
| 3D: Patient BMI level | |||||||
| BMI <20 | 64/34/30 | 10.0 (4.0–14.3) | 3.8 (1.0–11.1) | 0.023 | 113.0 (70.0–130.0) | 98.0 (60.0–165.0) | 0.876 |
| BMI 20–30 | 914/476/438 | 10.0 (4.0–18.6) | 6.9 (3.0–13.0) | <0.001 | 83.0 (60.0–120.0) | 124.0 (90.0–180.0) | <0.001 |
| BMI >30 | 381/169/212 | 10.0 (4.0–20.0) | 7.3 (3.1–13.0) | <0.001 | 80.0 (59.0–130.0) | 129.0 (95.0–170.0) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, L.; Blessberger, H.; Balsam, P.; De Potter, T.; Buchta, P.; Ernst, S.; Waldmann, V.; Costa, F.M.; Bogdan, S.; Nahler, A.; et al. Impact of Contact Force-Sensing Catheters on Fluoroscopy Time in Interventional Electrophysiology: A European Survey. J. Clin. Med. 2022, 11, 1322. https://doi.org/10.3390/jcm11051322
Fiedler L, Blessberger H, Balsam P, De Potter T, Buchta P, Ernst S, Waldmann V, Costa FM, Bogdan S, Nahler A, et al. Impact of Contact Force-Sensing Catheters on Fluoroscopy Time in Interventional Electrophysiology: A European Survey. Journal of Clinical Medicine. 2022; 11(5):1322. https://doi.org/10.3390/jcm11051322
Chicago/Turabian StyleFiedler, Lukas, Hermann Blessberger, Pawel Balsam, Tom De Potter, Piotr Buchta, Sabine Ernst, Victor Waldmann, Francisco Moscoso Costa, Stefan Bogdan, Alexander Nahler, and et al. 2022. "Impact of Contact Force-Sensing Catheters on Fluoroscopy Time in Interventional Electrophysiology: A European Survey" Journal of Clinical Medicine 11, no. 5: 1322. https://doi.org/10.3390/jcm11051322
APA StyleFiedler, L., Blessberger, H., Balsam, P., De Potter, T., Buchta, P., Ernst, S., Waldmann, V., Costa, F. M., Bogdan, S., Nahler, A., Hrncic, D., Lambert, T., Schönbauer, R., Pfeffer, M., Roithinger, F. X., Steinwender, C., & Kosiuk, J. (2022). Impact of Contact Force-Sensing Catheters on Fluoroscopy Time in Interventional Electrophysiology: A European Survey. Journal of Clinical Medicine, 11(5), 1322. https://doi.org/10.3390/jcm11051322






