Biliary Atresia in 2021: Epidemiology, Screening and Public Policy
Abstract
1. Introduction
2. Epidemiology and Pathogenesis
2.1. Seasonal Variability
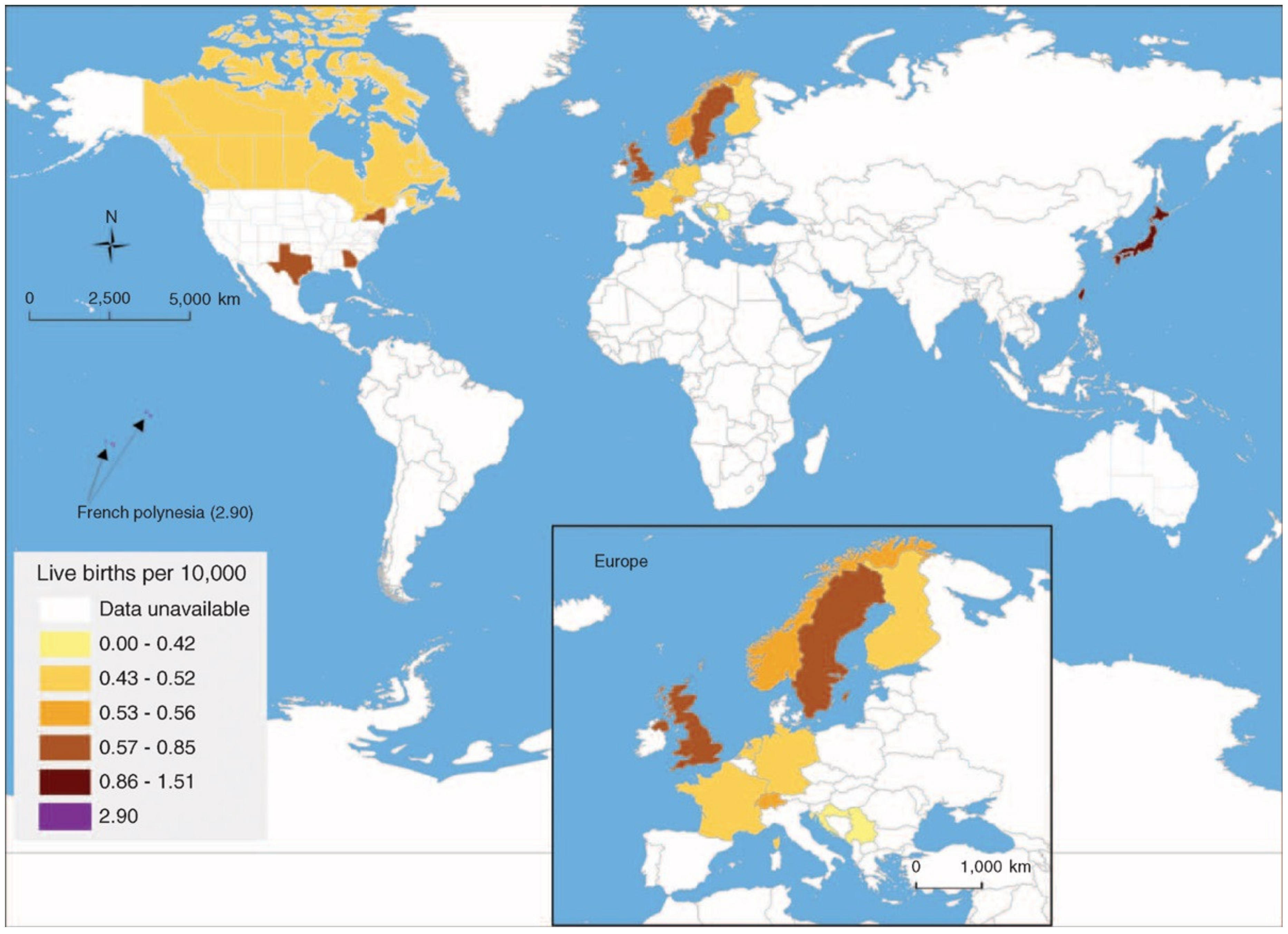
2.2. Geographic Variations
3. Early Intervention Is a Key Prognostic Indicator: The Need for Screening
3.1. KPE at Infant Age < 30 Days Is Optimal
3.2. The Problem of ‘Late’ Referral
4. Newborn Screening for Biliary Atresia
4.1. The UK “Yellow Alert” Educational Campaign
4.2. BA Screening Using a Stool Color Card
4.3. BA Screening Using a Stool Colour Smartphone App
4.4. BA Screening Using Conjugated or Direct (Fractionated) Bilirubin
5. BA Screening and Public Health Policy: The Challenge of Influencing Policymakers and Considerations for Program Implementation
6. Limitations of the Current BA Screening Programs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartley, J.L.; Davenport, M.; Kelly, D.A. Biliary atresia. Lancet 2009, 37, 1704–1713. [Google Scholar] [CrossRef]
- Sanchez-Valle, A.; Kassira, N.; Varela, V.C.; Radu, S.C.; Paidas, C.; Kirby, R.S. Biliary atresia: Epidemiology, genetics, clinical update and public health perspective. Adv. Pediatr. 2017, 64, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rivera, C.; Jolin-Dahel, K.S.; Fortinsky, K.J.; Gozdyra, P.; Benchimol, E.I. International incidence and outcomes of biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.H.Y.; Zheng, S.; Tam, P.K.H. Biliary atresia: East versus west. Semin. Pediatr. Surg. 2020, 29, 150950. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, J.; Zhang, R.; Yin, Y.; Ye, J.; Tan, L.; Xia, H. The perinatal infection of cytomegalovirus is an important etiology for biliary atresia in China. Clin. Pediatr. 2012, 51, 109–113. [Google Scholar] [CrossRef]
- Lakshminarayan, B.; Davenport, M. Biliary atresia: A comprehensive review. J. Autoimmun. 2016, 73, 1–9. [Google Scholar] [CrossRef]
- Schreiber, R.A.; Kleinman, R.E. Genetics, immunology and biliary atresia: An opening or a diversion. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 111–113. [Google Scholar] [CrossRef]
- Feldman, A.; Mack, C.L. Biliary atresia: Cellular dynamics and immune dysregulation. Semin. Pediatr. Surg. 2012, 21, 192–200. [Google Scholar] [CrossRef]
- Bezerra, J.A.; Wells, R.G.; Mack, C.L.; Karpen, S.J.; Hoofnagle, J.H.; Doo, E.; Sokol, R.J. Biliary atresia: Clinical and research challenges for the twenty first century. Hepatology 2018, 68, 1163–1173. [Google Scholar] [CrossRef]
- Strickland, A.D.; Shannon, K. Studies in the etiology of extrahepatic biliary atresia: Time-space clustering. J. Pediatr. 1982, 100, 749–753. [Google Scholar] [CrossRef]
- Yoon, P.W.; Bresee, J.S.; Olney, R.S.; James, L.M.; Khoury, M.J. Epidemiology of biliary atresia: A population-based study. Pediatrics 1997, 99, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, J.W.; Moon, J.S.; Ko, J.S. Epidemiology of biliary atresia in Korea. J. Korean Med. Sci. 2017, 32, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Nomden, M.; van Wessel, D.B.; Ioannou, S.; Verkade, H.J.; de Kleine, R.H.; Alizadeh, B.Z.; Bruggink, J.L.; Hulscher, J.B. A higher incidence of isolated biliary atresia in rural area: Results from an epidemiologic study in the Netherlands. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Livesey, E.; Borja, M.C.; Sharif, K.; Alizai, N.; McClean, P.; Kelly, D.; Hadzic, N.; Davenport, M. Epidemiology of biliary atresia in England and Wales (1999–2006). Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F451–F455. [Google Scholar] [CrossRef] [PubMed]
- Fischler, B.; Haglund, B.; Hjern, A. A population-based study on the incidence and possible pre- and perinatal etiologic risk factors of biliary atresia. J. Pediatr. 2002, 141, 217–222. [Google Scholar] [CrossRef]
- Caton, A.R.; Druschel, C.M.; McNutt, L.A. The epidemiology of extrahepatic biliary atresia in New York State, 1983–1998. Pediatr. Perinat. Epidemiol. 2004, 18, 97–105. [Google Scholar] [CrossRef]
- Nomden, M.; de Kleine, R.H.; Bruggink, J.L.; Verkade, H.J.; Burgerhof, J.G.; Hulscher, J.B. Unusual long absence of isolated biliary atresia in COVID lockdown: Coïncidence or association? J. Pediatr. Gastroenterol. Nutr. 2021, 74, e17–e18. [Google Scholar] [CrossRef]
- Kasai, M.; Suzuki, S. A new operation for, ‘non-correctable’ biliary atresia: Hepatic porto-enterostomy. Shuiyutsu 1959, 13, 733–739. [Google Scholar]
- Lilly, J.R.; Altman, R.P. Hepatic portoenterostomy (the Kasai operation) for biliary atresia. Surgery 1975, 78, 76–86. [Google Scholar]
- Shneider, B.L.; Magee, J.C.; Karpen, S.J.; Rand, E.B.; Narkewicz, M.R.; Bass, L.M.; Schwarz, K.; Whitington, P.F.; Bezerra, J.A.; Kerkar, N.; et al. Total serum bilirubin within 3 months of hepatopoetoenterostomy predicts native liver survival in biliary atresia. J. Pediatr. 2016, 170, 211–217. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, M.H.; Chen, H.L.; Ni, Y.H.; Hsu, H.Y.; Wu, J.F. Bilirubin level 1 week after hepatoportoenterostomy predicts native liver survival in biliary atresia. Pediatr. Res. 2020, 87, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Nio, M.; Sasaki, H.; Wada, M.; Kazama, T.; Nishi, K.; Tanaka, H. Impact of age of Kasai operation on short- and long- term outcomes of type III biliary atresia at a single institution. J. Pediatr. Surg. 2010, 45, 2361–2363. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, M.P.; Johansen, L.S.; Svensson, J.F.; Bjørnland, K.; Gatzinsky, V.; Stenström, P.; Koivusalo, A.; Kvist, N.; Almström, M.; Emblem, R.; et al. Outcomes of biliary atresia in the nordic countries- a mulitcenter study of 158 patients during 2005–2016. J. Pediatr. Surg. 2018, 53, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.; de Langen, Z.J.; Groen, H.; Scheenstra, R.; Peeters, P.M.; Hulscher, J.B.; Verkade, H.J. Biliary atresia in the Netherlands: Outcome of patients diagnosed between 1987 and 2008. J. Pediatr. 2012, 160, 638–644. [Google Scholar] [CrossRef]
- Fanna, M.; Masson, G.; Capito, C.; Girard, M.; Guerin, F.; Hermeziu, B.; Lachaux, A.; Roquelaure, B.; Gottrand, F.; Broue, P.; et al. Management of biliary atresia in France 1986–2015: Long-term results. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 416–424. [Google Scholar] [CrossRef]
- Schreiber, R.A.; Barker, C.C.; Roberts, E.A.; Martin, S.R.; Alvarez, F.; Smith, L.; Butzner, J.D.; Wrobel, I.; Mack, D.; Moroz, S.; et al. Biliary atresia: The Canadian experience. J. Pediatr. 2007, 151, 659–665. [Google Scholar] [CrossRef]
- Serinet, M.O.; Wildhaber, B.E.; Broue, P.; Lachaux, A.; Sarles, J.; Jacquemin, E.; Gauthier, F.; Chardot, C. Impact of age at Kasai operation on its results in late childhood and adolescence: A rational basis for biliary atresia screening. Pediatrics 2009, 123, 1280–1286. [Google Scholar] [CrossRef]
- Davenport, M.; Puricelli, V.; Farrant, P.; Hadzic, N.; Mieli-Vergani, G.; Portmann, B.; Howard, E.R. The outcome of the older (≥100 days) infant with biliary atresia. J. Pediatr. Surg. 2004, 39, 575–581. [Google Scholar] [CrossRef]
- Chardot, C.; Carton, M.; Spire-Bendelac, N.; Le Pommelet, C.; Golmard, J.L.; Reding, R.; Auvert, B. Is the Kasai operation still indicated in children older than 3 months diagnosed with biliary atresia. J. Pediatr. 2001, 138, 224–228. [Google Scholar] [CrossRef]
- Volpert, D.; White, F.; Finegold, M.J.; Molleston, J.; DeBaun, M.; Perlmutter, D.H. Outcome of early hepatic portoenterostomy for biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 265–269. [Google Scholar] [CrossRef]
- Calinescu, A.M.; Wilde, J.C.; Korff, S.; McLin, V.A.; Wildhaber, B.E. Perioperative complications after Kasai hepatoportoenterostomy: Data from the Swiss national biliary atresia registry. Eur. J. Pediatr. Surg. 2020, 30, 364–370. [Google Scholar] [CrossRef]
- Kelley-Quon, L.I.; Shue, E.; Burke, R.V.; Smith, C.; Kling, K.; Mahdi, E.; Ourshalimian, S.; Fenlon, M.; Dellinger, M.; Shew, S.B.; et al. The need for early Kasai portoenterostomy; a western pediatric surgery research consortium study. Pediatr. Surg. Int. 2021, 38, 193–199. [Google Scholar] [CrossRef]
- McKieran, P.J. Prompt diagnosis of biliary atresia education has not succeeded, time to move to universal screening. Arch. Dis. Child. 2020, 105, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Lin, N.; Xiao, Y.; Wang, Y.; Wen, J.; Zou, G.M.; Gu, X.; Cai, W. Elevated bile acids in newborns with biliary atresia. PLoS ONE 2012, 7, 49270. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.P.; Davidson, L.L.; Dick, M.C. Earilier identification of biliary atresia and hepatobiliary disease; selective screening in the third week of life. Arch. Dis. Child. 1995, 72, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A. Screening for biliary atresia. Pediatr. Surg. Int. 2017, 33, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Yokoyama, K.; Mizuta, K.; Tsuchioka, T.; Kudo, T.; Sasaki, H.; Nio, M.; Tang, J.; Ohkubo, T.; Matsui, A. Stool card screening for early detection of biliary atresia and long term native liver survival: A 19 year cohort study in Japan. J. Pediatr. 2015, 166, 897–902. [Google Scholar] [CrossRef]
- Chang, M.H. Screening for biliary atresia Chang Gung. Med. J. 2006, 29, 231–233. [Google Scholar]
- Hsiao, C.H.; Chang, M.H.; Chen, H.L.; Lee, H.C.; Wu, T.C.; Lin, C.C.; Yang, Y.J.; Chen, A.C.; Tiao, M.M.; Lau, B.H.; et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology 2008, 47, 1233–1240. [Google Scholar] [CrossRef]
- Lien, T.H.; Chang, M.H.; Wu, J.F.; Chen, H.L.; Lee, H.C.; Chen, A.C.; Tiao, M.M.; Wu, T.C.; Yang, Y.J.; Lin, C.C.; et al. Effects of the Infant stool colour card screening program on 5 year outcome of biliary atresia in Taiwan. Hepatology 2011, 53, 202–208. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Zhao, J.Q.; Wang, J.; Qui, L.; Yang, H.H.; Diao, M.; Li, L.; Gu, Y.H.; Matsui, A. Modified stool color card with digital images was efficient and feasible for early detection of biliary atresia—A pilot study in Beijing, China. World J. Pediatr. 2016, 12, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, J.P.; Schreiber, R.A.; Butler, A.E.; MacFarlane, J.; Kaczorowski, J.; Masucci, L.; Bryan, S.; Collet, J.P. Province-wide biliary atresia home screening program in British Columbia: Evaluation of first 2 years. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Liccardo, D.; Paoletti, M.; Pietrobattista, A.; Basso, M.S.; Mosca, A.; Safarikia, S.; Grimaldi, C.; Saffioti, M.C.; Candusso, M.; et al. A novel mobile phone application for infant sotol color recognition: An easy and effective tool to identify acholic stools in newborns. J. Med. Screen. 2021, 28, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Keffler, S.; Kelly, D.A.; Powell, J.E.; Green, A. Population screening for newborn liver disease: A feasibility study. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 306–311. [Google Scholar] [CrossRef]
- Powell, J.E.; Keffler, S.; Kelly, D.A.; Green, A. Population screening for neonatal liver disease. J. Med. Screen. 2003, 10, 112–116. [Google Scholar] [CrossRef]
- Gu, Y.H.; Zhao, J.Q.; Kong, Y.Y.; Yang, H.H.; Diao, M.; Li, L.; Nomachi, S.; Tezuka, M.; Hanai, J.; Matsui, A. Repeatability and reliability of home based stool color card screening for biliary atresia based on results in China and Japan Tohoku. Exp. Med. 2020, 252, 365–372. [Google Scholar] [CrossRef]
- Schreiber, R.A.; Masucci, L.; Kaczorowski, J.; Collet, J.P.; Lutley, P.; Espinosa, V.; Bryan, S. Home based screening for biliary atresia usinf infant stool colour cards: A large scale prospective cohort study and cost effectiveness analysis. J. Med. Screen. 2014, 21, 126–132. [Google Scholar] [CrossRef]
- Morinville, V.; Ahmed, N.; Ibberson, C.; Kovacs, L.; Kaczorowski, J.; Bryan, S.; Collet, J.P.; Schreiber, R. Home based screening for biliary atresia using infant stool colour cards in Canada: Quebec feasibility study. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 536–541. [Google Scholar] [CrossRef]
- Borgeat, M.; Korff, S.; Wildhaber, B. Newborn biliary atresia screening with the stool colour card. BMJ Paediatr. Open 2018, 2, e000269. [Google Scholar] [CrossRef]
- Bezerra, J.A. Biliary atresia in Brazil. J. Pediatr. 2010, 86, 445–447. [Google Scholar] [CrossRef][Green Version]
- El-Shaabrawi, M.H.; Baroudy, S.R.; Hassanin, F.S.; Farag, A.E. A pilot study of a stool color card as a diagnostic tool for extrahepatic biliary atresia at a single tertiary referral center n a low/middle income country. Arab J. Gasterol. 2021, 22, 61–65. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Wang, B.; Zhang, L. Biliary atresia screening in Shenzhen: Implementation and achievements. Arch. Dis. Child. 2020, 105, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, J.; Tavares, M.; Silva, E.S.; Lopes, A.I. The stool color card as a screening tool for biliary atresia in the digital version of the Portuguese child and youth health booklet. Acta Med. Port. 2021, 34, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Madadi-Sanjani, O.; Blaser, J.; Voigt, G.; Kuebler, J.F.; Petersen, C. Home based color card screening for biliary atresia: The first steps for implementation of a nationwide newborn screening in Germany. Pediatr. Surg. Int. 2019, 35, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Franciscovich, A.; Vaidya, D.; Doyle, J.; Bolinger, J.; Capdevila, M.; Rice, M.; Hancock, L.; Mahr, T.; Mogul, D.B. PoopMD, a mobile health application accurately identifies infant acholic stools. PLoS ONE 2015, 10, e0132270. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, E.; Hayashi, K.; Suzuki, M.; Obatake, M.; Urayama, K.Y.; Nakano, S.; Taura, Y.; Nio, M.; Takahashi, O. An iphone application using a novel stool color detection algorithm for biliary atresia screening. Pediatr. Surg. Int. 2017, 33, 1115–1121. [Google Scholar] [CrossRef]
- Shen, Z.; Zheng, S.; Dong, R.; Chen, G. Saturation of stool colour in HSV color model is a promising objective parameter for screening biliary atresia. J. Pediatr. Surg. 2016, 51, 2091–2094. [Google Scholar] [CrossRef]
- Harpavat, S.; Garcia-Prats, J.A.; Shneider, B.L. Newborn screening for biliary atresia. NEJM 2016, 375, 605–606. [Google Scholar] [CrossRef]
- Harpavat, S.; Garcia-Prats, J.A.; Anaya, C.; Brandt, M.L.; Lupo, P.J.; Finegold, M.J.; Obuobi, A.; ElHennawy, A.A.; Jarriel, W.S.; Shneider, B.L. Diagnostic yield of newborn screening for biliary atresia using direct or conjugated bilirubin measurements. JAMA 2020, 323, 1141–1150. [Google Scholar] [CrossRef]
- Rabbani, T.; Guthery, S.L.; Himes, R.; Shneider, B.L.; Harpavat, S. Newborn screening for biliary atresia: A review of current methods. Curr. Gastroenterol. Rep. 2021, 23, 28. [Google Scholar] [CrossRef]
- Mogul, D.; Zhou, M.; Intihar, P.; Schwarz, K.; Frick, K. Cost-effectiveness analysis of screening for biliary atresia with the stool color card. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Green, N.S.; Calonge, N.; Lam, W.K.; Comeau, A.M.; Goldenberg, A.J.; Ojodu, J.; Prosser, L.A.; Tanksley, S.; Bocchini, J.A., Jr. Decision-making process for conditions nominated to the recommended uniform screening panel: Statement of the US department of health and human services secretary’s advisory committee on heritable disorders in newborns and children. Genet. Med. 2014, 16, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, J.; Waisbren, S.E. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J. Inherit. Metab. Dis. 2006, 29, 677–682. [Google Scholar] [CrossRef] [PubMed]
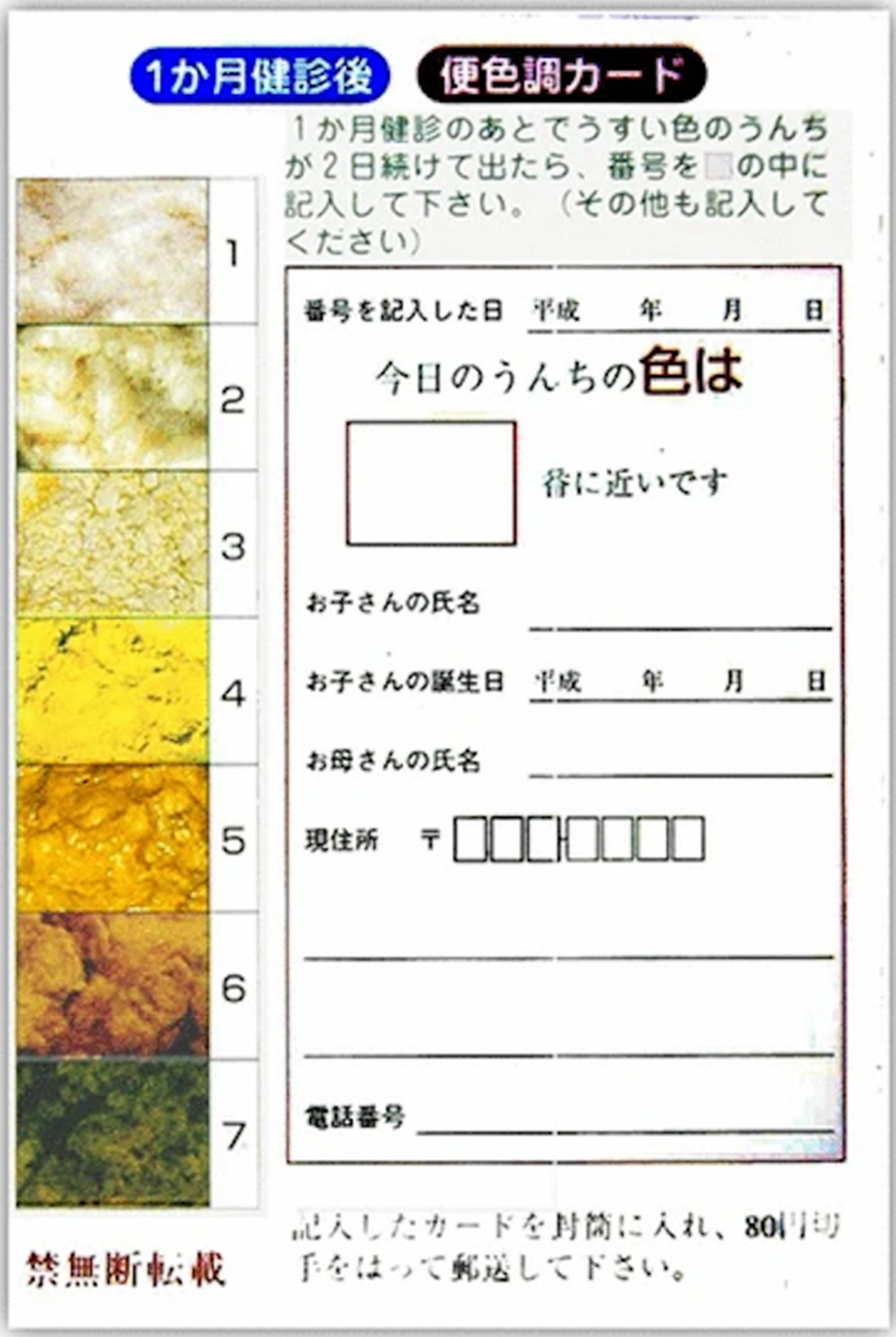
| Stool Colour Card Screening | ||||||||
|---|---|---|---|---|---|---|---|---|
| Country | Year | # Screened Patients | BA Cases | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | KPE Age Pre-/Post Screening |
| Taiwan * [39] Universal national program | 2004–2005 | 422,273 | 75 | 84 | 99.9 | 22.5 | 99.9 | <60 days: 47%/67% 0>91 days post screening |
| Japan # [37] TochigiPrefecture | 1994–2011 | 313,230 | 34 | 76.5 (62.2–90.7) | 99.9 (99.9–100.0) | 12.7 (8.2–7.3) | 99.9 (99.9–99.9) | 67/56 (median days) 25%/11%>80 days |
| Chaoyang District Beijing † [41] | 2013–2014 | 29,799 | 4 | 50 | 99.9 | 4.5 | 99.9 | n/a |
| Canada § [42] British Columbia | 2014–2016 | 87,583 | 6 | 83 | 99.9 | 6 | 99.9 | n/a |
| * Diagnostic accuracy statistics for detecting BA by 60 days of life; # Diagnostic accuracy statistics for detecting BA by 1 month of life; † Diagnostic accuracy statistics for detecting BA by 4 months of life; § Diagnostic accuracy statistics for detecting BA by 1 month of life; n/a = not available. | ||||||||
| Fractionated Bilirubin Screening | ||||||||
| Country | Year | # Screened Patients | BA Cases | Sensitivity | Specificity | PPV | NPV | KPE Age Pre-/Post Screening |
| UK * [43] | 1995–1997 | 23,214 | 100.0 (76–100) | 99.5 (99.5–99.6) | 10.3 (5–16) | n/a | n/a | |
| US # [44] | 2013–2014 | 11,636 | 2 | 100.0 (20–100) | 99.9 (99.8–99.9) | 18 (3–52) | n/a | n/a |
| US # [45] | 2015–2018 | 123,279 | 7 | 100.0 (56–100) | 99.9 (99.9–99.9) | 5.9 (3–12) | 100 (100–100) | 56/36 |
| * Diagnostic accuracy statistics for detecting BA by 28 days of life (last follow-up test for BA patients performed on day of life 22); # Diagnostic accuracy statistics for detecting BA by 2 weeks of life in a two-stage screening approach (first test in newborn period, second test at 2 weeks of life if first test abnormal); n/a = not available. | ||||||||
| Characteristics | PoopMD | Baby Poop | PopòApp |
|---|---|---|---|
| Year | 2015 | 2017 | 2020 |
| Country | USA | Japan | Italy |
| Reference | [14] | [16] | Current study |
| Programming language | Java | Java | |
| Operating system | iOS/Android | iOS | iOS/Android |
| Source of pictures | Previously validated and recorded | Pre-existing images | Newly acquired images taken with the Pop6App |
| Establishment of the gold standard for stool color | ISCC | Pre-existing BA and non-BA stool images | ISCC |
| Color analyzer system | RGB parameters | RGB and HSV parameters + ma chine learning process | RGB system + machine learning process |
| Clinical assessment of the App | Agreement between 6 doc-tors who revisited the pictures | Performance tested with pre-classi-fied images | Real-time assessment by 4 doctors who took the images (agreement between 4 doctors) |
| Classification of stool color | Acholic, cholic, indeterminate | Acholic, cholic | Acholic, cholic, uncertain, indeterminate |
| Number of pictures for Accuracy test of the App | 34 | 40 | 160 |
| – Acholic | 7 | 5 | 60 |
| – Normal | 24 | 35 | 63 |
| – Uncertain | 16 | ||
| – Indeterminate | 3 | 21 | |
| Sensitivity (95% CI) | 100% | 100% (48–100%) | 100% (93.9–100.0%) |
| Specificity (95% CI) | 89% | 100% (90–100%) | 99% (94.6–99.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiber, R.A.; Harpavat, S.; Hulscher, J.B.F.; Wildhaber, B.E. Biliary Atresia in 2021: Epidemiology, Screening and Public Policy. J. Clin. Med. 2022, 11, 999. https://doi.org/10.3390/jcm11040999
Schreiber RA, Harpavat S, Hulscher JBF, Wildhaber BE. Biliary Atresia in 2021: Epidemiology, Screening and Public Policy. Journal of Clinical Medicine. 2022; 11(4):999. https://doi.org/10.3390/jcm11040999
Chicago/Turabian StyleSchreiber, Richard A., Sanjiv Harpavat, Jan B. F. Hulscher, and Barbara E. Wildhaber. 2022. "Biliary Atresia in 2021: Epidemiology, Screening and Public Policy" Journal of Clinical Medicine 11, no. 4: 999. https://doi.org/10.3390/jcm11040999
APA StyleSchreiber, R. A., Harpavat, S., Hulscher, J. B. F., & Wildhaber, B. E. (2022). Biliary Atresia in 2021: Epidemiology, Screening and Public Policy. Journal of Clinical Medicine, 11(4), 999. https://doi.org/10.3390/jcm11040999






