Temporal Change in Renoprotective Effect of Tolvaptan on Patients with Heart Failure: AURORA Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
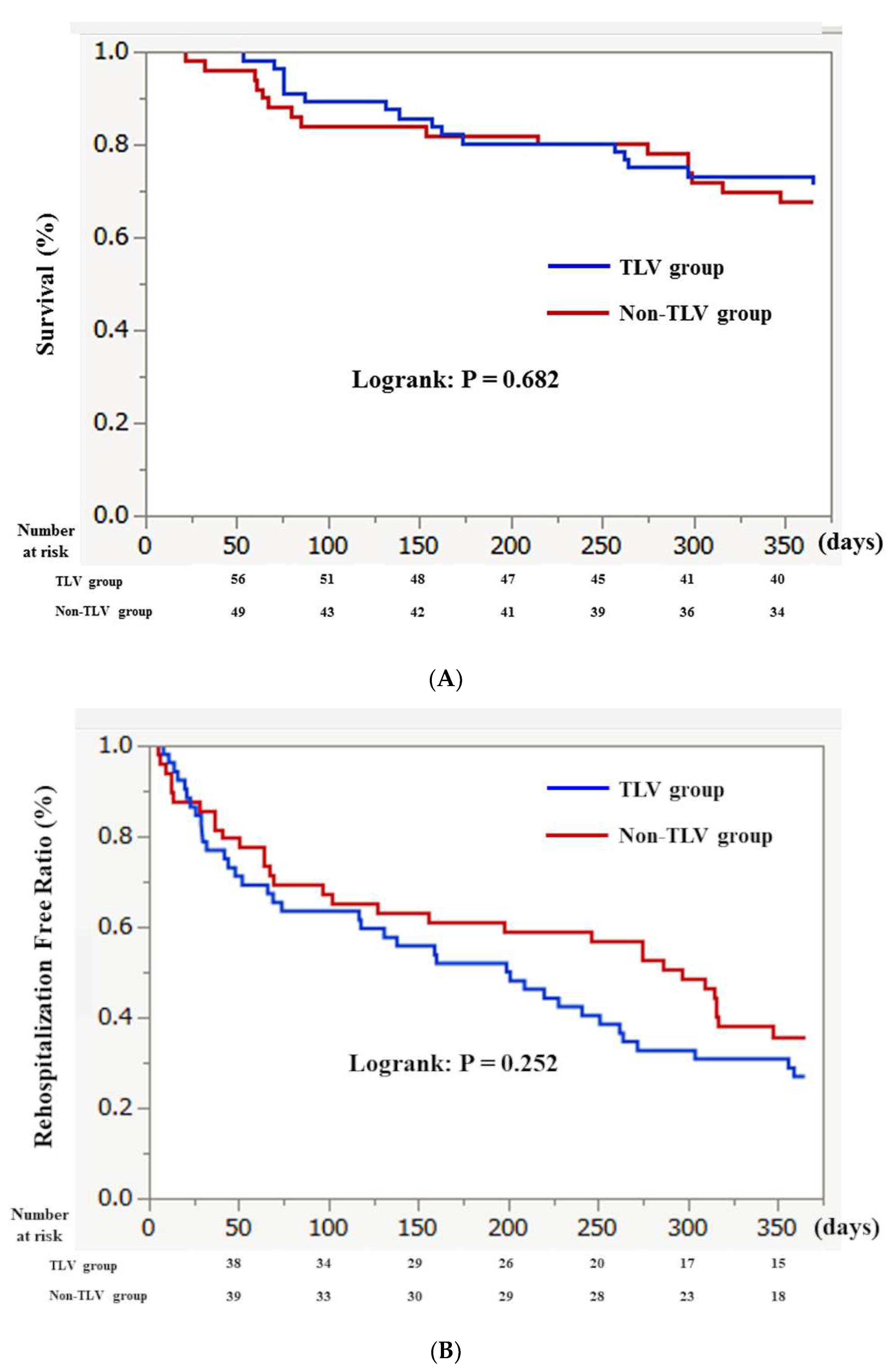
3. Results
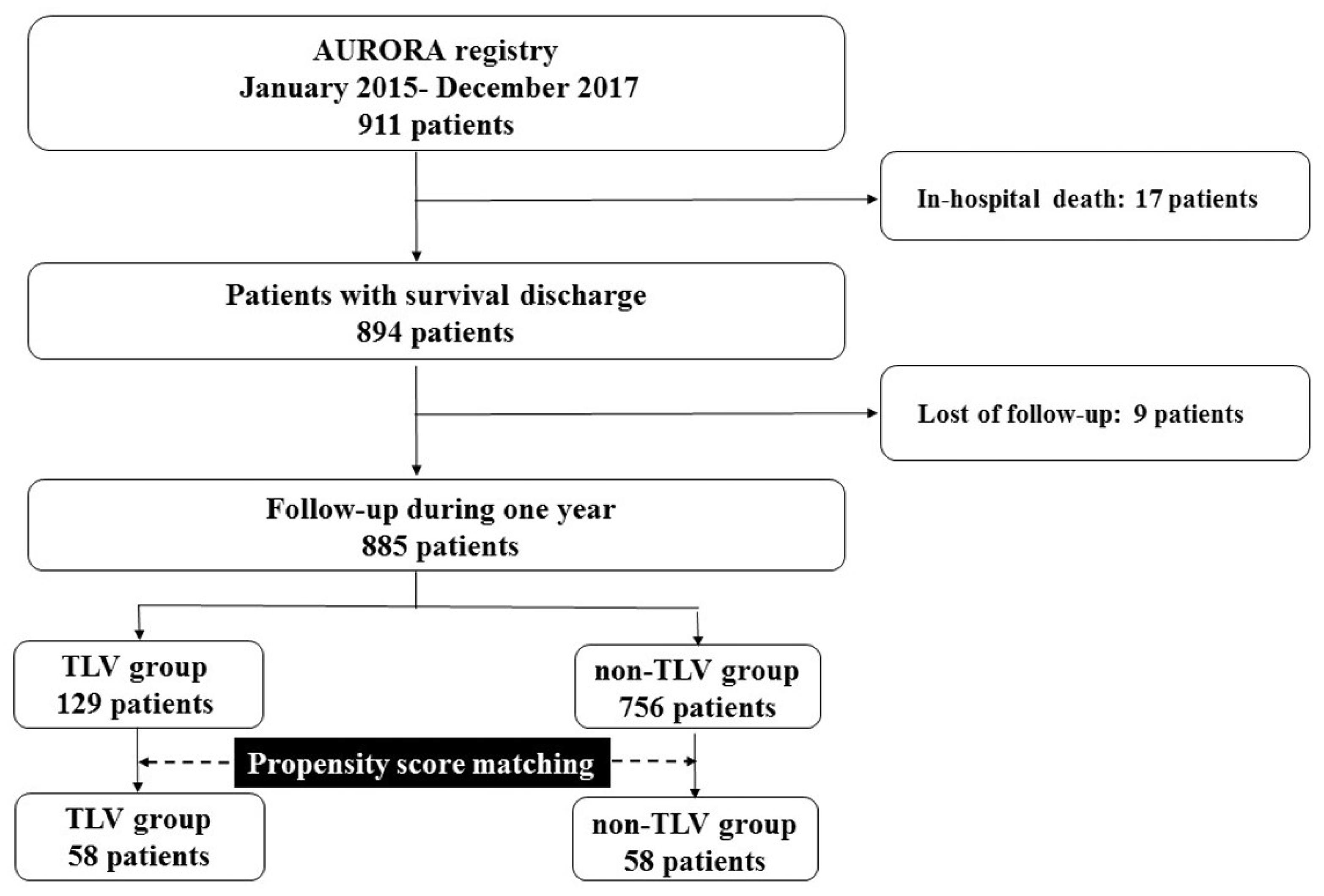
3.1. Study Cohort
3.2. Patient Characteristics
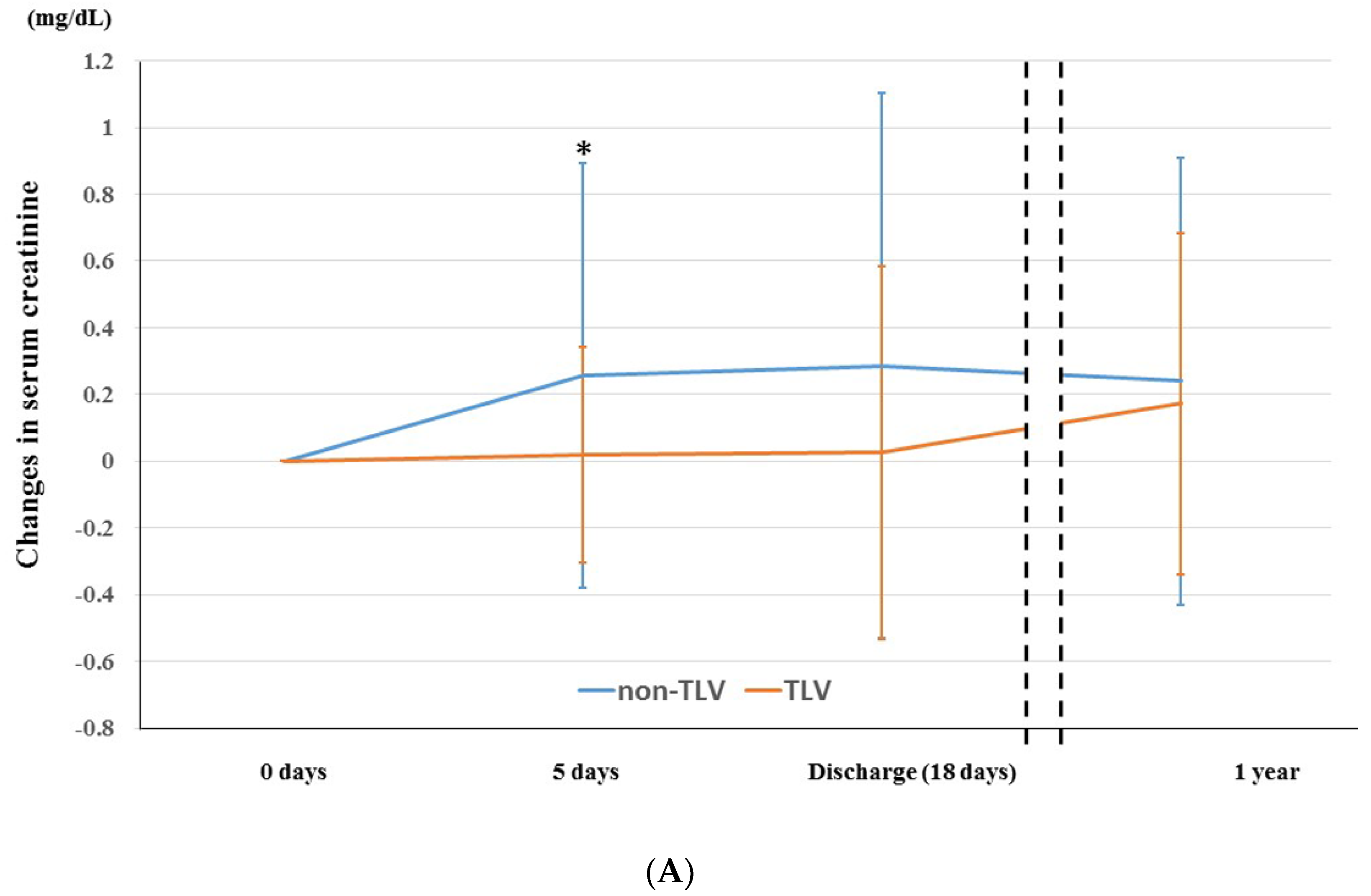
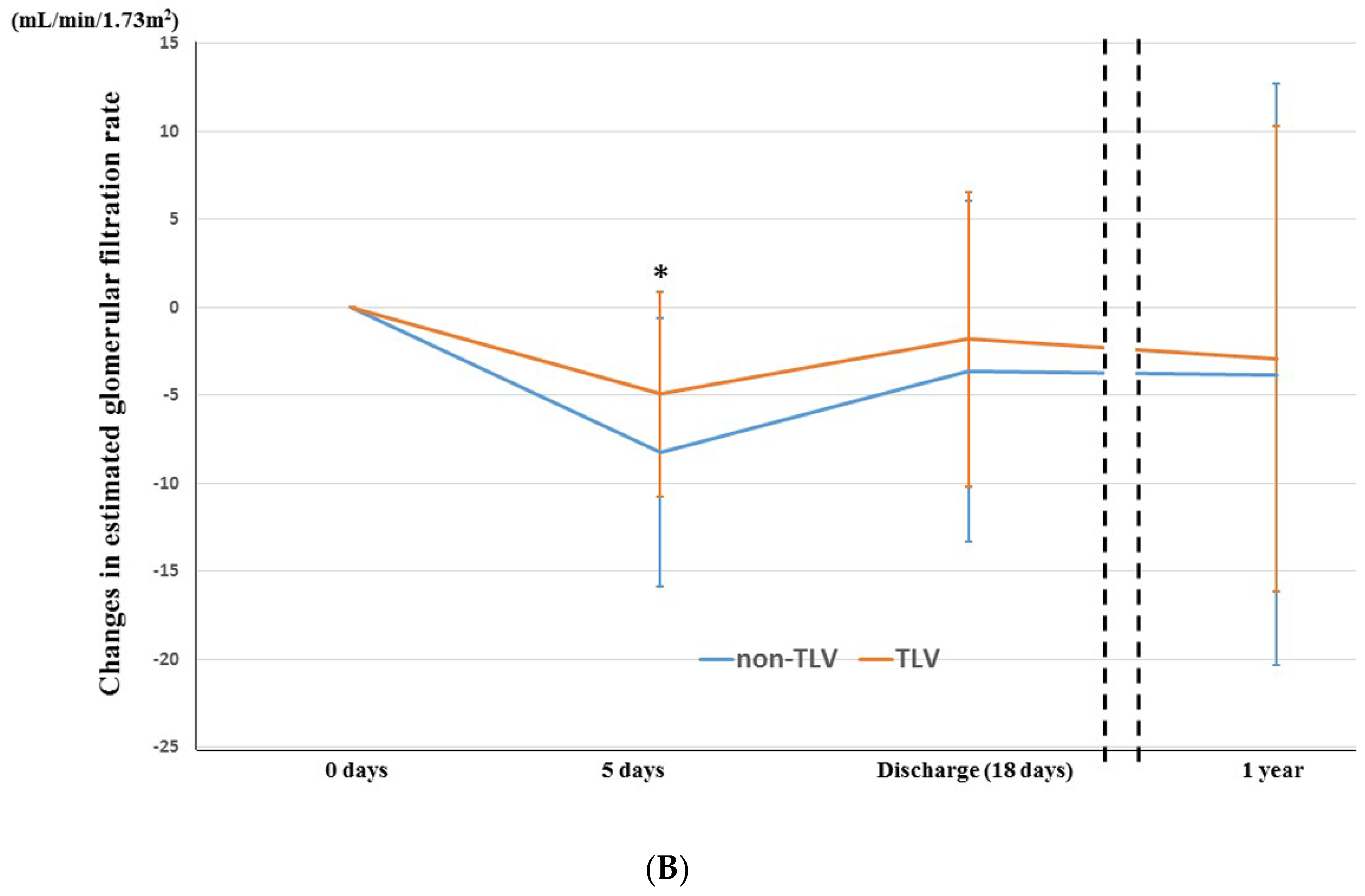
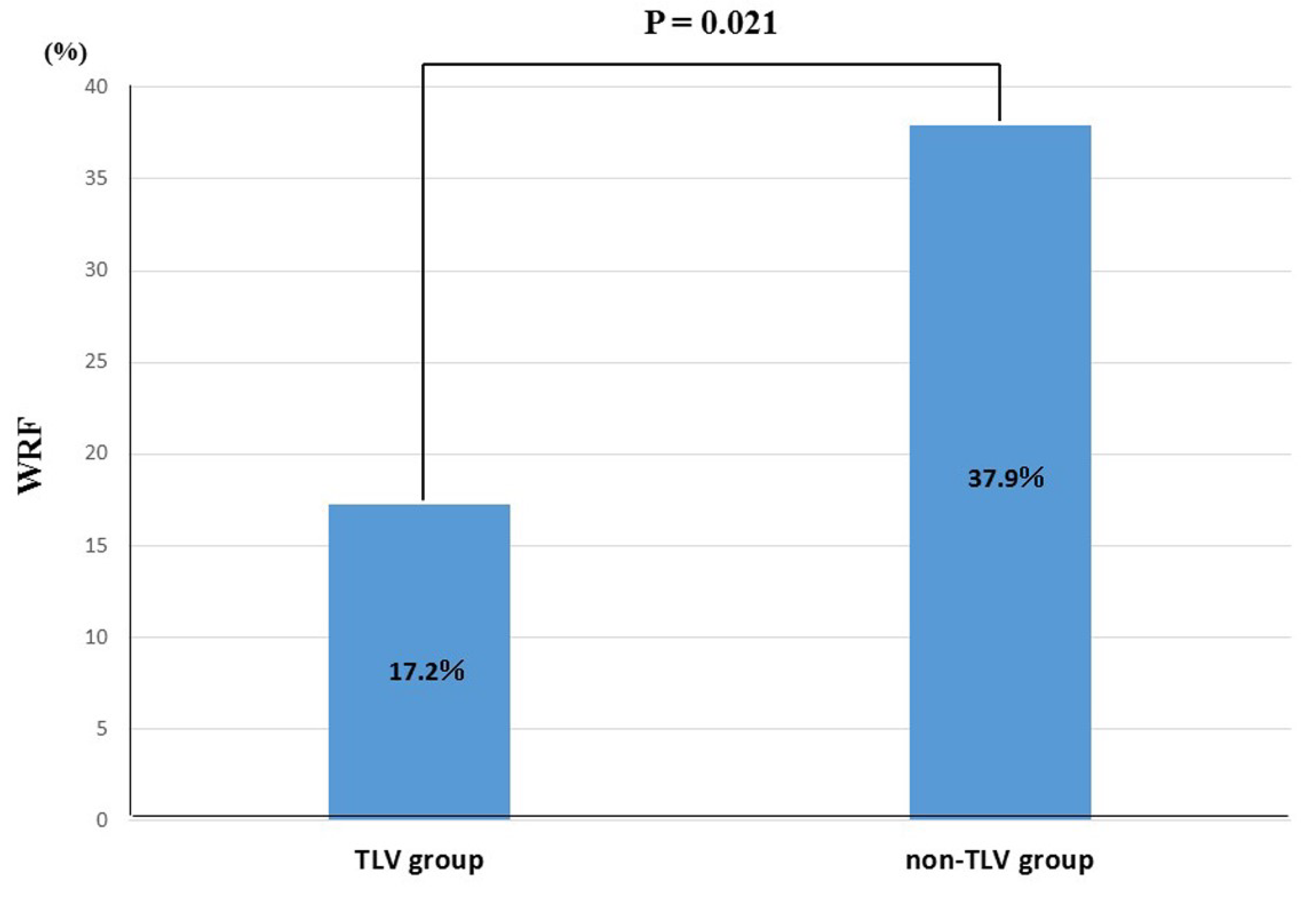
3.3. Temporal Change in Renoprotection between TLV and Non-TLV Groups
3.4. Clinical Outcome
4. Discussion
4.1. Temporal Change in Renoprotective Effect of TLV
4.2. Effect of TLV on One-Year All-Cause Mortality and Rehospitalizations
4.3. Optimal Duration and Suitable Dose of TLV
4.4. Clinical Implications
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Farmakis, D.; Parissis, J.; Karavidas, A.; Karvounis, C.; Triposkiadis, F.; Filippatos, G.; Lekakis, J. In-hospital management of acute heart failure: Practical recommendations and future perspectives. Int. J. Cardiol. 2015, 201, 231–236. [Google Scholar] [CrossRef]
- Goh, C.Y.; Vizzi, G.; De Cal, M.; Ronco, C. Cardiorenal syndrome: A complex series of combined heart/kidney disorders. Contrib. Nephrol. 2011, 174, 33–45. [Google Scholar] [PubMed]
- Kuragaichi, T.; Sato, Y. Temporal trends of a vasopressin V2 receptor antagonist in heart failure using a nationwide database in Japan. ESC Heart Fail. 2021, 8, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Sato, Y.; Yamada, T.; Morita, T.; Furukawa, Y.; Iwasaki, Y.; Kawasaki, M.; Kikuchi, A.; Kondo, T.; Ozaki, T.; et al. Tolvaptan Reduces the Risk of Worsening Renal Function in Patients with Acute Decompensated Heart Failure and Preserved Left Ventricular Ejection Fraction—Prospective Randomized Controlled Study. Circ. J. 2017, 81, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, T.; Kinugawa, K. Update of acute and long-term tolvaptan therapy. J. Cardiol. 2019, 73, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef]
- Nakamura, M.; Sunagawa, O.; Kugai, T.; Kinugawa, K. Amiodarone-Induced Hyponatremia Masked by Tolvaptan in a Patient with an Implantable Left Ventricular Assist Device. Int. Heart J. 2017, 58, 1004–1007. [Google Scholar] [CrossRef] [Green Version]
- Takimura, H.; Hada, T.; Kawano, M.; Yabe, T.; Takimura, Y.; Nishio, S.; Nakano, M.; Tsukahara, R.; Muramatsu, T. A novel validated method for predicting the risk of re-hospitalization for worsening heart failure and the effectiveness of the diuretic upgrading therapy with tolvaptan. PLoS ONE 2018, 13, e0207481. [Google Scholar] [CrossRef]
- Ho, K.K.; Pinsky, J.L.; Kannel, W.B.; Levy, D. The epidemiology of heart failure: The Framingham Study. J. Am. Coll. Cardiol. 1993, 22, 6a–13a. [Google Scholar] [CrossRef] [Green Version]
- Matsue, Y.; Suzuki, M.; Torii, S.; Yamaguchi, S.; Fukamizu, S.; Ono, Y.; Fujii, H.; Kitai, T.; Nishioka, T.; Sugi, K.; et al. Goldsmith SR Clinical Effectiveness of Tolvaptan in Patients with Acute Heart Failure and Renal Dysfunction. J. Card. Fail. 2016, 22, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Imamura, T.; Kinugawa, K.; Fujino, T.; Inaba, T.; Maki, H.; Hatano, M.; Yao, A.; Komuro, I. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ. J. 2014, 78, 2240–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voors, A.A.; Davison, B.A.; Felker, G.M.; Ponikowski, P.; Unemori, E.; Cotter, G.; Teerlink, J.R.; Greenberg, B.H.; Filippatos, G.; Teichman, S.L.; et al. Early drop in systolic blood pressure and worsening renal function in acute heart failure: Renal results of Pre-RELAX-AHF. Eur. J. Heart Fail. 2011, 13, 961–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, D.E.; Butler, J.; Wang, Y.; Abraham, W.T.; O’Connor, C.M.; Gottlieb, S.S.; Loh, E.; Massie, B.M.; Rich, M.W.; Stevenson, L.W.; et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol. 2004, 43, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metra, M.; Nodari, S.; Parrinello, G.; Bordonali, T.; Bugatti, S.; Danesi, R.; Fontanella, B.; Lombardi, C.; Milani, P.; Verzura, G.; et al. Worsening renal function in patients hospitalised for acute heart failure: Clinical implications and prognostic significance. Eur. J. Heart Fail. 2008, 10, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Logeart, D.; Tabet, J.Y.; Hittinger, L.; Thabut, G.; Jourdain, P.; Maison, P.; Tartiere, J.M.; Solal, A.C. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int. J. Cardiol. 2008, 127, 228–232. [Google Scholar] [CrossRef]
- Jujo, K.; Saito, K.; Ishida, I.; Furuki, Y.; Kim, A.; Suzuki, Y.; Sekiguchi, H.; Yamaguchi, J.; Ogawa, H.; Hagiwara, N. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail. 2016, 3, 177–188. [Google Scholar] [CrossRef]
- Kin, H.; Matsumura, K.; Yamamoto, Y.; Fujii, K.; Otagaki, M.; Takahashi, H.; Park, H.; Yoshioka, K.; Yokoi, M.; Sugiura, T.; et al. Renoprotective effect of tolvaptan in patients with new-onset acute heart failure. ESC Heart Fail. 2020, 7, 1764–1770. [Google Scholar] [CrossRef]
- Costello-Boerrigter, L.C.; Smith, W.B.; Boerrigter, G.; Ouyang, J.; Zimmer, C.A.; Orlandi, C.; Burnett, J.C., Jr. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am. J. Physiol. Renal Physiol. 2006, 290, F273–F278. [Google Scholar] [CrossRef] [Green Version]
- Vallon, V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol. Sci. 2003, 18, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Hasselblad, V.; Gattis Stough, W.; Shah, M.R.; Lokhnygina, Y.; O’Connor, C.M.; Califf, R.M.; Adams, K.F., Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the ESCAPE trial. Eur. J. Heart Fail. 2007, 9, 1064–1069. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felker, G.M.; Mentz, R.J.; Cole, R.T.; Adams, K.F.; Egnaczyk, G.F.; Fiuzat, M.; Patel, C.B.; Echols, M.; Khouri, M.G.; Tauras, J.M.; et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kiernan, M.; Chandler, A.; Dhingra, R.; Mody, F.V.; Eisen, H.; Haught, W.H.; Wagoner, L.; Gupta, D.; Patten, R.; et al. Short-Term Effects of Tolvaptan in Patients with Acute Heart Failure and Volume Overload. J. Am. Coll. Cardiol. 2017, 69, 1409–1419. [Google Scholar] [CrossRef]
- Kinugawa, K.; Imamura, T.; Komuro, I. Experience of a vasopressin receptor antagonist, tolvaptan, under the unique indication in Japanese heart failure patients. Clin. Pharmacol. Ther. 2013, 94, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, K.; Sato, N.; Inomata, T.; Yasuda, M.; Shimakawa, T.; Fukuta, Y. Real-World Effectiveness and Tolerability of Tolvaptan in Patients with Heart Failure—Final Results of the Samsca Post-Marketing Surveillance in Heart Failure (SMILE). Study Circ. J. 2019, 83, 1520–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momomura, S.I. Tolvaptan, Is It a Trump to Worsening Renal Function? Circ. J. 2017, 81, 642–644. [Google Scholar] [CrossRef] [Green Version]
- Imamura, T.; Kinugawa, S.; Muramatsu, T.; Shiga, T.; Ogimoto, A.; Anzai, T.; Hagiwara, N.; Tsutsui, H.; Komuro, I.; Kinugawa, K. Long-Term Tolvaptan Treatment in Refractory Heart Failure. Circ. Rep. 2019, 1, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, M.; Rao, V.S.; Ivey-Miranda, J.; Fleming, J.; Mahoney, D.; Maulion, C.; Suda, N.; Siwakoti, K.; Ahmad, T.; Jacoby, D.; et al. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation 2020, 142, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Miura, K.; Iwao, H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J. Pharmacol. Sci. 2014, 124, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Overall | Propensity Score-Matching | |||||
|---|---|---|---|---|---|---|
| TLV Group (n = 129) | Non-TLV Group (n = 756) | p Value | TLV Group (n = 58) | Non-TLV Group (n = 58) | p Value | |
| Clinical data | ||||||
| Age, years | 79 (73–86) | 79 (71–85) | 0.594 | 79 (74–86) | 77 (70–85) | 0.338 |
| Male, n (%) | 85 (62.0) | 398 (70.0) | 0.026 | 34 (58.6) | 29 (50) | 0.456 |
| Hypertension, n (%) | 79 (57.7) | 542 (63.2) | 0.005 | 26 (44.8) | 43 (74.1) | 0.002 |
| Diabetes mellitus, n (%) | 56 (40.9) | 286 (37.0) | 0.390 | 25 (43.1) | 23 (39.7) | 0.851 |
| Dyslipidemia, n (%) | 42 (30.7) | 273 (356.3) | 0.286 | 21 (36.2) | 26 (44.8) | 0.450 |
| Chronic kidney disease, n (%) | 105 (76.7) | 369 (47.7) | <0.001 | 42 (72.4) | 34 (58.6) | 0.171 |
| Smoker, n (%) | 65 (47.0) | 339 (43.8) | 0.456 | 22 (37.9) | 22 (37.9) | 1.000 |
| Past history of HF admission, n (%) | 109 (73.6) | 328 (42.4) | <0.001 | 48 (82.8) | 35 (60.3) | 0.013 |
| Length of stay, days | 20 (13–26) | 16 (12–24) | <0.001 | 18 (13–23) | 17 (14–25) | 0.862 |
| Systolic blood pressure, mmHg | 117 (100–133) | 126 (108–149) | 0.028 | 115 (99–130) | 115 (102–130) | 0.330 |
| Diastolic blood pressure, mmHg | 62 (51–69) | 63 (56–72) | 0.018 | 65 (55–79) | 71 (64–82) | 0.040 |
| Body weight, kg | 53 (33–57) | 56 (47–66) | 0.170 | 55 (47–63) | 54 (48–64) | 0.993 |
| Electrocardiographic data at discharge | ||||||
| Heart rate | 73 (65–88) | 80 (71–95) | <0.001 | 73 (62–88) | 73 (66–83) | 0.067 |
| AF, n (%) | 48 (35.0) | 231 (29.8) | 0.359 | 19 (32.8) | 17.1 (29.3) | 0.841 |
| Laboratory data at discharge | ||||||
| CRP, mg/L | 0.36 (0.16–1.02) | 0.47 (0.18–1.46) | 0.094 | 0.40 (0.12–0.88) | 0.55 (0.20–1.95) | 0.639 |
| BNP, pg/mL | 1298 (628–1570) | 809 (426–1329) | <0.001 | 947 (532–1511) | 1004 (548–1695) | 0.178 |
| Hemoglobin, g/dL | 8.1 (7.0–9.6) | 11.1 (9.8–13.0) | 0.003 | 10.7 (9.4–12.5) | 10.8 (9.28–12.10) | 0.865 |
| Creatinine, mg/dL | 1.54 (1.23–2.19) | 1.10 (0.86–1.81) | <0.001 | 1.51 (1.29–2.01) | 1.47 (0.996–2.28) | 0.489 |
| eGFR, mL/min/1.73 m2 | 28.3 (20.2–39.8) | 33.6 (22.1–56.2) | 0.004 | 28.5 (22.7–39.9) | 30.7 (21.56–47.7) | 0.628 |
| Albumin, g/dL | 3.7 (3.4–3.9) | 3.5 (3.2–3.8) | 0.121 | 3.7 (3.4–3.9) | 3.5 (3.3–3.9) | 0.121 |
| Sodium, mEq/L | 138 (134–140) | 140 (137–142) | <0.001 | 137 (134–140) | 139 (137–141) | 0.058 |
| Potassium, mEq/L | 4.5 (3.7–4.6) | 4.2 (3.8–4.7) | <0.001 | 4.5 (4.1–4.8) | 4.2 (3.8–4.8) | 0.355 |
| Echocardiographic data at discharge | ||||||
| LVDd, mm | 55 (47–64) | 52 (47–58) | 0.030 | 58 (46–55) | 53 (43–62) | 0.661 |
| LVDs, mm | 43 (30–54) | 38 (30–49) | 0.030 | 46 (29–55) | 39 (31–53) | 1.000 |
| LVEF, % | 42 (31–63) | 51 (37–65) | 0.012 | 44 (33–65) | 51 (31–63) | 0.792 |
| LAD, mm | 51 (46–55) | 49 (45–53) | 0.121 | 52 (47–55) | 51 (44–56) | 0.618 |
| E/e’ | 17.4 (13.8–23.3) | 17.8 (13.3–23.3) | 0.620 | 17.7 (14.3–27.3) | 17.5 (13.7–23.1) | 0.635 |
| MR, n (%) | 11 (8.0) | 115 (14.9) | 0.031 | 8 (13.8) | 9 (15.5) | 1.000 |
| AR, n (%) | 11 (8.0) | 38 (4.9) | 0.149 | 6 (10.3) | 3 (5.2) | 0.490 |
| TR, n (%) | 22 (16.1) | 84 (10.9) | 0.084 | 10 (17.2) | 13 (22.49) | 0.642 |
| Medication at discharge | ||||||
| β blocker, n (%) | 103 (75.2) | 492 (63.4) | 0.008 | 45 (77.6) | 44 (75.9) | 1.000 |
| ACEI/ARB, n (%) | 64 (46.7) | 448 (57.9) | 0.019 | 22 (37.9) | 38 (65.5) | 0.005 |
| MRA, n (%) | 73 (53.3) | 309 (39.9) | 0.005 | 33 (56.9) | 29 (50.0) | 0.577 |
| Loop diuretics, n (%) | 133 (97.0) | 597 (77.1) | <0.001 | 58 (100.0) | 58 (100.0) | 1.000 |
| Loop diuretics dose (mg/day) | 60 (40–80) | 30 (20–60) | <0.001 | 60 (40–80) | 40 (23–80) | 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishino, M.; Egami, Y.; Tanaka, A.; Kawanami, S.; Sugae, H.; Ukita, K.; Kawamura, A.; Nakamura, H.; Matsuhiro, Y.; Yasumoto, K.; et al. Temporal Change in Renoprotective Effect of Tolvaptan on Patients with Heart Failure: AURORA Study. J. Clin. Med. 2022, 11, 977. https://doi.org/10.3390/jcm11040977
Nishino M, Egami Y, Tanaka A, Kawanami S, Sugae H, Ukita K, Kawamura A, Nakamura H, Matsuhiro Y, Yasumoto K, et al. Temporal Change in Renoprotective Effect of Tolvaptan on Patients with Heart Failure: AURORA Study. Journal of Clinical Medicine. 2022; 11(4):977. https://doi.org/10.3390/jcm11040977
Chicago/Turabian StyleNishino, Masami, Yasuyuki Egami, Akihiro Tanaka, Shodai Kawanami, Hiroki Sugae, Kohei Ukita, Akito Kawamura, Hitoshi Nakamura, Yutaka Matsuhiro, Koji Yasumoto, and et al. 2022. "Temporal Change in Renoprotective Effect of Tolvaptan on Patients with Heart Failure: AURORA Study" Journal of Clinical Medicine 11, no. 4: 977. https://doi.org/10.3390/jcm11040977
APA StyleNishino, M., Egami, Y., Tanaka, A., Kawanami, S., Sugae, H., Ukita, K., Kawamura, A., Nakamura, H., Matsuhiro, Y., Yasumoto, K., Tsuda, M., Okamoto, N., Matsunaga-Lee, Y., Yano, M., & Tanouchi, J. (2022). Temporal Change in Renoprotective Effect of Tolvaptan on Patients with Heart Failure: AURORA Study. Journal of Clinical Medicine, 11(4), 977. https://doi.org/10.3390/jcm11040977






