Chinese Admission Warning Strategy for Predicting the Hospital Discharge Outcome in Patients with Traumatic Brain Injury
Abstract
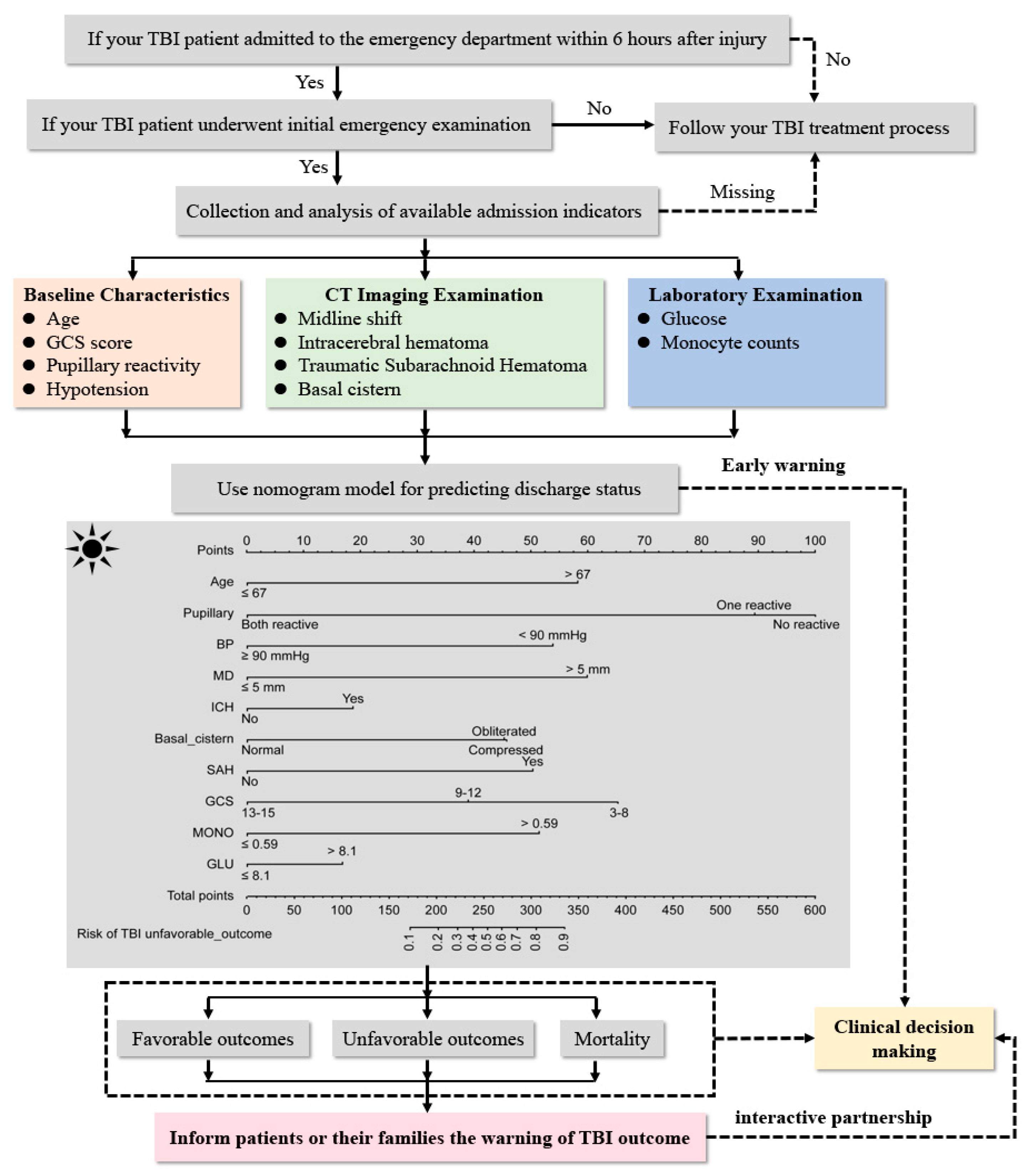
:1. Introduction
2. Patients and Methods
2.1. Participants
2.2. Data Collection and Patient Management
2.3. Outcome Assessment
2.4. Statistical Analysis (Developing and Validating Phase)
3. Results
3.1. Participants
3.2. Feature Selection and Admission Warning Strategy Development
4. Clinical Use and Validation
4.1. Internal Validation
4.2. External Validation
5. Discussion
6. Limitations and Future Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.Y.; Gao, G.Y.; Feng, J.F.; Mao, Q.; Chen, L.G.; Yang, X.F.; Liu, J.F.; Wang, Y.H.; Qiu, B.H.; Huang, X.J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef]
- Gao, G.; Wu, X.; Feng, J.; Hui, J.; Mao, Q.; Lecky, F.; Lingsma, H.; Maas, A.I.R.; Jiang, J. China CENTER-TBI Registry Participants. Clinical characteristics and outcomes in patients with traumatic brain injury in China: A prospective, multicentre, longitudinal, observational study. Lancet Neurol. 2020, 19, 670–677. [Google Scholar] [CrossRef]
- Puffer, R.C.; Yue, J.K.; Mesley, M.; Billigen, J.B.; Sharpless, J.; Fetzick, A.L.; Puccio, A.; Diaz-Arrastia, R.; Okonkwo, D.O. Long-term outcome in traumatic brain injury patients with midline shift: A secondary analysis of the Phase 3 COBRIT clinical trial. J. Neurosurg. 2018, 131, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Chinese Head Trauma Study, C. Chinese Head Trauma Data Bank: Effect of Gender on the Outcome of Patients With Acute Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1164–1167. [Google Scholar] [CrossRef]
- Van der Naalt, J.; Timmerman, M.E.; de Koning, M.E.; van der Horn, H.J.; Scheenen, M.E.; Jacobs, B.; Hageman, G.; Yilmaz, T.; Roks, G.; Spikman, J.M. Early predictors of outcome after mild traumatic brain injury (UPFRONT): An observational cohort study. Lancet Neurol. 2017, 16, 532–540. [Google Scholar] [CrossRef]
- Retel Helmrich, I.R.A.; Lingsma, H.F.; Turgeon, A.F.; Yamal, J.M.; Steyerberg, E.W. Prognostic Research in Traumatic Brain Injury: Markers, Modeling, and Methodological Principles. J. Neurotrauma 2020, 38, 2502–2513. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Mushkudiani, N.; Perel, P.; Butcher, I.; Lu, J.; McHugh, G.S.; Murray, G.D.; Marmarou, A.; Roberts, I.; Habbema, J.D.; et al. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008, 5, e165. [Google Scholar] [CrossRef] [Green Version]
- Zetterberg, H.; Blennow, K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 2016, 12, 563–574. [Google Scholar] [CrossRef]
- Van de Zande, N.; Manivannan, S.; Sharouf, F.; Shastin, D.; Abdulla, M.; Chumas, P.D.; Zaben, M. Demographics, presentation, and clinical outcomes after traumatic bifrontal contusions: A systematic review. Neurosurg. Rev. 2020, 43, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, M.; Koskinen, L.D.; Jonasson, P.; Levi, R.; Stalnacke, B.M. Computed tomography and clinical outcome in patients with severe traumatic brain injury. Brain Inj. 2017, 31, 351–358. [Google Scholar] [CrossRef]
- Kurtz, P.; Rocha, E.E.M. Nutrition Therapy, Glucose Control, and Brain Metabolism in Traumatic Brain Injury: A Multimodal Monitoring Approach. Front. Neurosci. 2020, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Toffolo, K.; Osei, J.; Kelly, W.; Poulsen, A.; Donahue, K.; Wang, J.; Hunter, M.; Bard, J.; Wang, J.; Poulsen, D. Circulating microRNAs as biomarkers in traumatic brain injury. Neuropharmacology 2019, 145, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.G.F.; Milliron, E.; Ho, M.L.; Hu, H.H.; Rusin, J.; Leonard, J.; Sribnick, E.A. Advanced neuroimaging in traumatic brain injury: An overview. Neurosurg. Focus 2019, 47, E17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, J.K.; Yuh, E.L.; Korley, F.K.; Winkler, E.A.; Sun, X.; Puffer, R.C.; Deng, H.; Choy, W.; Chandra, A.; Taylor, S.R.; et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: A prospective multicentre study. Lancet Neurol. 2019, 18, 953–961. [Google Scholar] [CrossRef]
- Collaborators, M.C.T.; Perel, P.; Arango, M.; Clayton, T.; Edwards, P.; Komolafe, E.; Poccock, S.; Roberts, I.; Shakur, H.; Steyerberg, E.; et al. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 2008, 336, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Dijkland, S.A.; Foks, K.A.; Polinder, S.; Dippel, D.W.J.; Maas, A.I.R.; Lingsma, H.F.; Steyerberg, E.W. Prognosis in Moderate and Severe Traumatic Brain Injury: A Systematic Review of Contemporary Models and Validation Studies. J. Neurotrauma 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Wilson, J.T.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- Lingsma, H.F.; Roozenbeek, B.; Steyerberg, E.W.; Murray, G.D.; Maas, A.I. Early prognosis in traumatic brain injury: From prophecies to predictions. Lancet Neurol. 2010, 9, 543–554. [Google Scholar] [CrossRef]
- Creeden, S.; Ding, V.Y.; Parker, J.J.; Jiang, B.; Li, Y.; Lanzman, B.; Trinh, A.; Khalaf, A.; Wolman, D.; Halpern, C.H.; et al. Interobserver Agreement for the Computed Tomography Severity Grading Scales for Acute Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1445–1451. [Google Scholar] [CrossRef]
- Au, A.K.; Clark, R.S.B. Paediatric traumatic brain injury: Prognostic insights and outlooks. Curr. Opin. Neurol. 2017, 30, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.L.; Yamal, J.M.; Chan, W.; Robertson, C.S. Prognosis of Six-Month Glasgow Outcome Scale in Severe Traumatic Brain Injury Using Hospital Admission Characteristics, Injury Severity Characteristics, and Physiological Monitoring during the First Day Post-Injury. J. Neurotrauma 2019, 36, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Y.; Yang, H.; Yu, H.; Wang, T.; Chen, Z.; Yao, R.; Liang, Z. Development and validation a nomogram for predicting the risk of severe COVID-19: A multi-center study in Sichuan, China. PLoS ONE 2020, 15, e0233328. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [Green Version]
- Pencina, M.J.; D’Agostino, R.B. Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat. Med. 2004, 23, 2109–2123. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med. Inform. Decis. Mak. 2008, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, G.; Cong, D.; Zeng, Y.; Fan, W.; Shen, Y. A traumatic injury mortality prediction (TRIMP) based on a comprehensive assessment of abbreviated injury scale 2005 predot codes. Sci. Rep. 2021, 11, 21757. [Google Scholar] [CrossRef]
- Erlebach, R.; Pagnamenta, A.; Klinzing, S.; Stretti, F.; Cottini, S.; Schupbach, R.; Steiger, P.; Brandi, G. Age-related outcome of patients after traumatic brain injury: A single-center observation. Minerva Anestesiol. 2017, 83, 1169–1177. [Google Scholar] [CrossRef]
- Shibahashi, K.; Sugiyama, K.; Okura, Y.; Tomio, J.; Hoda, H.; Hamabe, Y. Defining Hypotension in Patients with Severe Traumatic Brain Injury. World Neurosurg. 2018, 120, e667–e674. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Song, J.; Li, S.; Cao, C.; Fang, L.; Wang, C.; Xu, G. Helsinki Computed Tomography Scoring System Can Independently Predict Long-Term Outcome in Traumatic Brain Injury. World Neurosurg. 2017, 101, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Siironen, J.; Skrifvars, M.B.; Hernesniemi, J.; Kivisaari, R. Predicting outcome in traumatic brain injury: Development of a novel computerized tomography classification system (Helsinki computerized tomography score). Neurosurgery 2014, 75, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Mata-Mbemba, D.; Mugikura, S.; Nakagawa, A.; Murata, T.; Ishii, K.; Li, L.; Takase, K.; Kushimoto, S.; Takahashi, S. Early CT findings to predict early death in patients with traumatic brain injury: Marshall and Rotterdam CT scoring systems compared in the major academic tertiary care hospital in northeastern Japan. Acad. Radiol. 2014, 21, 605–611. [Google Scholar] [CrossRef]
- Talari, H.R.; Hamidian, Y.; Moussavi, N.; Fakharian, E.; Abedzadeh-Kalahroudi, M.; Akbari, H.; Taher, E.B. The Prognostic Value of Rotterdam Computed Tomography Score in Predicting Early Outcomes Among Children with Traumatic Brain Injury. World Neurosurg. 2019, 125, e139–e145. [Google Scholar] [CrossRef] [PubMed]
- Rovlias, A.; Kotsou, S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000, 46, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.; Scaife, E.R.; Hansen, K.W.; Downey, E.C. Hyperglycemia and outcomes from pediatric traumatic brain injury. J. Trauma 2003, 55, 1035–1038. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Wu, X.; Yu, J.; Yuan, Q.; Du, Z.; Hu, J. Admission circulating monocytes level is an independent predictor of outcome in traumatic brain injury. Brain Inj. 2018, 32, 515–522. [Google Scholar] [CrossRef]
- Lu, H.Y.; Li, T.C.; Tu, Y.K.; Tsai, J.C.; Lai, H.S.; Kuo, L.T. Predicting long-term outcome after traumatic brain injury using repeated measurements of Glasgow Coma Scale and data mining methods. J. Med. Syst. 2015, 39, 14. [Google Scholar] [CrossRef]
- Thelin, E.P.; Nelson, D.W.; Vehvilainen, J.; Nystrom, H.; Kivisaari, R.; Siironen, J.; Svensson, M.; Skrifvars, M.B.; Bellander, B.M.; Raj, R. Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: An observational, multicenter study. PLoS Med. 2017, 14, e1002368. [Google Scholar] [CrossRef]
- Kahraman, S.; Hu, P.; Stein, D.M.; Stansbury, L.G.; Dutton, R.P.; Xiao, Y.; Hess, J.R.; Scalea, T.M. Dynamic three-dimensional scoring of cerebral perfusion pressure and intracranial pressure provides a brain trauma index that predicts outcome in patients with severe traumatic brain injury. J. Trauma 2011, 70, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.T.; Song, E.S.; Yoon, B.C.; Choi, Y.H.; Kim, K.; Kim, D.J. Changes in the gray and white matter of patients with ischemic-edematous insults after traumatic brain injury. J. Neurosurg. 2018, 131, 1243–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variables | Primary Cohort (n = 605) | Sub Cohort One (n = 180) | Sub Cohort Two (n = 107) |
|---|---|---|---|
| Age (years) (mean ± sd) | 60.1 ± 18.0 | 60.6 ± 17.0 | 59.3 ± 15.8 |
| Sex (n, %) | |||
| Male | 401 (66.3%) | 117 (65.0%) | 61 (57.0%) |
| Female | 204 (33.7%) | 63 (35.0%) | 46 (43.0%) |
| Mechanism of head injury (n, %) | |||
| Traffic incident | 242 (40.0%) | 64 (25.6%) | |
| Fall | 318 (52.6%) | 96 (53.3%) | 39 (36.4%) |
| Other cause | 45 (7.4%) | 20 (11.1%) | 57 (53.3%) |
| Time from injury to admission (h) (median, iqr) | 6 (3–12) | 6 (3–12) | 6 (3–12) |
| Pupillary reactivity at admission (n, %) | |||
| Normal | 536 (88.6%) | 157 (87.2%) | 92 (86.0%) |
| Unilateral abnormality | 21 (3.5%) | 10 (5.6%) | 5 (4.7%) |
| Bilateral abnormality | 48 (7.9%) | 13 (7.2%) | 10 (9.3%) |
| Gcs score at admission | |||
| 14–15 | 406 (67.1%) | 121 (67.2%) | 75 (70.1%) |
| 9–13 | 104 (17.2%) | 27 (15.0%) | 15 (14.0%) |
| ≤8 | 95 (15.7%) | 32 (17.8%) | 17 (15.9%) |
| Hypotension at admission (<90 mmhg) (n, %) | |||
| Yes | 61 (10.1%) | 21 (11.7%) | 9 (8.4%) |
| No | 544 (89.9%) | 159 (88.3%) | 98 (91.6%) |
| Combined extracranial injuries (number) (mean ± sd) | 1.3 ± 1.6 | 1.4 ± 1.6 | 1.2 ± 1.5 |
| Combined underlying diseases (number) (mean ± sd) | 0.9 ± 1.1 | 1.0 ± 1.2 | 0.9 ± 1.0 |
| Neurosurgical procedure (n, %) | |||
| Yes | 145 (24.0%) | 52 (28.9%) | 20 (18.7%) |
| No | 460 (76.0%) | 128 (71.1%) | 87 (81.3%) |
| GOSE at discharge (n, %) | |||
| Favorable outcome for 5–8 | 494 (81.7%) | 146 (81.1%) | 88 (82.2%) |
| Unfavorable outcome for 1–4 | 111 (18.3%) | 34 (18.9%) | 19 (17.8%) |
| Mortality | 74 (12.23%) | 13 (7.2%) | 10 (9.3%) |
| Death within one month (n, %) | |||
| Yes | 74 (12.2%) | 7 (3.9%) | 7 (6.5%) |
| No | 531 (87.8%) | 173 (96.1%) | 100 (93.5%) |
| CT characteristics at admission | |||
| midline shift (n, %) | |||
| Yes | 91 (15.0%) | 28 (15.6%) | 13 (12.1%) |
| No | 514 (85.0%) | 152 (84.4%) | 94 (87.9%) |
| Intracranial lesion (n, %) | |||
| traumatic subarachnoid hemorrhage | 353 (58.3%) | 109 (60.1%) | 55 (51.4%) |
| epidural hematoma | 104 (17.2%) | 25 (13.9%) | 10 (9.3%) |
| subdural hematoma | 333 (55.0%) | 98 (54.4%) | 57 (53.3%) |
| intraparenchymal lesion | 304 (50.2%) | 93 (51.7%) | 55 (51.4%) |
| Lesion size ≥ 25 mL (n, %) | |||
| yes | 81 (13.4%) | 19 (10.6%) | 12 (11.2%) |
| no | 524 (86.6%) | 161 (89.4%) | 95 (88.8%) |
| Basal cistern (n, %) | |||
| normal | 462 (76.4%) | 141 (78.3%) | 84 (78.5%) |
| compression | 86 (14.2%) | 23 (12.8%) | 13 (12.1%) |
| occlusion | 57 (9.4%) | 16 (8.9%) | 10 (9.3%) |
| Marshall classification on admission CT (n, %) | |||
| I–II | 397 (65.6%) | 115 (63.9%) | 74 (69.2%) |
| III–IV | 48 (7.9%) | 18 (10.0%) | 11 (10.3%) |
| V–VI | 175 (28.9%) | 47 (26.1%) | 22 (20.6%) |
| Laboratory examination at admission | |||
| hemoglobin level (G/L) (mean ± sd) | 131.7 ± 20.2 | 133.0 ± 20.0 | 129.7 ± 20.4 |
| blood glucose level (MMOL/L) (mean ± sd) | 7.9 ± 3.2 | 7.9 ± 3.6 | 7.9 ± 3.3 |
| white blood cell count (×109/L) (mean ± sd) | 11.9 ± 5.3 | 12.5 ± 5.5 | 11.8 ± 5.3 |
| monocyte count (×109/L) (mean ± sd) | 0.6 ± 0.6 | 0.6 ± 0.4 | 0.57 ± 0.43 |
| monocyte ratio (×100%) (mean ± sd) | 5.1 ± 2.3 | 4.9 ± 2.5 | 5.2 ± 3.2 |
| neutrophil count (×109/L) (mean ± sd) | 10.0 ± 5.2 | 10.5 ± 5.4 | 10.2 ± 6.0 |
| lymphocyte count (×109/L) (mean ± sd) | 1.4 ± 1.2 | 1.3 ± 1.0 | 1.4 ± 1.8 |
| lactate level (MMOL/L) (mean ± sd) | 2.2 ± 1.5 | 2.2 ± 1.3 | 2.1 ± 1.3 |
| Intercept and Variable | Prediction Ability | ||
|---|---|---|---|
| β | Odds Ratio (95% CI) | p-Value | |
| Age | 1.563 | 4.772 (2.019–11.281) | <0.001 |
| GCS score of 3–8 points | — | — | 0.013 |
| GCS score of 9–12 points | −0.711 | 0.491 (0.148–1.672) | 0.245 |
| GCS score of 13–15 points | −1.754 | 0.173 (0.052-0.580) | 0.004 |
| Normal pupil | — | — | 0.001 |
| Unilateral pupil reaction | 2.398 | 11.004 (1.089–111.151) | 0.042 |
| No pupil reaction | 2.685 | 14.663 (3.131–68.660) | 0.001 |
| Hypotension (≤90 mmHg) | 1.445 | 4.240 (1.250–14.380) | 0.020 |
| Midline shift (≥5 mm) | 1.607 | 4.986 (1.693–14.688) | 0.004 |
| Intracerebral hematoma | 0.497 | 1.645 (0.677–3.995) | 0.272 |
| Subarachnoid Hematoma | 1.352 | 3.864 (1.053–14.186) | 0.042 |
| Basal cistern—Normal | — | — | 0.063 |
| Basal cistern—Compression | 1.227 | 3.411 (1.205–9.655) | 0.021 |
| Basal cistern—Occlusion | 1.216 | 3.373 (0.755–15.062) | 0.111 |
| Glucose level (>8.1 mmol/L) | 0.448 | 1.565 (0.674–3.636) | 0.298 |
| Monocyte count (>0.59 × 109/L) | 1.381 | 3.977 (1.640–9.643) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Zhuang, Z.; Zhao, C.; Zhao, Z.; Yang, X.; Zhou, Y.; Pan, S.; Chen, K.; Li, K.; Huang, Q.; et al. Chinese Admission Warning Strategy for Predicting the Hospital Discharge Outcome in Patients with Traumatic Brain Injury. J. Clin. Med. 2022, 11, 974. https://doi.org/10.3390/jcm11040974
Zheng R, Zhuang Z, Zhao C, Zhao Z, Yang X, Zhou Y, Pan S, Chen K, Li K, Huang Q, et al. Chinese Admission Warning Strategy for Predicting the Hospital Discharge Outcome in Patients with Traumatic Brain Injury. Journal of Clinical Medicine. 2022; 11(4):974. https://doi.org/10.3390/jcm11040974
Chicago/Turabian StyleZheng, Ruizhe, Zhongwei Zhuang, Changyi Zhao, Zhijie Zhao, Xitao Yang, Yue Zhou, Shuming Pan, Kui Chen, Keqin Li, Qiong Huang, and et al. 2022. "Chinese Admission Warning Strategy for Predicting the Hospital Discharge Outcome in Patients with Traumatic Brain Injury" Journal of Clinical Medicine 11, no. 4: 974. https://doi.org/10.3390/jcm11040974
APA StyleZheng, R., Zhuang, Z., Zhao, C., Zhao, Z., Yang, X., Zhou, Y., Pan, S., Chen, K., Li, K., Huang, Q., Wang, Y., & Ma, Y. (2022). Chinese Admission Warning Strategy for Predicting the Hospital Discharge Outcome in Patients with Traumatic Brain Injury. Journal of Clinical Medicine, 11(4), 974. https://doi.org/10.3390/jcm11040974






