A Longitudinal Study of Mitral Regurgitation Detected after Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
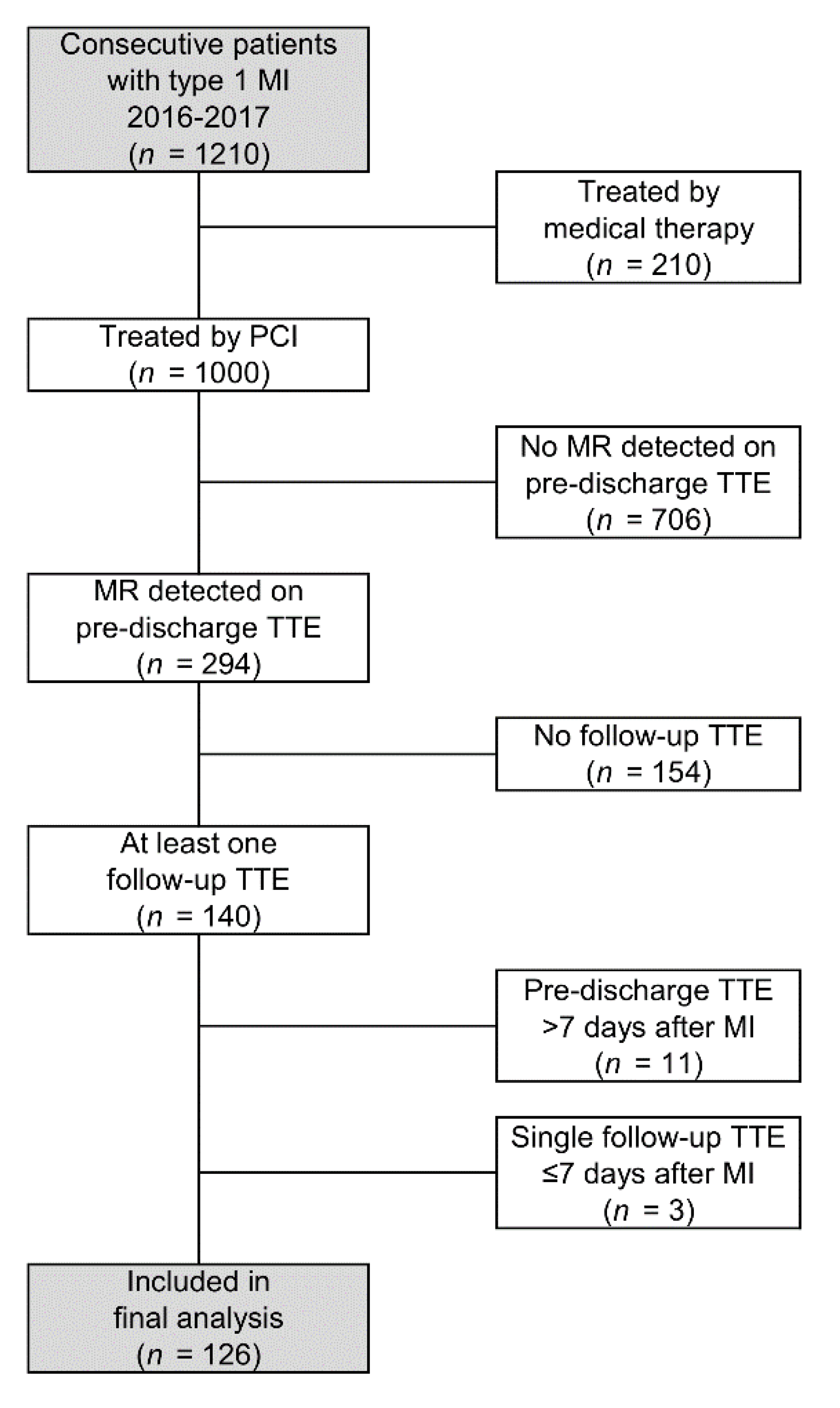
2.1. Patient Selection
2.2. Data Collection
2.3. MR and Chamber Assessment
2.4. Intervention and Medical Treatment
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
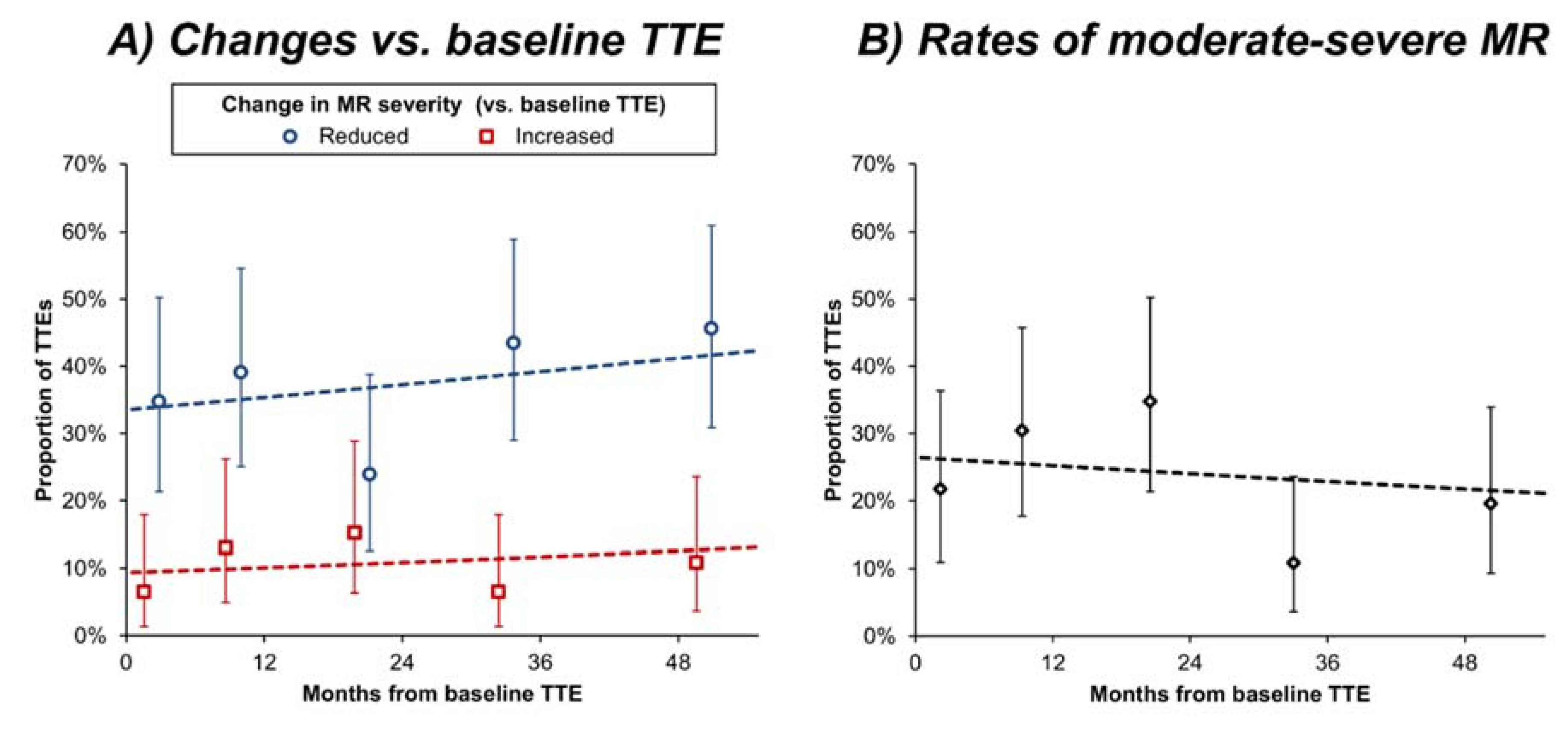
3.2. Changes in MR Severity
3.3. Associations with Changes in MR Severity
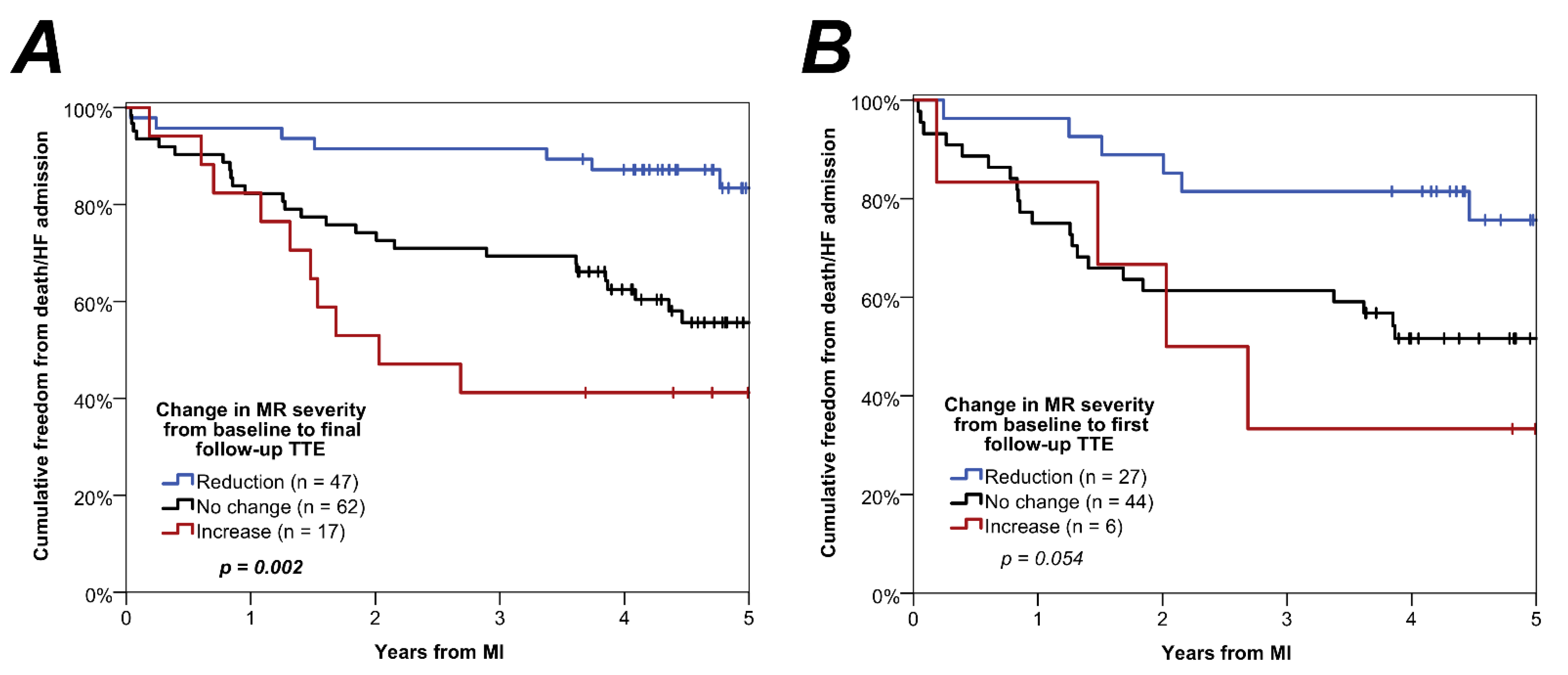
3.4. Associations with Patient Outcome
3.5. Assessment of Selection Bias
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, H.; Radhakrishnan, A.; Nightingale, P.; Brown, S.; May, J.; O’Connor, K.; Shakeel, I.; Zia, N.; Doshi, S.N.; Townend, J.N.; et al. Mitral regurgitation following acute myocardial infarction treated by percutaneous coronary intervention-prevalence, risk factors and predictors of outcome. Am. J. Cardiol. 2021, (in press). [Google Scholar] [CrossRef] [PubMed]
- Nishino, S.; Watanabe, N.; Kimura, T.; Enriquez-Sarano, M.; Nakama, T.; Furugen, M.; Koiwaya, H.; Ashikaga, K.; Kuriyama, N.; Shibata, Y. The course of ischemic mitral regurgitation in acute myocardial infarction after primary percutaneous coronary intervention. Circ. Cardiovasc. Imaging 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentias, A.; Raza, M.Q.; Barakat, A.F.; Hill, E.; Youssef, D.; Krishnaswamy, A.; Desai, M.Y.; Griffin, B.; Ellis, S.; Menon, V.; et al. Outcomes of ischaemic mitral regurgitation in anterior versus inferior ST elevation myocardial infarction. Open Heart 2016, 3, e000493. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Smith, R.L.; Schiattarella, G.G.; Trimarco, B.; Esposito, G.; Grayburn, P.A. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: A systematic review and meta-analysis. JAMA Cardiol. 2017, 2, 1130. Available online: https://europepmc.org/articles/PMC (accessed on 1 December 2020). [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 00, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Wharton, G.; Steeds, R.; Allen, J.; Phillips, H.; Jones, R.; Kanagala, P.; Lloyd, G.; Masani, N.; Mathew, T.; Oxborough, D.; et al. A minimum dataset for a standard adult transthoracic echocardiogram: A guideline protocol from the British Society of echocardiography. Echo Res. Pract. 2015, 2, G9–G24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Morisco, C.; di Panzillo, E.A.; Izzo, R.; Trimarco, B. Reverse left ventricular remodeling after acute myocardial infarction: The prognostic impact of left ventricular global torsion. Int. J. Cardiovasc. Imaging 2012, 29, 787. Available online: https://link.springer.com/article/10.1007/s10554-012-0159 (accessed on 16 November 2021). [CrossRef] [PubMed]
- Doughty, R.N.; Whalley, G.A.; Gamble, G.; MacMahon, S.; Sharpe, N. Left Ventricular Remodeling With Carvedilol in Patients With Congestive Heart Failure Due to Ischemic Heart Disease. J. Am. Coll. Cardiol. 1997, 29, 1060–1066. [Google Scholar] [CrossRef] [Green Version]
- Konstam, M.A.; Rousseau, M.F.; Kronenberg, M.W.; E Udelson, J.; Melin, J.; Stewart, D.; Dolan, N.; Edens, T.R.; Ahn, S.; Kinan, D. Effects of the Angiotensin Converting Enzyme Inhibitor Enalapril on the Long-term Progression of Left Ventricular Dysfunction in Patients With Heart Failure. Circulation 1992, 86, 431–438. Available online: http://ahajournals.org (accessed on 16 November 2021). [CrossRef] [PubMed] [Green Version]
- Anon. Heart Valve Disease Presenting in Adults: Investigation and Management NICE Guideline. Available online: www.nice.org.uk/guidance/ng (accessed on 16 December 2021).

| n | Statistic | |
|---|---|---|
| Age at MI (years) | 126 | 70.9 ± 11.4 |
| Sex (% male) | 126 | 81 (64%) |
| BSA (m2) | 126 | 1.84 ± 0.23 |
| Hypertension | 126 | 87 (69%) |
| Diabetes mellitus | 126 | 50 (40%) |
| Creatinine clearance (mL/min) | 126 | 70 ± 34 |
| Peak troponin (ng/L) | 126 | 647 (106–3430) |
| Type of MI | ||
| NSTEMI | 126 | 76 (60%) |
| STEMI | 126 | 50 (40%) |
| Coronary artery involvement | ||
| Left main coronary artery | 126 | 9 (7%) |
| Left anterior descending coronary artery | 126 | 97 (77%) |
| Left circumflex coronary artery | 126 | 63 (50%) |
| Right coronary artery | 126 | 61 (48%) |
| Baseline TTE | ||
| Days from MI to baseline TTE | 126 | 2 (1–3) |
| LVEDVi (mL/m2) | 115 | 50 (41–69) |
| LVESVi (mL/m2) | 115 | 27 (17–39) |
| LAVi (mL/m2) | 122 | 32 (25–44) |
| LVEF (%) | 125 | 50 ± 15 |
| Follow-up TTEs | ||
| Number of follow-up TTEs | 126 | |
| 1 | 71 (56%) | |
| 2 | 24 (19%) | |
| 3 | 20 (16%) | |
| 4 | 6 (5%) | |
| 5 | 4 (3%) | |
| 6 | 0 (0%) | |
| 7 | 1 (1%) | |
| Months from baseline to final TTE | 126 | 25 (7–45) |
| Evidence of medication optimisation * | 120 | 60 (50%) |
| Baseline TTE | First Follow-up TTE | Final Follow-up TTE | |
|---|---|---|---|
| Months from baseline TTE | - | 9 (3–23) | 25 (7–45) |
| MR severity | |||
| None | - | 34 (27%) | 34 (27%) |
| Mild | 94 (75%) | 60 (48%) | 60 (48%) |
| Moderate | 30 (24%) | 29 (23%) | 28 (22%) |
| Severe | 2 (2%) | 3 (2%) | 4 (3%) |
| Change in MR severity (vs. baseline) * | |||
| Reduced | - | 45 (36%) | 47 (37%) |
| No change | - | 68 (54%) | 62 (49%) |
| Increased | - | 13 (10%) | 17 (13%) |
| p-Value | p < 0.001 | p < 0.001 | |
| (a) | ||||
| MR Severity: Baseline TTE | MR Severity: Final Follow-up TTE | |||
| None | Mild | Moderate | Severe | |
| None | - | - | - | - |
| Mild | 30 | 47 | 15 | 2 |
| Moderate | 4 | 13 | 13 | 0 |
| Severe | 0 | 0 | 0 | 2 |
| (b) | ||||
| MR Severity: First Follow-up TTE | MR Severity: Final Follow-up TTE | |||
| None | Mild | Moderate | Severe | |
| None | 8 | 6 | 0 | 0 |
| Mild | 5 | 14 | 6 | 1 |
| Moderate | 1 | 6 | 8 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Change in MR Severity from Baseline to Final TTE | |||||
|---|---|---|---|---|---|
| n | Reduction | No change | Increase | p-Value | |
| Age at MI (years) | 126 | 66.7 ± 11.5 | 72.9 ± 11.5 | 75.2 ± 6.6 | 0.003 |
| Sex | 126 | 0.660 | |||
| Female | 16 (36%) | 26 (58%) | 3 (7%) | ||
| Male | 31 (38%) | 36 (44%) | 14 (17%) | ||
| BSA (m2) | 126 | 1.89 ± 0.24 | 1.81 ± 0.22 | 1.81 ± 0.25 | 0.065 |
| Hypertension | 126 | 0.005 | |||
| No | 21 (54%) | 16 (41%) | 2 (5%) | ||
| Yes | 26 (30%) | 46 (53%) | 15 (17%) | ||
| Diabetes mellitus | 126 | 0.173 | |||
| No | 32 (42%) | 35 (46%) | 9 (12%) | ||
| Yes | 15 (30%) | 27 (54%) | 8 (16%) | ||
| Creatinine clearance (mL/min) | 126 | 81 ± 40 | 64 ± 29 | 60 ± 22 | 0.015 |
| Peak troponin (ng/L) | 126 | 892 (86–3979) | 546 (191–2837) | 730 (38–3926) | 0.736 |
| Type of MI | 126 | 0.195 | |||
| NSTEMI | 26 (34%) | 37 (49%) | 13 (17%) | ||
| STEMI | 21 (42%) | 25 (50%) | 4 (8%) | ||
| Evidence of medication optimisation * | 120 | 0.577 | |||
| No | 23 (38%) | 29 (48%) | 8 (13%) | ||
| Yes | 20 (33%) | 31 (52%) | 9 (15%) | ||
| Baseline TTE parameters | |||||
| LVEDVi (mL/m2) | 126 | 51 (42–61) | 50 (38–72) | 55 (41–89) | 0.630 |
| LVESVi (mL/m2) | 126 | 25 (17–35) | 27 (16–42) | 27 (22–61) | 0.317 |
| LAVi (mL/m2) | 126 | 31 (24–41) | 32 (24–44) | 36 (30–48) | 0.344 |
| LVEF (%) | 125 | 52 ± 13 | 50 ± 15 | 45 ± 19 | 0.196 |
| Change in TTE parameters from baseline to final follow-up TTE ** | |||||
| Change in LVEDVi (mL/m2 per year) ** | 110 | −0.8 (−3.4, 2.5) | 2.5 (−3.9, 13.1) | 2.9 (2.1, 15.4) | 0.013 |
| Change in LVESVi (mL/m2 per year) ** | 110 | −1.2 (−3.1, 0.7) | 0.2 (−6.3, 6.2) | 3.5 (0.8, 14.6) | 0.016 |
| Change in LVEF (PP per year) ** | 118 | 0.7 (−2.4, 4.8) | 0.0 (−4.1, 7.4) | −0.3 (−5.9, 3.6) | 0.316 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.; Yuan, M.; Shakeel, I.; Hodson, J.; Radhakrishnan, A.; Brown, S.; May, J.; O’Connor, K.; Zia, N.; Doshi, S.N.; et al. A Longitudinal Study of Mitral Regurgitation Detected after Acute Myocardial Infarction. J. Clin. Med. 2022, 11, 965. https://doi.org/10.3390/jcm11040965
Sharma H, Yuan M, Shakeel I, Hodson J, Radhakrishnan A, Brown S, May J, O’Connor K, Zia N, Doshi SN, et al. A Longitudinal Study of Mitral Regurgitation Detected after Acute Myocardial Infarction. Journal of Clinical Medicine. 2022; 11(4):965. https://doi.org/10.3390/jcm11040965
Chicago/Turabian StyleSharma, Harish, Mengshi Yuan, Iqra Shakeel, James Hodson, Ashwin Radhakrishnan, Samuel Brown, John May, Kieran O’Connor, Nawal Zia, Sagar N. Doshi, and et al. 2022. "A Longitudinal Study of Mitral Regurgitation Detected after Acute Myocardial Infarction" Journal of Clinical Medicine 11, no. 4: 965. https://doi.org/10.3390/jcm11040965
APA StyleSharma, H., Yuan, M., Shakeel, I., Hodson, J., Radhakrishnan, A., Brown, S., May, J., O’Connor, K., Zia, N., Doshi, S. N., Hothi, S. S., Townend, J. N., Myerson, S. G., Ludman, P. F., Steeds, R. P., & Nadir, M. A. (2022). A Longitudinal Study of Mitral Regurgitation Detected after Acute Myocardial Infarction. Journal of Clinical Medicine, 11(4), 965. https://doi.org/10.3390/jcm11040965







