Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry
Abstract
:1. Introduction
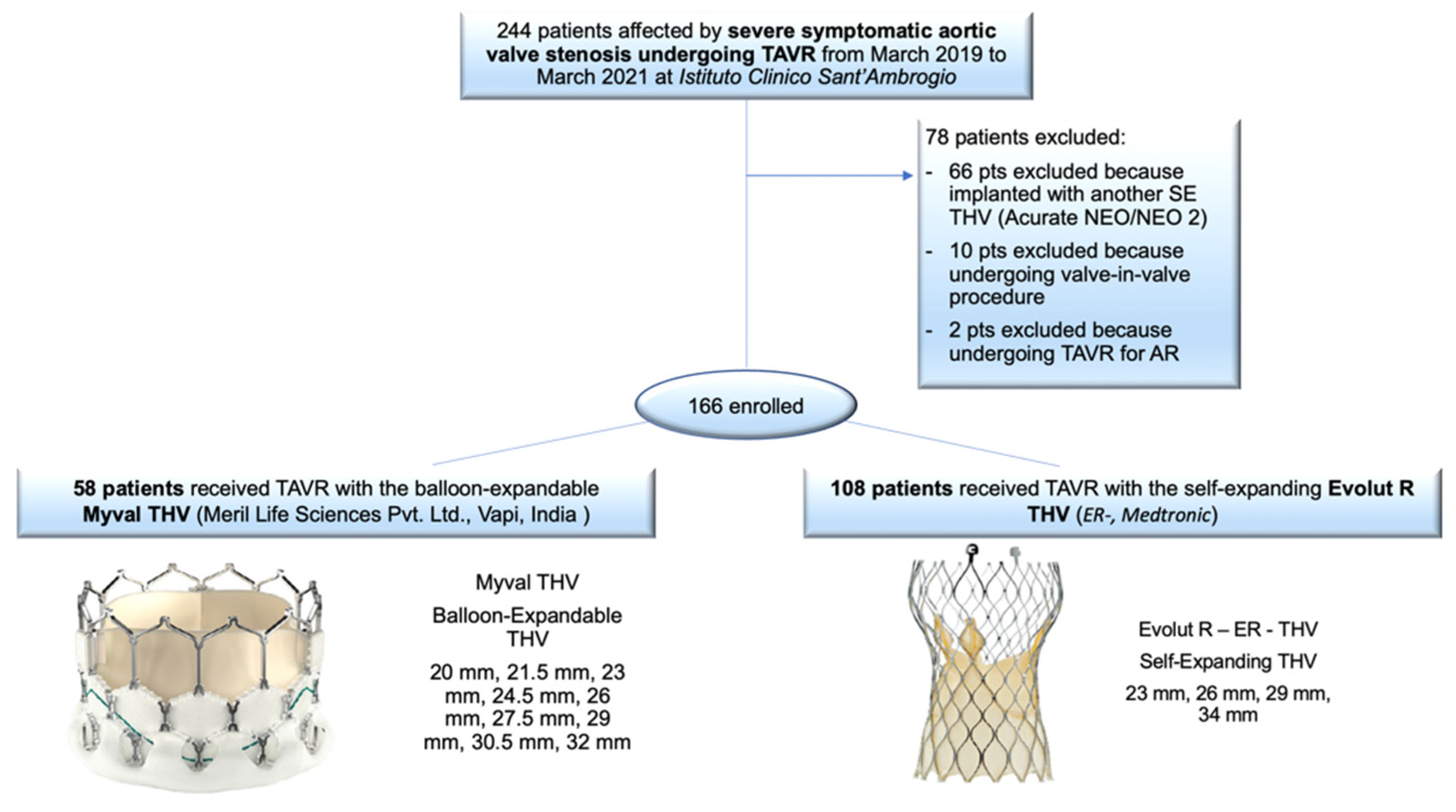
2. Materials and Methods
Statistical Analysis
3. Results
Clinical Outcome at the Six-Month Follow-Up
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 17, 1597–1607. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Craig, D.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis Abstract. N. Engl. J. Med. 2014, 19, 1790–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tölg, R.; Zachow, D.; Guerra, E.; Massberg, S.; Schäfer, U.; et al. Comparison of balloon-expandable vs. self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA—J. Am. Med. Assoc. 2014, 311, 1503–1514. [Google Scholar] [CrossRef]
- Van Belle, E.; Vincent, F.; Labreuche, J.; Auffret, V.; Debry, N.; Lefèvre, T.; Eltchaninoff, H.; Manigold, T.; Gilard, M.; Verhoye, J.P.; et al. Balloon-Expandable Versus Self-Expanding Transcatheter Aortic Valve Replacement: A Propensity-Matched Comparison from the FRANCE-TAVI Registry. Circulation 2020, 141, 243–259. [Google Scholar] [CrossRef]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Lauten, A.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Lanz, J.; Kim, W.-K.; Walther, T.; Burgdorf, C.; Möllmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: A randomised non-inferiority trial. Lancet 2019, 394, 1619–1628. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, R.; Chandra, P.; Goel, P.; Bharadwaj, P.; Joseph, G.; Jose, J.; Mahajan, A.; Mehrotra, S.; Sengottovelu, G.; et al. First-in-human evaluation of a novel balloon-expandable transcatheter heart valve in patients with severe symptomatic native aortic stenosis: The MyVal-1 study. EuroIntervention 2020, 16, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Soliman, O.; Wang, R.; Ono, M.; Hara, H.; Gao, C.; Zeller, E.; Thakkar, A.; Tamburino, C.; Bedogni, F.; et al. Rationale and design of a randomized clinical trial comparing safety and efficacy of myval transcatheter heart valve versus contemporary transcatheter heart valves in patients with severe symptomatic aortic valve stenosis: The Landmark trial. Am. Heart J. 2021, 232, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart diseaseDeveloped by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 1–72. [Google Scholar] [CrossRef]
- Barbanti, M.; Yang, T.H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Sponga, S.; Perron, J.; Dagenais, F.; Mohammadi, S.; Baillot, R.; Doyle, D.; Nalli, C.; Voisine, P. Impact of residual regurgitation after aortic valve replacement. Eur. J. Cardiothorac. Surg. 2012, 42, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, H.C.; Pibarot, P.; Hueter, I.; Gertz, Z.M.; Stewart, W.J.; Kapadia, S.; Tuzcu, E.M.; Babaliaros, V.; Thourani, V.; Szeto, W.Y.; et al. Predictors of Mortality and Outcomes of Therapy in Low-Flow Severe Aortic Stenosis. Circulation 2013, 127, 2316–2326. [Google Scholar] [CrossRef] [Green Version]
- Karyofillis, P.; Kostopoulou, A.; Thomopoulou, S.; Habibi, M.; Livanis, E.; Karavolias, G.; Voudris, V. Conduction abnormalities after transcatheter aortic valve implantation. J. Geriatr. Cardiol. 2018, 15, 105–112. [Google Scholar] [CrossRef]
- García-Gómez, M.; Delgado-Arana, J.R.; Halim, J.; De Marco, F.; Trani, C.; Martin, P.; Won-Keun, K.; Montorfano, M.; den Heijer, P.; Bedogni, F.; et al. Next-generation balloon-expandable Myval transcatheter heart valve in low-risk aortic stenosis patients. Catheter. Cardiovasc. Interv. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Arana, J.R.; Gordillo-Monge, M.X.; Halim, J.; De Marco, F.; Trani, C.; Martin, P.; Infusino, F.; Ancona, M.; Den Heijer, P.; Bedogni, F.; et al. Early clinical and haemodynamic matched comparison of balloon-expandable valves. Heart 2021, 1–8. [Google Scholar] [CrossRef]
- Kawashima, H.; Wang, R.; Mylotte, D.; Jagielak, D.; De Marco, F.; Ielasi, A.; Onuma, Y.; Den Heijer, P.; Terkelsen, C.J.; Wijns, W.; et al. Quantitative Angiographic Assessment of Aortic Regurgitation after Transcatheter Aortic Valve Implantation among Three Balloon-Expandable Valves Quantitative Angiographic Assessment of Aortic Regurgitation after Transcatheter Aortic Valve Implantation among Three Balloon-Expandable Valves. Glob. Heart 2021, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Mangi, A.A.; Popma, J.J.; Khabbaz, K.; Reardon, M.J.; Kleiman, N.S.; Yakubov, S.J.; Watson, D.; Kodali, S.; George, I.; et al. Early Outcomes With the Evolut PRO Repositionable Self-Expanding Transcatheter Aortic Valve with Pericardial Wrap. JACC Cardiovasc. Interv. 2018, 11, 160–168. [Google Scholar] [CrossRef] [PubMed]
| Self-Expanding Valve (Evolut R) n = 108 | Balloon-Expandable Valve (Myval) n = 58 | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 83 ± 5.7 | 82 ± 6 | 0.66 |
| Male gender, n (%) | 66/108 (61) | 29/58 (50) | 0.17 |
| STS-PROM score (%), mean ± SD | 3.9 ± 2.5 | 3.3 ± 1.8 | 0.07 |
| PAD, n (%) | 15/108 (14) | 18/58 (31) | 0.01 * |
| CAD, n (%) | 52/108 (48) | 35/58 (60) | 0.1 |
| Prior MI, n (%) | 19/108 (18) | 8/58 (14) | 0.5 |
| Prior PCI, n (%) | 38/108 (35) | 25/58 (43) | 0.3 |
| Prior CABG, n (%) | 11/108 (10) | 3/58 (5) | 0.4 |
| Atrial fibrillation, n (%) | 41/108 (38) | 18/58 (31) | 0.1 |
| Prior PM/ICD, n (%) | 17/108 (16) | 3/58 (5) | 0.04 * |
| Prior stroke, n (%) | 11/108 (10) | 3/58 (5) | 0.3 |
| Renal insufficiency (GFR < 60 mL/min), n (%) | 53/108 (49) | 28/58 (48) | 0.9 |
| Pulmonary hypertension (PAPs > 60 mmHg), n (%) | 7/108 (6.5) | 4/58 (7) | 0.9 |
| COPD, n (%) | 19/108 (18) | 5/58 (9) | 0.08 |
| Cardiovascular risk factors | |||
| - Diabetes mellitus, n (%) | 33/108 (31) | 12/58 (21) | 0.1 |
| - Arterial hypertension, n (%) | 87/108 (81) | 52/58 (90) | 0.1 |
| - Hypercholesterolemia, n (%) | 54/108 (50) | 35/58 (60) | 0.2 |
| - Former smoker, n (%) | 29/108 (27) | 18/58 (31) | 0.7 |
| - Obesity (BMI kg/m2), n (%) | 18/108 (17) | 4/58 (7) | 0.07 |
| New York Heart Association class, n (%) | |||
| I | 7/108 (6.5) | 1/58 (1.7) | 0.2 |
| II | 53/108 (49) | 28/58 (48) | 0.9 |
| III | 42/108 (39) | 24/58 (41) | 0.8 |
| IV | 6/108 (6) | 5/58 (9) | 0.4 |
| Self-Expanding Valve (Evolut R) n = 108 | Balloon-Expandable Valve (Myval) n = 58 | p-Value | |
|---|---|---|---|
| Transthoracic echocardiography | |||
| Aortic Valve Area (cm2), mean ± SD | 0.75 ± 0.15 | 0.7 ± 0.18 | 0.2 |
| Aortic Valve Area indexed (cm2/m2), mean ± SD | 0.42 ± 0.1 | 0.41 ± 0.1 | 0.4 |
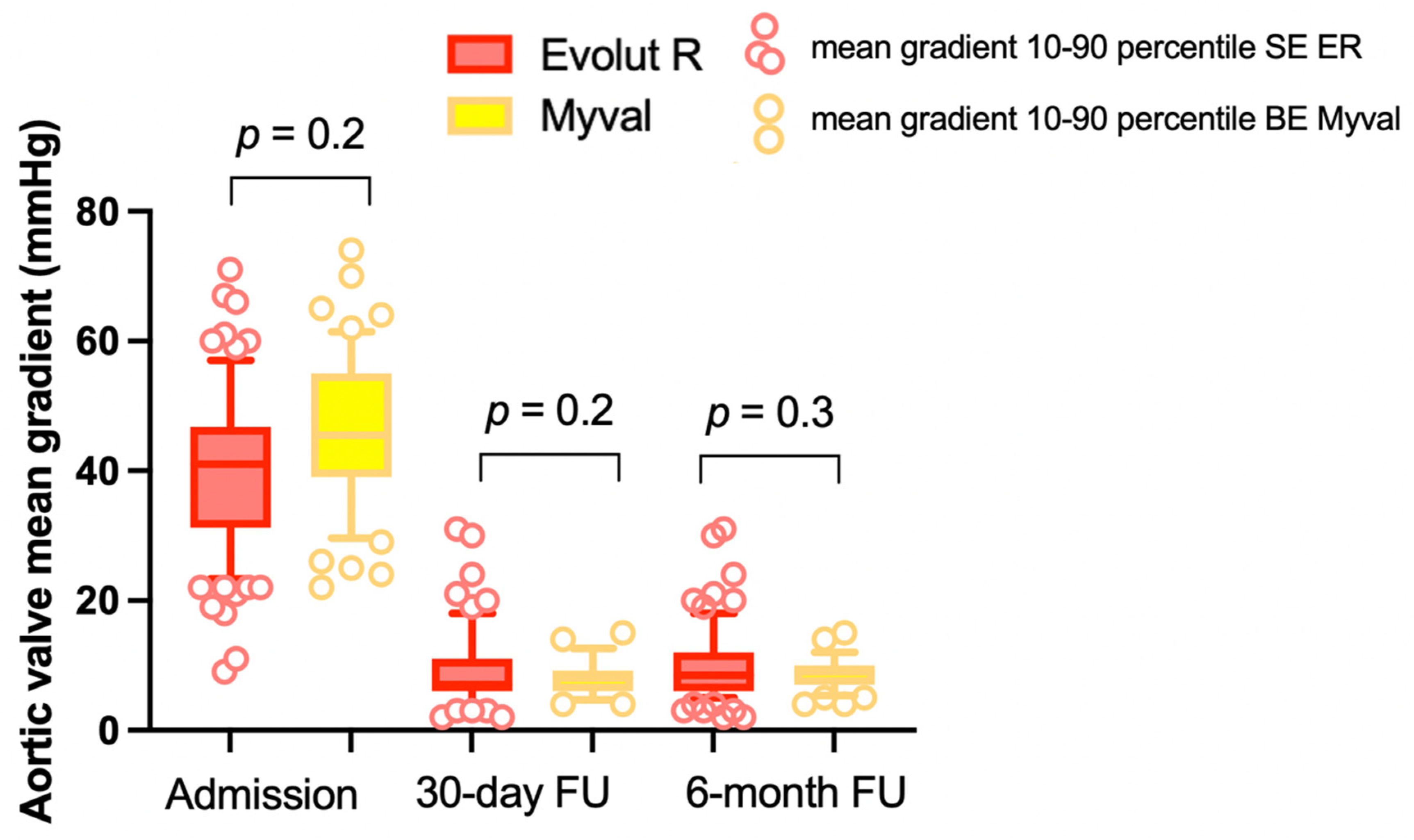
| Aortic Valve mean gradient (mmHg), mean ± SD | 41 ± 13 | 43.3 ± 13.8 | 0.2 |
| LVEF (%), mean ± SD | 52 ± 11 | 55.7 ± 10.2 | 0.07 |
| Aortic regurgitation ≥moderate, n (%) | 9/108 (8) | 8/58 (14) | 0.3 |
| Multislice Computed Tomography | |||
| Aortic annulus diameter (mm), mean ± SD | 23.9 ± 3.1 | 23.8 ± 2.3 | 0.9 |
| Aortic annulus area (mm2), mean ± SD | 460.8 ± 105.7 | 466.9 ± 108.9 | 0.74 |
| Aortic annulus average perimeter (mm), mean ± SD | 77.3 ± 9.4 | 74.7 ± 7.4 | 0.3 |
| Degree of aortic leaflets calcification (≥moderate), n (%) | 56/108 (52) | 32/58 (55) | 0.7 |
| Degree of annulus calcification (≥moderate), n (%) | 16/108 (15) | 9/58 (16) | 0.9 |
| Degree of LVOT calcification (≥moderate), n (%) | 7/108 (6.5) | 4/58 (7) | 0.9 |
| Height of coronary artery (mm), mean ± SD | |||
| Left main | 14 ± 3 | 13.7 ± 3.3 | 0.9 |
| Right | 15 ± 3.9 | 16.6 ± 3.5 | 0.2 |
| Self-Expanding Valve (Evolut R) n = 108 | Balloon-Expandable Valve (Myval) n = 58 | p-Value | |
|---|---|---|---|
| Transfemoral TAVR, n (%) | 107/108 (99) | 56/58 (97) | 0.27 |
| Procedure time, min (mean ± SD) | 113.2 ± 38 | 112.15 ± 38 | 0.6 |
| Fluoroscopy time, min (mean ± SD) | 32.2 ± 13.2 | 26.3 ± 9.8 | 0.005 * |
| Total contrast volume, mL | 180 ± 61 | 179 ± 72 | 0.9 |
| General anesthesia, n (%) | 0/108 (0) | 1/58 (1.7) | 0.2 |
| Pre-dilation, n (%) | 31/108 (29) | 54/58 (93) | <0.001 * |
| Cerebral protection device, n (%) | 4/108 (3.7) | 3/58 (5.2) | 0.6 |
| Implantation of multiple valves, n (%) | 1/108 (0.9) | 0/58 (0) | 0.5 |
| Need for SAVR, n (%) | 0/108 (0) | 0/58 (0) | >0.9 |
| Post-dilatation, n (%) | 27/108 (25) | 2/58 (3.4) | 0.005 * |
| Valve size (mm) | |||
| 21.5 | 5/58 (8.6) | ||
| 23 | 10/108 (9) | 11/58 (19) | 0.14 |
| 23.5 | |||
| 24.5 | 13/58 (22.5) | ||
| 26 | 22/108 (20.3) | 14/58 (24) | 0.7 |
| 27.5 | 4/58 (7) | ||
| 29 | 30/108 (27.7) | 7/58 (12.1) | 0.03 * |
| 30.5 | 2/58 (3.4) | ||
| 32 | 2/58 (3.4) | 0.28 | |
| 34 | 46/108 (43) |
| Self-Expanding Valve (Evolut R) n = 108 | Balloon-Expandable Valve (Myval) n = 58 | p-Value | |
|---|---|---|---|
| Procedural death, n (%) | 0/108 (0) | 0/58 (0) | >0.9 |
| Valve embolization, n (%) | 1/108 (0.9) | 0/58 (0) | 0.5 |
| Coronary artery obstruction, n (%) | 3/108 (2.8) | 1/58 (1.7) | 0.6 |
| Periprocedural MI, n (%) | 2/108 (1.8) | 1/58 (1.7) | 0.9 |
| Cardiac tamponade, n (%) | 0/108 (0) | 0/58 (0) | >0.9 |
| Annular rupture, n (%) | 0/108 (0) | 0/58 (0) | >0.9 |
| LV perforation, n (%) | 0/108 (0) | 0/58 (0) | >0.9 |
| Ventricular arrhythmias, n (%) | 3/108 (2.8) | 1/58 (1.7) | 0.6 |
| Final PVL | |||
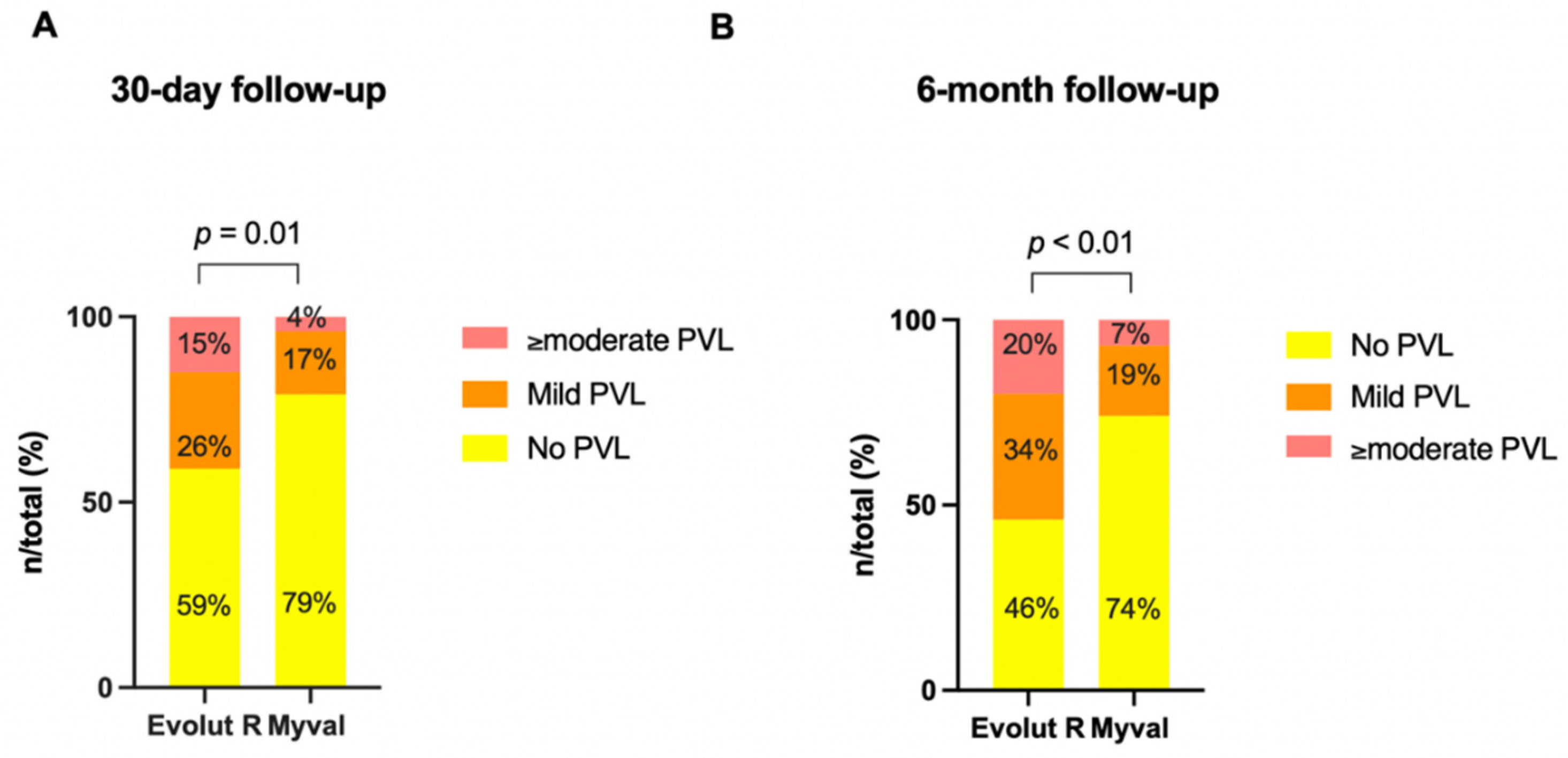
| None/trace | 64/108 (59.2) | 46/58 (79.3) | 0.01 * |
| Mild | 28/108 (26) | 10/58 (17.3) | 0.2 |
| ≥Moderate | 16/108 (14.8) | 2/58 (3.4) | 0.03 * |
| Technical success (VARC3), n (%) | 105/108 (97.2) | 57/58 (98.3) | >0.9 |
| Self-Expanding Valve (Evolut R) n = 108 | Balloon-Expandable Valve (Myval) n = 58 | p-Value | |
|---|---|---|---|
| Device success (VARC 3) | |||
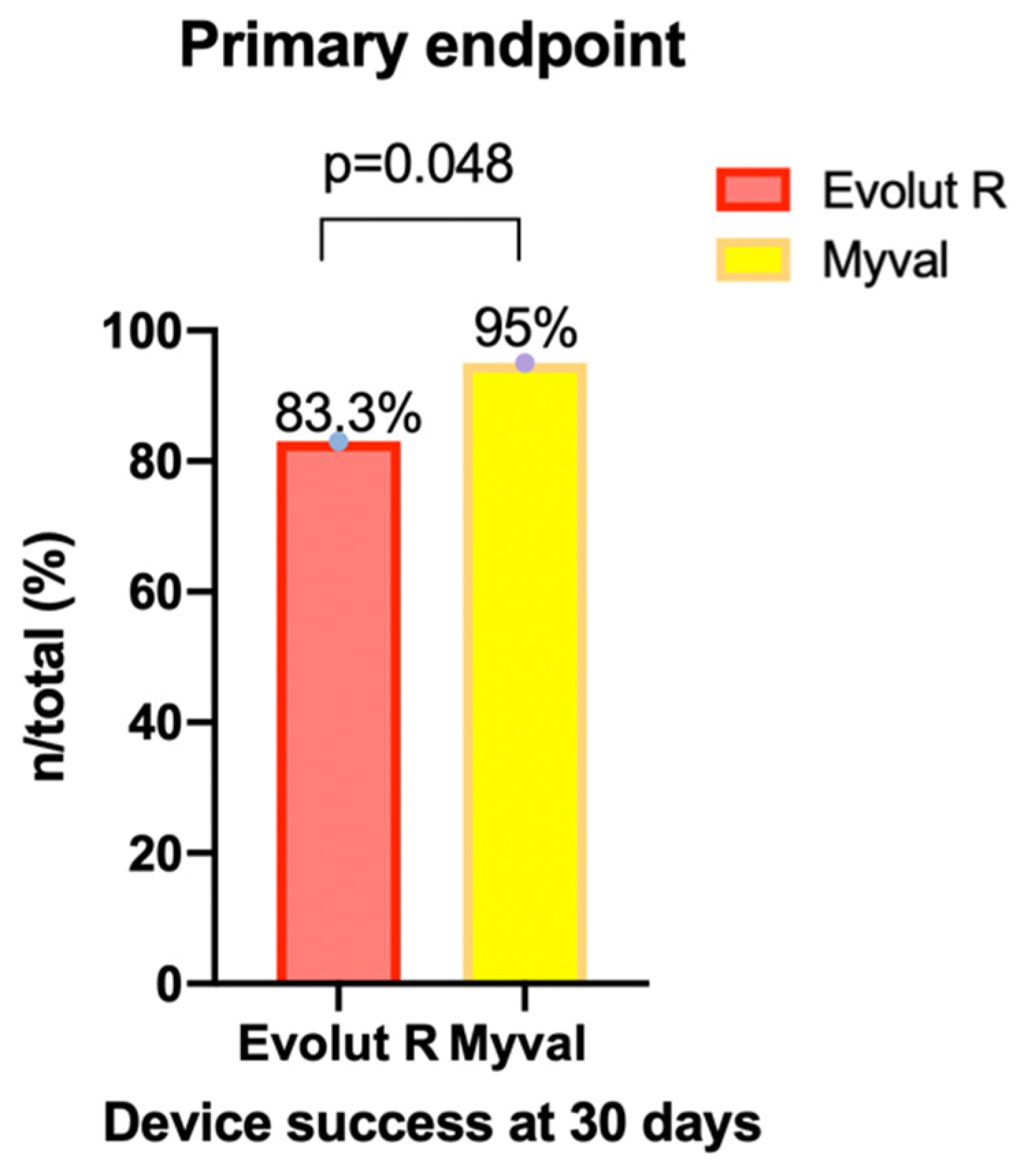
| Primary endpoint, n (%) | 90/108 (83.3) | 55/58 (94.8) | 0.048 * |
| Early safety (VARC 3), n (%) | 71/108 (66) | 47/58 (81) | 0.048 * |
| All-cause mortality, n (%) | 3/108 (2.8) | 1/58 (1.7) | >0.9 |
| Cardiovascular mortality, n (%) | 2/108 (1.8) | 0/58 (0) | 0.5 |
| Cardiovascular hospitalization, n (%) | 5/108 (4.6) | 0/58 (0) | 0.2 |
| Neurologic events, n (%) | 2/108 (1.8) | 2/58 (3.4) | 0.6 |
| Disabling stroke, n (%) | 0/108 (0) | 1/58 (1.7) | 0.3 |
| Non disabling stroke, n (%) | 1/108 (0.9) | 1/58 (1.7) | >0.9 |
| Transient ischemic attack, n (%) | 1/108 (0.9) | 0/58 (0) | >0.9 |
| Bleeding (VARC 3), n (%) | 19/108 (17.6) | 12/58 (20.7) | 0.7 |
| Type 1, n (%) | 10/108 (9.3) | 9/58 (15.5) | 0.3 |
| Type 2, n (%) | 7/108 (6.5) | 3/58 (5.2) | >0.9 |
| Type 3, n (%) | 1/108 (0.9) | 0/58 (0) | >0.9 |
| Type 4, n (%) | 1/108 (0.9) | 0/58(0) | >0.9 |
| Vascular complications (VARC 3), n (%) | 20/108 (18.5) | 6/58 (10.3) | 0.2 |
| Major, n (%) | 1/108 (0.9) | 1/58 (1.7) | >0.9 |
| Minor, n (%) | 19/108 (17.6) | 5/58 (8.6) | 0.1 |
| Access-related non-vascular complications, n (%) | 1/108 (0.9) | 0/58 (0) | >0.9 |
| Conduction disturbances, n (%) | 48/108 (44.4) | 16/58 (27.6) | 0.04 * |
| 1st degree AV block, n (%) | 6/108 (5.6) | 1/58 (1.7) | 0.42 |
| 2nd degree AV block, n (%) | 4/108 (3.7) | 2/58 (3.4) | >0.9 |
| 3rd degree AV block, n (%) | 16/108 (14.8) | 3/58 (5.2) | 0.07 |
| LBBB, n (%) | 13/108 (12) | 10/58 (17) | 0.4 |
| Ventricular arrhythmias, n (%) | 3/108 (2.8) | 0/58 (0) | 0.5 |
| Atrial fibrillation or flutter, n (%) | 2/108 (1.8) | 0/58 (0) | 0.5 |
| Permanent Pacemaker implantation, n (%) | 22/91 (24.2) | 6/55 (11) | 0.053 |
| Acute Kidney Injury, n (%) | 2/108 (1.8) | 1/58 (1.7) | >0.9 |
| Prosthetic valve thrombosis, n (%) | 0/108 (0) | 0/58 (0) | >0.9 |
| Prosthetic valve endocarditis, n (%) | 0/108 (0) | 0/58 (0) | >0.9 |
| ≥moderate paravalvular leak, n (%) | 16/108 (14.8) | 2/58 (3.4) | 0.03 * |
| Aortic Valve mean gradient (mmHg), mean ± SD | 9.3 ± 4.9 | 8 ± 2.7 | 0.2 |
| Self-Expanding Valve (Evolut R) n = 106/108 | Balloon-Expandable Valve (Myval) n = 58/58 | p-Value | |
|---|---|---|---|
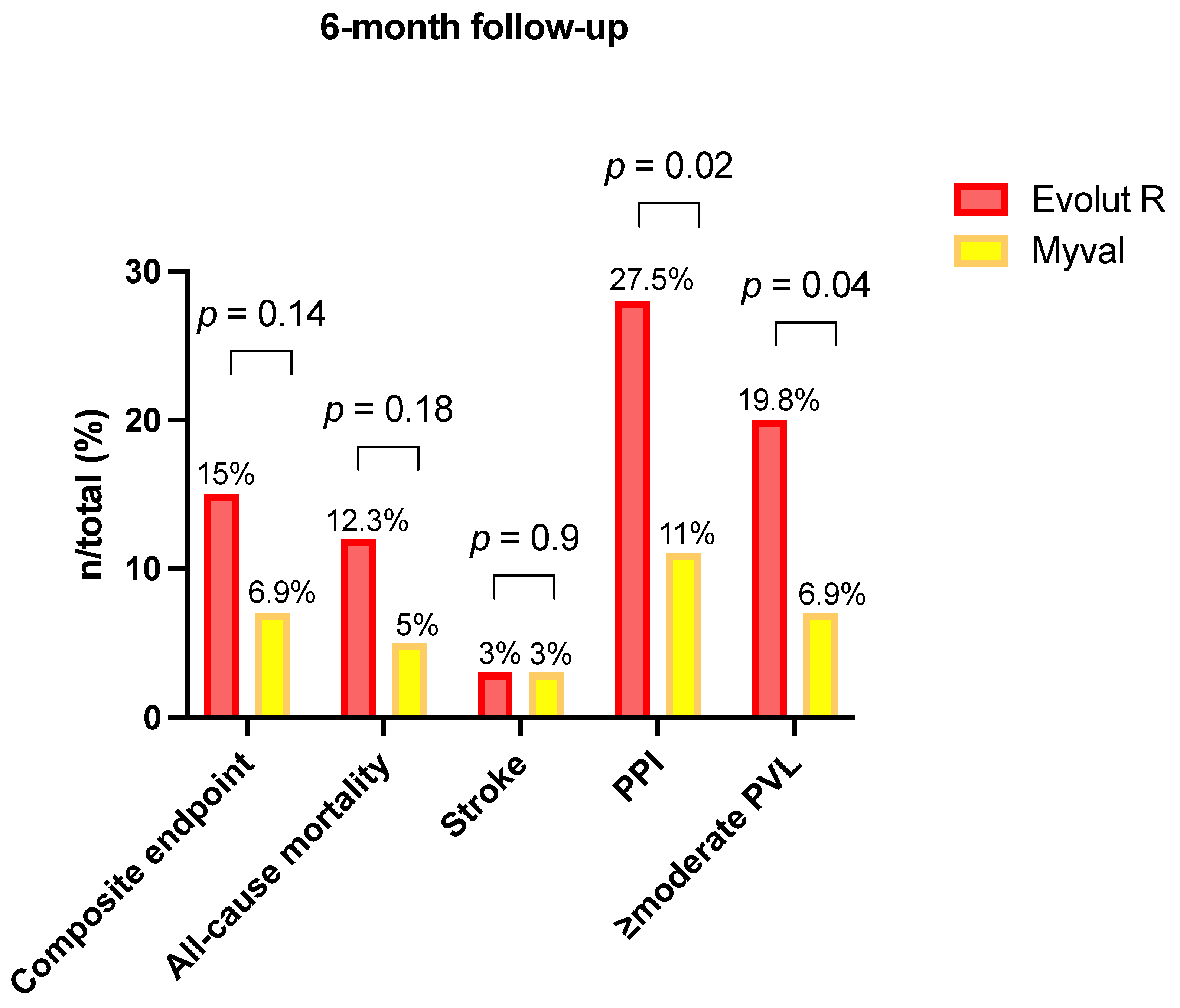
| Primary endpoint, n (%) | 16/106 (15.1) | 4/58 (6.9) | 0.14 |
| All-cause mortality, n (%) | 13/106 (12.3) | 3/58 (5.2) | 0.18 |
| Cardiovascular mortality, n (%) | 10/106 (9.4) | 2/58 (3.4) | 0.22 |
| Cardiovascular hospitalization, n (%) | 8/106 (7.5) | 3/55 (5.2) | 0.75 |
| Neurologic events, n (%) | 3/106 (2.8) | 2/58 (3.4) | >0.99 |
| Disabling stroke, n (%) | 1/106 (0) | 1/58 (1.7) | >0.99 |
| Non disabling stroke, n (%) | 1/106 (0.9) | 1/58 (1.7) | >0.99 |
| Transient ischemic attack, n (%) | 1/106 (0.9) | 0/58 (0) | >0.99 |
| Permanent Pacemaker implantation, n (%) | 25/91 (27.5) | 6/55 (11) | 0.02 * |
| Prosthetic valve thrombosis, n (%) | 0/106 (0) | 0/58 (0) | >0.99 |
| Prosthetic valve endocarditis, n (%) | 0/106 (0) | 1/58 (1.7) | >0.99 |
| Paravalvular leak | 57/106 (53.8) | 15/58 (26) | <0.001 * |
| None/trace, n (%) | 49/106 (46.2) | 43/58 (74) | <0.001 * |
| Mild, n (%) | 36/106 (34) | 11/58 (19) | 0.0138 * |
| ≥moderate, n (%) | 21/106 (19.8) | 4/58 (7) | 0.0396* |
| Aortic Valve mean gradient (mmHg), mean ± SD | 10 ± 5 | 9.2 ± 3 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barki, M.; Ielasi, A.; Buono, A.; Maliandi, G.; Pellicano, M.; Bande, M.; Casilli, F.; Messina, F.; Uccello, G.; Briguglia, D.; et al. Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry. J. Clin. Med. 2022, 11, 959. https://doi.org/10.3390/jcm11040959
Barki M, Ielasi A, Buono A, Maliandi G, Pellicano M, Bande M, Casilli F, Messina F, Uccello G, Briguglia D, et al. Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry. Journal of Clinical Medicine. 2022; 11(4):959. https://doi.org/10.3390/jcm11040959
Chicago/Turabian StyleBarki, Monica, Alfonso Ielasi, Andrea Buono, Gabriele Maliandi, Mariano Pellicano, Marta Bande, Francesco Casilli, Francesca Messina, Giuseppe Uccello, Daniele Briguglia, and et al. 2022. "Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry" Journal of Clinical Medicine 11, no. 4: 959. https://doi.org/10.3390/jcm11040959
APA StyleBarki, M., Ielasi, A., Buono, A., Maliandi, G., Pellicano, M., Bande, M., Casilli, F., Messina, F., Uccello, G., Briguglia, D., Medda, M., Tespili, M., & Donatelli, F. (2022). Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry. Journal of Clinical Medicine, 11(4), 959. https://doi.org/10.3390/jcm11040959






