Efficacy of Biologics on Refractory Eosinophilic Otitis Media Associated with Bronchial Asthma or Severe Uncontrolled CRSwNP
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
- Male or female aged 18–75 years;
- Confirmed diagnosis of refractory eosinophilic otitis media (EOM);
- Ongoing treatment with biologics for SEA and/or severe uncontrolled CRSwNP;
- Willingness and ability to provide written informed consent;
- Observational follow-up period of at least 6 months.
- History of genetic, congenital or acquired immunodeficiency;
- Autoimmune diseases;
- Current malignancy;
- Previous radiotherapy for head and neck cancer.
- SNOT-22. We used the validated Italian version of SNOT-22. Possible total score range: 0–110. A SNOT-22 score < 20 was suggestive of mild symptoms. During follow-up time, the minimal clinically important difference (MCID) in SNOT-22 scores was assumed for an 8.9-point increase as reported in previous studies [19].
- Nasal endoscopy (Nasal Polyp Score). Each side of the nasal cavity was separately evaluated and scored in a range from 0–4 (0 = no polyps; 1 = small polyps in the middle meatus not reaching below the inferior border of the middle turbinate; 2 = polyps reaching below the lower border of the middle turbinate; 3 = large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle turbinate; and 4 = large polyps causing complete obstruction of the inferior nasal cavity). The sum of the scores for both nasal cavities was recorded as the NPS value [20].
- VAS for symptoms. Intensity of symptoms was measured on a horizontal 10 cm line. A mean score for each symptom analyzed was obtained using the average value of the scores assigned to all patients for the same symptom [20].
- Cellular infiltration of nasal mucosa at nasal cytology. Nasal leukocyte counts were performed on nasal scraped tissue, obtained from the inferior turbinate bilaterally. Scraping was performed by rhinoprobe (Farmark s.n.c, Milan, Italy). The sample was gently spread on glass slides and immediately fixed in 95% ethyl alcohol and stained with May–Grunwald–Giemsa. The slides were examined under oil immersion by light microscopy at a magnification of 400×. The slides were examined under oil immersion by light microscopy at a magnification of 1000×. Nasal tissue eosinophil infiltration was measured as “eosinophil count per high power field (Ec-hpf)” and reported as the mean of at least 10 high powered fields observed at nasal cytology [21].
- Sniffin’Sticks identification test. It consists of 16 blue pens with black numbers. Each pen is presented only once and an interval of at least 30 s is observed between each presentation to avoid olfactory desensitization. For each odorant pen, the subject must make a forced choice from a list of 4 written proposals. The identification score corresponds to the number of correct responses out of 16 total score. The results were associated with smell function as follows: 0–5 anosmia, 6–11 hyposmia, and 12–16 normal smell [22].
- Otitis severity score (see Table 1), a scoring system used to evaluate the severity of EOM (Iino et al.). The degree of severity of EOM was evaluated according to five items: (1) quantity of middle ear effusion (MEE) or otorrhea; (2) condition of the middle ear mucosa; (3) frequency of intratympanic injection of triamcinolone acetonide; (4) frequency of administration of systemic corticosteroids; and (5) frequency of administration of antibiotics. These items were scored on a scale from 0 to 2 [23].
- Pure tone average (PTA) at audiometry, average of hearing threshold levels at a set of specified frequencies: typically, 500, 1000, 2000 and 4000 Hz. Patients’ PTA was measured at pure tone audiometry test conducted before biological therapy and after 6 month of follow-up time.
- Chronic otitis media outcome test (COMOT-15). We used the validated Italian version of COMOT-15. The COMOT-15 consists of 3 subscales, categorized as ear symptoms (questions 1–6), hearing function (questions 7–9) and mental health (questions 10–13), which form the overall score. In addition to questions from 1 to 13, the COMOT-15 contains two other questions: a general evaluation of the impact of chronic otitis media on QoL (question 14) and a question on the frequency of ENT visits as a result of chronic otitis media in the previous 6 months (question 15). Possible total score range 0–75 [24].
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Population
3.2. Changes in Specific SinoNasal Outcomes, Need of OCS and Antibiotics, and Biomarkers
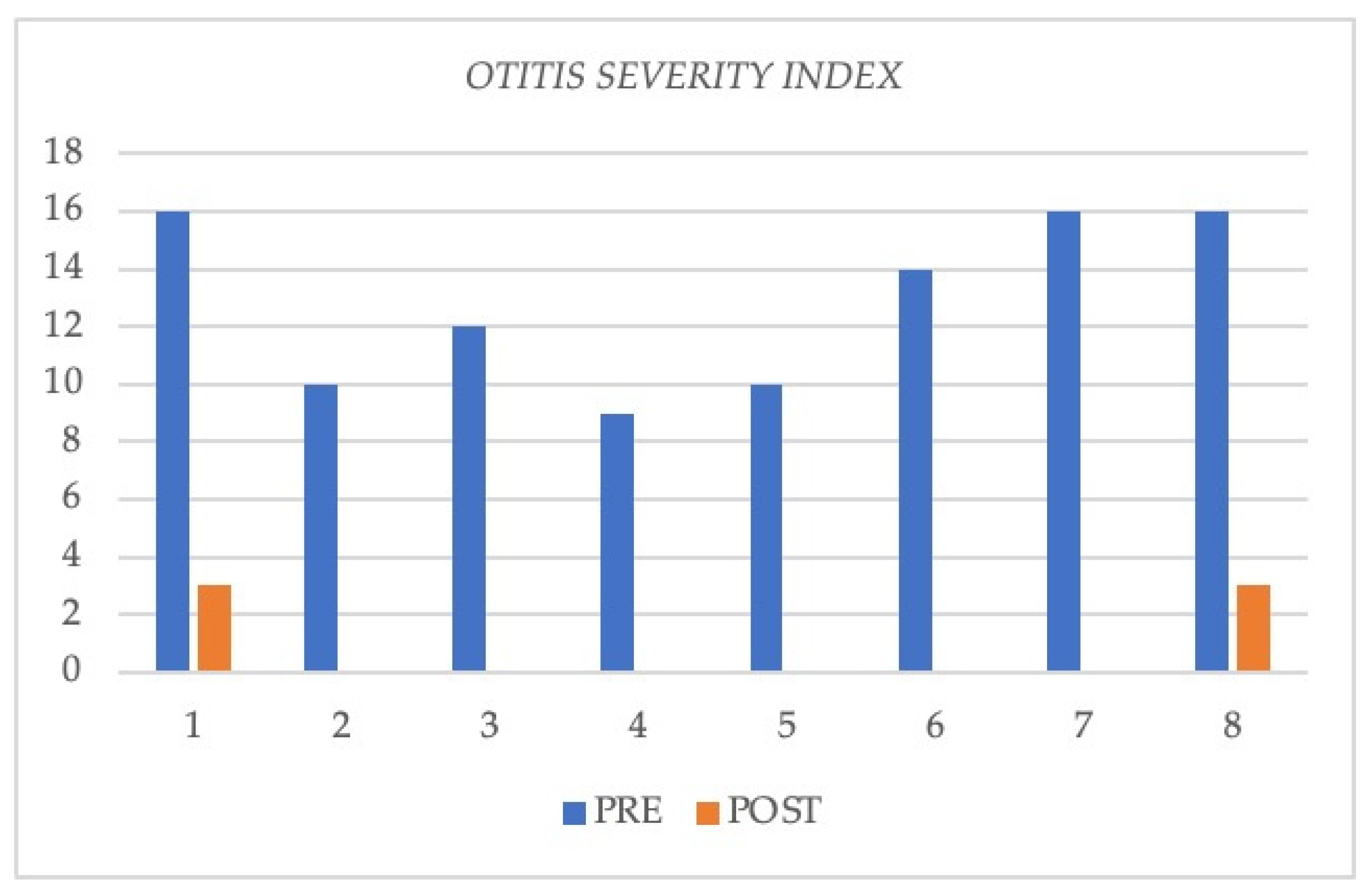
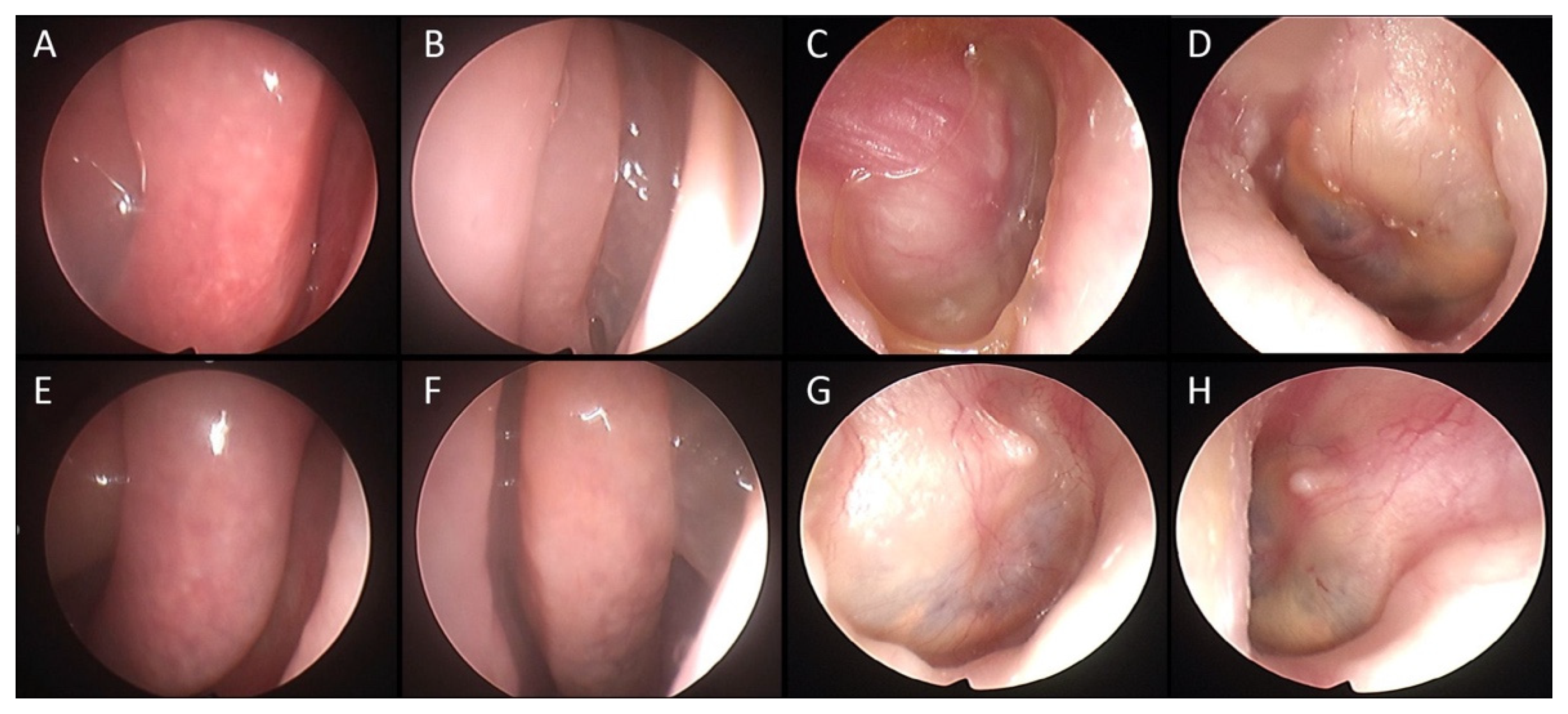
3.3. Changes in Severity of Otitis during Treatments with Biologics
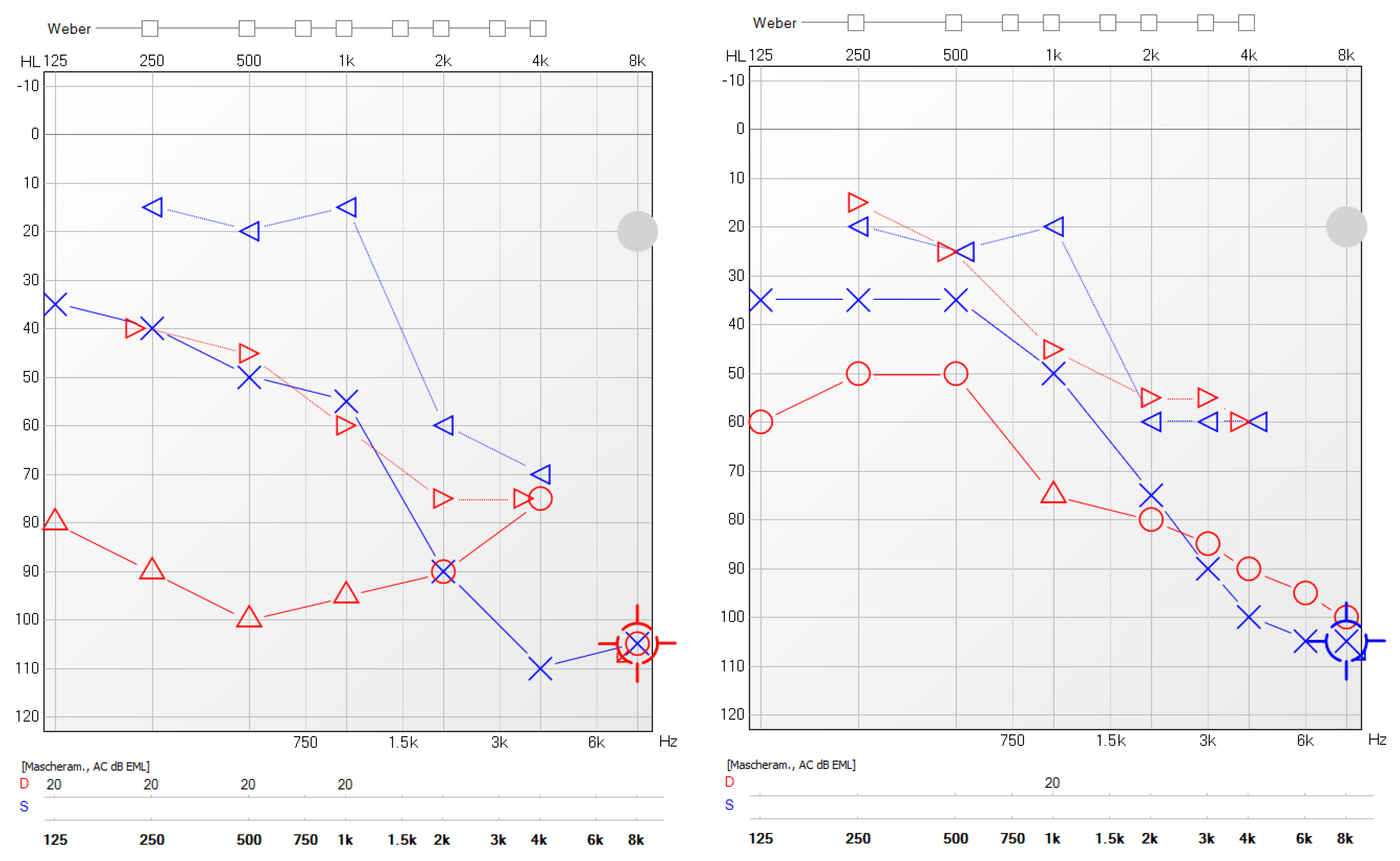
3.4. Efficacy of Biologics on Hearing Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iino, Y.; Tomioka-Matsutani, S.; Matsubara, A.; Nakagawa, T.; Nonaka, M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx 2011, 38, 456–461. [Google Scholar] [CrossRef]
- Van der Lans, R.J.L.; Spronsen, E.; Fokkens, W.J.; Reitsma, S. Complete Remission of Severe Eosinophilic Otitis Media With Dupilumab: A Case Report. Laryngoscope 2021, 131, 2649–2651. [Google Scholar] [CrossRef]
- De Corso, E.; Lucidi, D.; Battista, M.; Romanello, M.; De Vita, C.; Baroni, S.; Autilio, C.; Galli, J.; Paludetti, G. Prognostic value of nasal cytology and clinical factors in nasal polyps development in patients at risk: Can the beginning predict the end? Int. Forum Allergy Rhinol. 2017, 7, 861–867. [Google Scholar] [CrossRef] [PubMed]
- De Corso, E.; Baroni, S.; Battista, M.; Romanello, M.; Penitente, R.; Di Nardo, W.; Passali, G.C.; Sergi, B.; Fetoni, A.R.; Bussu, F.; et al. Nasal fluid release of eotaxin-3 and eotaxin-2 in persistent sinonasal eosinophilic inflammation. Int. Forum Allergy Rhinol. 2014, 4, 617–624. [Google Scholar] [CrossRef] [PubMed]
- De Corso, E.; Baroni, S.; Romitelli, F.; Luca, L.; Di Nardo, W.; Passali, G.C.; Paludetti, G. Nasal lavage CCL24 levels correlate with eosinophils trafficking and symptoms in chronic sino-nasal eosinophilic inflammation. Rhinol. J. 2011, 49, 174–179. [Google Scholar] [CrossRef] [PubMed]
- De Corso, E.; Baroni, S.; Lucidi, D.; Battista, M.; Romanello, M.; Autilio, C.; Morelli, R.; Di Nardo, W.; Passali, G.C.; Sergi, B.; et al. Nasal lavage levels of granulocyte-macrophage colony-stimulating factor and chronic nasal hypereosinophilia. Int. Forum Allergy Rhinol. 2015, 5, 557–562. [Google Scholar] [CrossRef]
- De Corso, E.; Cantone, E.; Galli, J.; Seccia, V.; Lucidi, D.; Di Cesare, T.; Ottaviano, G.; Sergi, B.; Paludetti, G.; Fetoni, A.R. Otitis media in children: Which phenotypes are most linked to allergy? A systematic review. Pediatr. Allergy Immunol. 2021, 32, 524–534. [Google Scholar] [CrossRef]
- De Corso, E.; Lucidi, D.; Cantone, E.; Ottaviano, G.; Di Cesare, T.; Seccia, V.; Paludetti, G.; Galli, J. Clinical Evidence and Biomarkers Linking Allergy and Acute or Chronic Rhinosinusitis in Children: A Systematic Review. Curr. Allergy Asthma Rep. 2020, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nonaka, M.; Yamamura, Y.; Tagaya, E.; Pawankar, R.; Yoshihara, T. Improvement of Eosinophilic Otitis Media by Optimized Asthma Treatment. Allergy. Asthma Immunol. Res. 2013, 5, 175. [Google Scholar] [CrossRef][Green Version]
- De Corso, E.; Anzivino, R.; Galli, J.; Baroni, S.; Di Nardo, W.; De Vita, C.; Salvati, A.; Autilio, C.; Settimi, S.; Mele, D.; et al. Antileukotrienes improve naso-ocular symptoms and biomarkers in patients with NARES and asthma. Laryngoscope 2019, 129, 551–557. [Google Scholar] [CrossRef]
- De Corso, E.; Settimi, S.; Tricarico, L.; Mele, D.A.; Mastrapasqua, R.F.; Di Cesare, T.; Salvati, A.; Trozzi, L.; De Vita, C.; Romanello, M.; et al. Predictors of Disease Control After Endoscopic Sinus Surgery Plus Long-Term Local Corticosteroids in CRSwNP. Am. J. Rhinol. Allergy 2021, 35, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Sekine, Y.; Yoshida, S.; Kikuchi, S. Dupilumab therapy for patients with refractory eosinophilic otitis media associated with bronchial asthma. Auris Nasus Larynx 2021, 48, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Hayashi, M.; Kato, H.; Nakagawa, M.; Imaizumi, K.; Okazawa, M. IL13 May Play an Important Role in Developing Eosinophilic Chronic Rhinosinusitis and Eosinophilic Otitis Media with Severe Asthma. Int. J. Mol. Sci. 2021, 22, 11209. [Google Scholar] [CrossRef]
- Tomioka, S.; Kobayashi, T.; Takasaka, T. Intractable otitis media in patients with bronchial asthma (eosinophilic otitis media). In Cholesteatoma and Mastoid Surgery; Sanna, M., Ed.; CIC Edizioni Internazionali: Rome, Italy, 1997; pp. 851–853. [Google Scholar]
- Global Initiative for Asthma. Global strategy for Asthma Management and Prevention. 2020. Available online: www.ginasthma.org (accessed on 26 December 2021).
- Global Initiative for Asthma. Global strategy for Asthma Management and Prevention. 2021. Available online: www.ginasthma.org (accessed on 26 December 2021).
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinol. J. 2012, 50, 87. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinol. J. 2020, 20, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Russo, F.; Mozzanica, F.; Preti, A.; Bandi, F.; Costantino, C.; Gera, R.; Ottaviani, F.; Castelnuovo, P. Prognostic value of the Sinonasal Outcome Test 22 (SNOT-22) in chronic rhinosinusitis. Acta Otorhinolaryngol. Ital. 2020, 40, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.; Hellings, P.; Alobid, I.; Bachert, C.; Fokkens, W.; Wijk, R.G.; Gevaert, P.; Guilemany, J.; Kalogjera, L.; Lund, V.; et al. Diagnostic tools in Rhinology EAACI position paper. Clin. Transl. Allergy 2011, 1, 2. [Google Scholar] [CrossRef]
- Singhvi, P.; Baisakhiya, N.; Singh, G. Study the Role of Nasal Scrap Cytology in Allergic Rhinitis Patients in Rural Population. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 2057–2064. [Google Scholar] [CrossRef]
- Passali, G.C.; Passali, D.; Cingi, C.; Ciprandi, G. Smell impairment in patients with chronic rhinosinusitis: A real-life study. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 773–777. [Google Scholar] [CrossRef]
- Iino, Y.; Hara, M.; Hasegawa, M.; Matsuzawa, S.; Shinnabe, A.; Kanazawa, H.; Yoshida, N. Clinical Efficacy of Anti-IgE Therapy for Eosinophilic Otitis Media. Otol. Neurotol. 2012, 33, 1218–1224. [Google Scholar] [CrossRef]
- Cavaliere, M.; Capriglione, P.; Cavaliere, F.; De Corso, E.; Zanoletti, E.; Motta, G.; Iengo, M.; Cantone, E. Cross-cultural adaptation and Italian validation of chronic otitis media outcome test 15 (COMOT-15). Acta Otorhinolaryngol. Ital. 2021, 41, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, G.; Ciprandi, G.; Canonica, G.W. The nose-lung interaction in allergic rhinitis and asthma: United airways disease. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Ogino, E.; Yasuba, H. Cycling therapy with benralizumab and dupilumab for severe eosinophilic asthma with eosinophilic chronic rhinosinusitis and eosinophilic otitis media. Allergol. Int. 2021, 70, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Kato, H.; Yoshioka, S.; Okazawa, M. Rapid and remarkable effectiveness of benralizumab for treating severe bronchial asthma with intractable eosinophilic rhinosinusitis and eosinophilic otitis media: A case report. Respir. Med. Case Rep. 2021, 32, 101336. [Google Scholar] [CrossRef] [PubMed]
- De Corso, E.; Bellocchi, G.; De Benedetto, M.; Lombardo, N.; Macchi, A.; Malvezzi, L.; Motta, G.; Pagella, F.; Vicini, C.; Passali, D. Biologics for severe uncontrolled chronic rhinosinusitis with nasal polyps: A change management approach. Consensus of the Joint Committee of Italian Society of Otorhinolaryngology on biologics in rhinology. Acta Otorhinolaryngol. Ital. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Nonaka, M.; Yamamura, Y.; Pawankar, R.; Tagaya, E. Optimal control of asthma improved eosinophilic otitis media. Asia Pac. Allergy 2018, 8, e5. [Google Scholar] [CrossRef] [PubMed]
- Kagoshima, H.; Hori, R.; Kojima, T.; Okanoue, Y.; Taguchi, A.; Yamamoto, H.; Hasebe, K.; Shoji, K. Successful treatment of eosinophilic chronic rhinosinusitis and eosinophilic otitis media using the anti-IL-5 receptor monoclonal antibody benralizumab: A case report. Respir. Med. Case Rep. 2020, 30, 101135. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.E.; Kang, Y.S.; Cho, Y.; Park, M.K. Eosinophilic Otitis Media Treated with Anti-IgE Monoclonal Antibodies and A Bone Conduction Implant. J. Int. Adv. Otol. 2018, 14, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Breslin, N.K.; Heindel, N.H.; Haberman, R.S. Role of Interleukin 5 Inhibition in the Treatment of Eosinophilic Otitis Media. OTO Open 2021, 5, 2473974X2199144. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Takahashi, E.; Ida, S.; Kikuchi, S. Clinical efficacy of anti-IL-5 monoclonal antibody mepolizumab in the treatment of eosinophilic otitis media. Auris Nasus Larynx 2019, 46, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Okude, A.; Tagaya, E.; Kondo, M.; Nonaka, M.; Tamaoki, J. A Case of Severe Asthma with Eosinophilic Otitis Media Successfully Treated with Anti-IgE Monoclonal Antibody Omalizumab. Case Rep. Pulmonol. 2012, 2012, 340525. [Google Scholar] [CrossRef]


| Mucosal Condition | |
|---|---|
| 0 | Nearly normal or slightly edematous |
| 1 | Edematous or slightly thickened |
| 2 | Highly thickened or granulated to an extent beyond the position of a normal eardrum |
| Quantity of MEE/otorrhea | |
| 0 | No MEE 1 |
| 1 | MEE with intratympanic aeration in a case without eardrum Perforation or otorrhea limited to the mesotympanum in a case with perforation |
| 2 | Mesotympanum totally filled with MEE in a case without perforation or otorrhea coming out from the mesotympanum to the external auditory canal in a case with perforation |
| Frequency of intratympanic administration of corticosteroid | |
| 0 | None |
| 1 | Once in the previous 3 months |
| 2 | Two or more times in the previous 3 months |
| Frequency of systemic administration of corticosteroids | |
| 0 | None. |
| 1 | 7 days or less in the previous 3 months. |
| 2 | More than 7 days in the previous 3 months. |
| Frequency of systemic antibiotics | |
| 0 | None. |
| 1 | 7 days or less in the previous 3 months. |
| 2 | More than 7 days in the previous 3 months. |
| Epidemiology | |
|---|---|
| Age | 54.38 ± 17.89; range 34–64 years |
| Female | 5/8 (62.5%) |
| Male | 3/8 (37.5%) |
| Phenotyping | |
| Concomitant allergy | 6/8 (75%) |
| Concomitant CRSwNP | 8/8 (100%) |
| Concomitant asthma Peripheral blood hyper-eosinophilia Previous sinonasal surgery | 8/8 (100%) 6/8 (75%) 8/8 (100%) |
| ASA sensitivity Smoking SNOT-22, mean Nasal polyp score, mean | 1/8 (12.5%) 0/8 69.5 ± 22.39 5.38 ± 1.4 |
| Sniffin’Sticks identification test, mean PNIF, mean Otorrhea Perforated ear drum Middle ear effusion PTA at baseline mean right PTA at baseline mean left COMOT-15, mean Otitis severity score, mean | 5.75 ± 4.62 61.23 ± 20.31 3/8 (37.5%) 3/8 (37.5%) 5/8 (62.5%) 59.1 ± 14.63 42.25 ± 17.27 57.63 ± 9.91 12.88 ± 3 |
| Case | Sex, Age | Ongoing Biologics | Primary Indication for Biologics | Previous Treatments in the Last Years | Previous Surgeries | Associated Diseases | Type of EOM |
|---|---|---|---|---|---|---|---|
| 1 | M, 64 | Dupilumab | Severe uncontrolled CRSwNP | 3 brief cycles of OCS and antibiotics, previous treatment with omalizumab | Almost 20 NS | Mild–moderate asthma | Bilateral perforation |
| 2 | F, 63 | Dupilumab | Severe uncontrolled CRSwNP | 4 brief cycles of OCS, antibiotics | 1 NS | Mild moderate asthma | COM |
| 3 | F, 49 | Mepolizumab | SEA | 3 brief cycles of OCS, antibiotics | 2 NS | CRSwNP Severe hyper eosinophilia | COM |
| 4 | M, 57 | Benralizumab | SEA | 2 brief cycles of OCS, antibiotics | 2 NS | CRSwNP | COM |
| 5 | M, 62 | Omalizumab | SEA | 2 brief cycles of OCS, antibiotics | 7 NS | CRSwNP | COM |
| 6 | F, 48 | Dupilumab | Severe uncontrolled CRSwNP | 2 brief cycles of OCS, antibiotics | 2 NS | Aspirin-intolerant Mild–moderate asthma | Monolateral perforation with granulation |
| 7 | F, 34 | Dupilumab | Severe uncontrolled CRSwNP | More than 3 brief cycles of OCS, antibiotics | 1 NS >2 ES | Mild–moderate asthma | Monolateral perforation with granulation |
| 8 | F, 58 | Dupilumab | Severe uncontrolled CRSwNP | More than 3 brief cycles of OCS, antibiotics | 1 NS | Mild–moderate asthma | COM |
| Case | Sex, Age | NPS Pre | NPS Post | SNOT-22 Pre | SNOT-22 Post | Sniffin’Sticks Pre-IT | Sniffin’Sticks Post-IT | PNIF Pre (L/min) | PNIF Post (L/min) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 64 | 8 | 4 | 63 | 15 | 0 | 6 | 40 | 150 |
| 2 | F, 63 | 6 | 0 | 81 | 35 | 0 | 8 | 50 | 100 |
| 3 | F, 49 | 4 | 1 | 88 | 54 | 11 | 14 | 50 | 100 |
| 4 | M, 57 | 4 | 0 | 80 | 16 | 4 | 13 | 50 | 100 |
| 5 | M, 62 | 4 | 1 | 81 | 36 | 4 | 9 | 50 | 100 |
| 6 | F, 48 | 6 | 2 | 21 | 10 | 6 | 12 | 80 | 100 |
| 7 | F, 34 | 5 | 2 | 57 | 23 | 12 | 14 | 70 | 100 |
| 8 | F, 58 | 6 | 3 | 85 | 75 | 9 | 13 | 100 | 150 |
| Case | Sex, Age | Otitis Severity Index Pre | Otitis Severity Index Post | PTA Pre | PTA Post | Cycles of OCS/Antibiotics Need Pre | Cycles of OCS/Antibiotics Need Post | COMOT 15 Pre | COMOT 15 Post |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 64 | 16 | 3 | 90 R 76.25 L | 73.75 R 65 L | 3 brief cycles of OCS and antibiotics, previous treatment with omalizumab | No | 63 | 34 |
| 2 | F, 63 | 10 | 0 | 65.25 R 60.5 L | 35.3 R 33.2 L | 4 brief cycles of OCS, antibiotics | No | 56 | 15 |
| 3 | F, 49 | 12 | 0 | 40 R 38.75 L | 23.75 R 35 L | 3 brief cycles of OCS, antibiotics | No | 65 | 29 |
| 4 | M, 57 | 9 | 0 | 51.2 R 37.5 L | 29.2 R 32.5 L | 2 brief cycles of OCS, antibiotics | No | 46 | 5 |
| 5 | M, 62 | 10 | 0 | 55 R 30 L | 25.3 R 20 L | 2 brief cycles of OCS, antibiotics | No | 47 | 19 |
| 6 | F, 48 | 14 | 0 | 56.25 R 32.5 L | 40.5 R 28.75 L | 2 brief cycles of OCS, antibiotics | No | 47 | 24 |
| 7 | F, 34 | 16 | 0 | 52.5 R 37.5 L | 37.25 R 32.5 L | >3 brief cycles of OCS, antibiotics | No | 67 | 43 |
| 8 | F, 58 | 16 | 3 | 62.5 R 25 L | 52.5 R 25 L | >3 brief cycles of OCS, antibiotics | No | 70 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Corso, E.; Montuori, C.; Settimi, S.; Mele, D.A.; Cantiani, A.; Corbò, M.; Cantone, E.; Paludetti, G.; Galli, J. Efficacy of Biologics on Refractory Eosinophilic Otitis Media Associated with Bronchial Asthma or Severe Uncontrolled CRSwNP. J. Clin. Med. 2022, 11, 926. https://doi.org/10.3390/jcm11040926
De Corso E, Montuori C, Settimi S, Mele DA, Cantiani A, Corbò M, Cantone E, Paludetti G, Galli J. Efficacy of Biologics on Refractory Eosinophilic Otitis Media Associated with Bronchial Asthma or Severe Uncontrolled CRSwNP. Journal of Clinical Medicine. 2022; 11(4):926. https://doi.org/10.3390/jcm11040926
Chicago/Turabian StyleDe Corso, Eugenio, Claudio Montuori, Stefano Settimi, Dario Antonio Mele, Alessandro Cantiani, Marco Corbò, Elena Cantone, Gaetano Paludetti, and Jacopo Galli. 2022. "Efficacy of Biologics on Refractory Eosinophilic Otitis Media Associated with Bronchial Asthma or Severe Uncontrolled CRSwNP" Journal of Clinical Medicine 11, no. 4: 926. https://doi.org/10.3390/jcm11040926
APA StyleDe Corso, E., Montuori, C., Settimi, S., Mele, D. A., Cantiani, A., Corbò, M., Cantone, E., Paludetti, G., & Galli, J. (2022). Efficacy of Biologics on Refractory Eosinophilic Otitis Media Associated with Bronchial Asthma or Severe Uncontrolled CRSwNP. Journal of Clinical Medicine, 11(4), 926. https://doi.org/10.3390/jcm11040926









