Cigarette Smoking Associated with Colorectal Cancer Survival: A Nationwide, Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
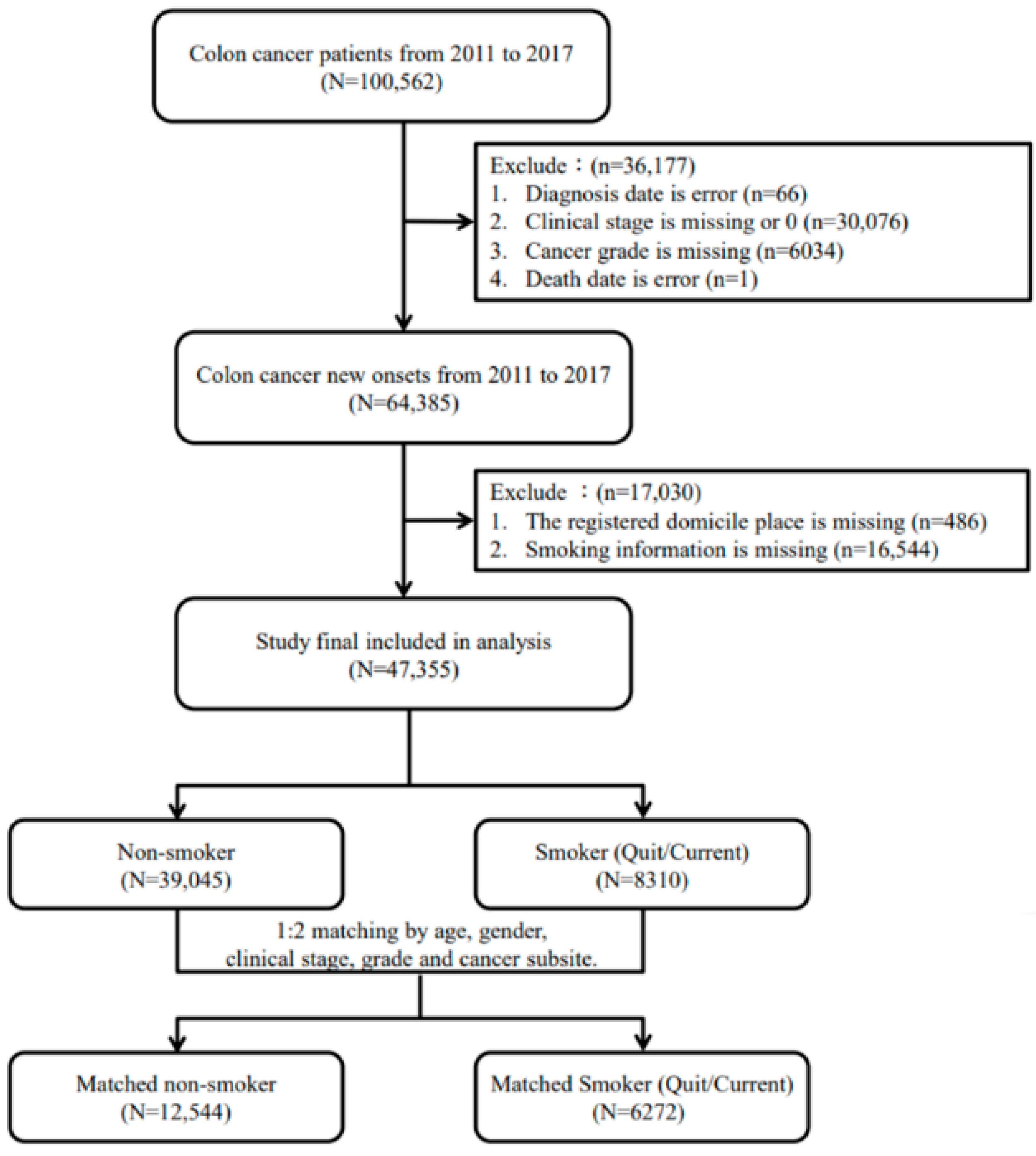
2.2. Study Population
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
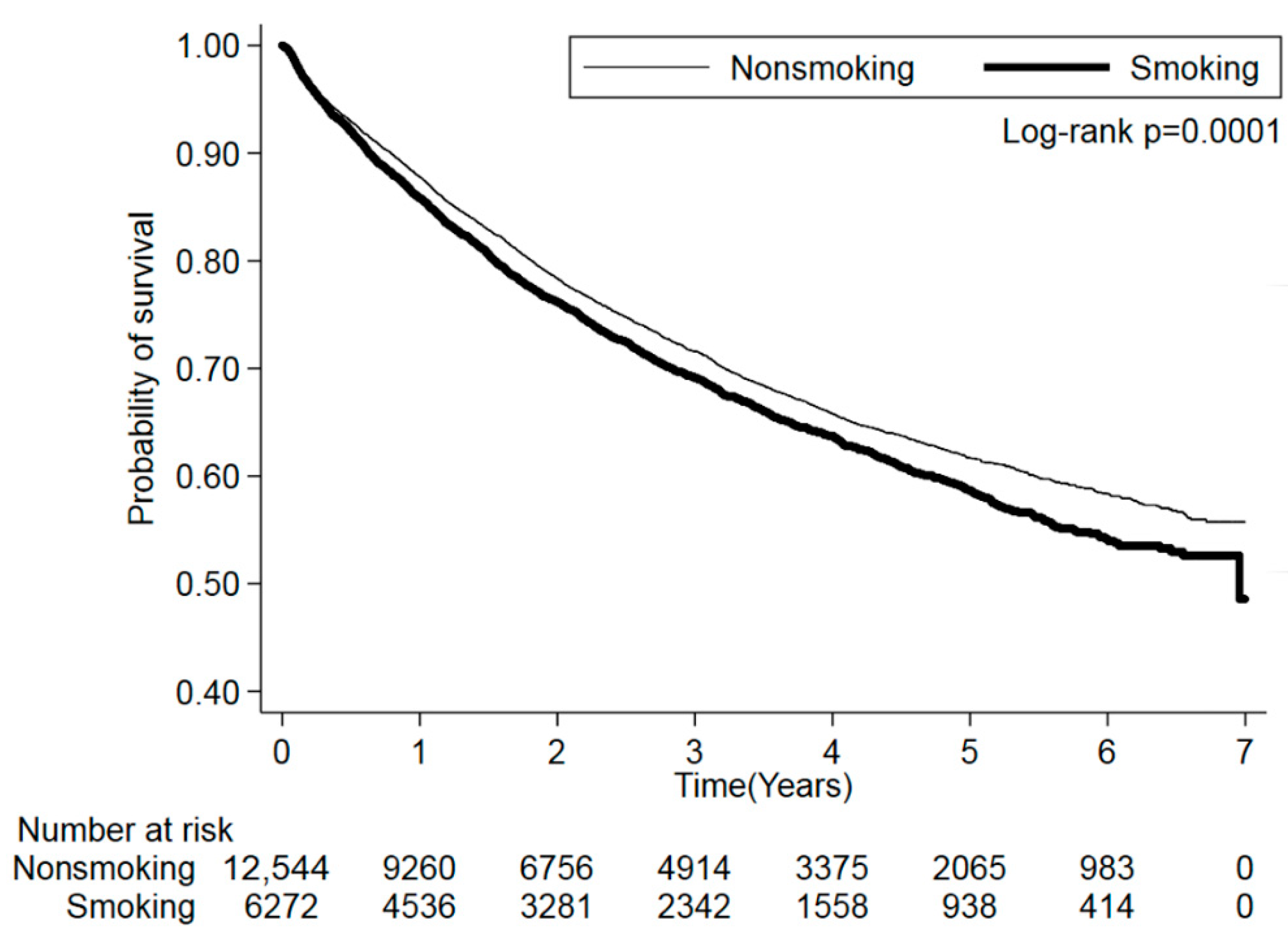
3.2. Cigarette Smoking and Mortality Risk
3.3. Cigarette Smoking and Mortality Risk Stratified by Sex
3.4. Cigarette Smoking and Mortality Risk Stratified by Cancer Subsite
3.5. Cigarette Smoking and Mortality Risk Stratified by Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Walter, V.; Jansen, L.; Hoffmeister, M.; Ulrich, A.; Chang-Claude, J.; Brenner, H. Smoking and survival of colorectal cancer patients: Population-based study from Germany. Int. J. Cancer 2015, 137, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Hayakawa, N.; Kojima, M.; Tamakoshi, K.; Watanabe, Y.; Suzuki, K.; Hashimoto, S.; Tokudome, S.; Toyoshima, H.; Ito, Y.; et al. Smoking and colorectal cancer in a non-Western population: A prospective cohort study in Japan. J. Epidemiol. 2003, 13, 323–332.e1-5. [Google Scholar] [CrossRef]
- Wei, P.L.; Kuo, L.J.; Huang, M.T.; Ting, W.C.; Ho, Y.S.; Wang, W.; An, J.; Chang, Y.J. Nicotine enhances colon cancer cell migration by induction of fibronectin. Ann. Surg. Oncol. 2011, 18, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.P.; Yu, L.; Lam, E.K.; Tai, E.K.; Wu, W.K.; Cho, C.H. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol. Appl. Pharmacol. 2007, 221, 261–267. [Google Scholar] [CrossRef]
- NIH State-of-the-Science Conference Statement on Tobacco Use: Prevention, Cessation, and Control. NIH Consens. State Sci. Statements 2006, 23, 1–26.
- Chao, A.; Thun, M.J.; Jacobs, E.J.; Henley, S.J.; Rodriguez, C.; Calle, E.E. Cigarette smoking and colorectal cancer mortality in the cancer prevention study II. J. Natl. Cancer Inst. 2000, 92, 1888–1896. [Google Scholar] [CrossRef]
- Giovannucci, E.; Colditz, G.A.; Stampfer, M.J.; Hunter, D.; Rosner, B.A.; Willett, W.C.; Speizer, F.E. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. women. J. Natl. Cancer Inst. 1994, 86, 192–199. [Google Scholar] [CrossRef]
- Limburg, P.J.; Vierkant, R.A.; Cerhan, J.R.; Yang, P.; Lazovich, D.; Potter, J.D.; Sellers, T.A. Cigarette smoking and colorectal cancer: Long-term, subsite-specific risks in a cohort study of postmenopausal women. Clin. Gastroenterol. Hepatol. 2003, 1, 202–210. [Google Scholar] [CrossRef]
- Paskett, E.D.; Reeves, K.W.; Rohan, T.E.; Allison, M.A.; Williams, C.D.; Messina, C.R.; Whitlock, E.; Sato, A.; Hunt, J.R. Association between cigarette smoking and colorectal cancer in the Women’s Health Initiative. J. Natl. Cancer Inst. 2007, 99, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, S.R.; Wang, P.P.; Savas, S.; Wish, T.; Zhao, J.; Green, R.; Woods, M.; Sun, Z.; Roebothan, B.; et al. Influence of pre-diagnostic cigarette smoking on colorectal cancer survival: Overall and by tumour molecular phenotype. Br. J. Cancer 2014, 110, 1359–1366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giovannucci, E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 725–731. [Google Scholar]
- Liang, P.S.; Chen, T.Y.; Giovannucci, E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2406–2415. [Google Scholar] [CrossRef]
- Tsoi, K.K.; Pau, C.Y.; Wu, W.K.; Chan, F.K.; Griffiths, S.; Sung, J.J. Cigarette smoking and the risk of colorectal cancer: A meta-analysis of prospective cohort studies. Clin. Gastroenterol. Hepatol. 2009, 7, 682–688. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, Y.; Wang, X.; Wang, J.; Yan, Z.; Gong, G.; Li, G.; Li, C. Meta-analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur. J. Cancer Prev. 2015, 24, 6–15. [Google Scholar] [CrossRef]
- Hannan, L.M.; Jacobs, E.J.; Thun, M.J. The association between cigarette smoking and risk of colorectal cancer in a large prospective cohort from the United States. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3362–3367. [Google Scholar] [CrossRef]
- Colangelo, L.A.; Gapstur, S.M.; Gann, P.H.; Dyer, A.R. Cigarette smoking and colorectal carcinoma mortality in a cohort with long-term follow-up. Cancer 2004, 100, 288–293. [Google Scholar] [CrossRef]
- Parajuli, R.; Bjerkaas, E.; Tverdal, A.; Le Marchand, L.; Weiderpass, E.; Gram, I.T. Smoking increases rectal cancer risk to the same extent in women as in men: Results from a Norwegian cohort study. BMC Cancer 2014, 14, 321. [Google Scholar] [CrossRef]
- Parajuli, R.; Bjerkaas, E.; Tverdal, A.; Selmer, R.; Le Marchand, L.; Weiderpass, E.; Gram, I.T. The increased risk of colon cancer due to cigarette smoking may be greater in women than men. Cancer Epidemiol. Biomark. Prev. 2013, 22, 862–871. [Google Scholar] [CrossRef]
- Rex, D.K.; Johnson, D.A.; Anderson, J.C.; Schoenfeld, P.S.; Burke, C.A.; Inadomi, J.M. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am. J. Gastroenterol. 2009, 104, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.J.; Bentley, A.H.; Ackland, C.; Boyle, P.J. Smoking compromises cause-specific survival in patients with operable colorectal cancer. Clin. Oncol. 2006, 18, 436–440. [Google Scholar] [CrossRef]
- Phipps, A.I.; Baron, J.; Newcomb, P.A. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: The Seattle Colon Cancer Family Registry. Cancer 2011, 117, 4948–4957. [Google Scholar] [CrossRef]
- Park, S.M.; Lim, M.K.; Shin, S.A.; Yun, Y.H. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J. Clin. Oncol. 2006, 24, 5017–5024. [Google Scholar] [CrossRef]
- Yu, G.P.; Ostroff, J.S.; Zhang, Z.F.; Tang, J.; Schantz, S.P. Smoking history and cancer patient survival: A hospital cancer registry study. Cancer Detect. Prev. 1997, 21, 497–509. [Google Scholar] [PubMed]
- Limsui, D.; Vierkant, R.A.; Tillmans, L.S.; Wang, A.H.; Weisenberger, D.J.; Laird, P.W.; Lynch, C.F.; Anderson, K.E.; French, A.J.; Haile, R.W.; et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J. Natl. Cancer Inst. 2010, 102, 1012–1022. [Google Scholar] [CrossRef]
- Walter, V.; Jansen, L.; Hoffmeister, M.; Brenner, H. Smoking and survival of colorectal cancer patients: Systematic review and meta-analysis. Ann. Oncol. 2014, 25, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Hoffmann, I. The changing cigarette, 1950–1995. J. Toxicol. Environ. Health 1997, 50, 307–364. [Google Scholar] [CrossRef]
- Hajiasgharzadeh, K.; Somi, M.H.; Sadigh-Eteghad, S.; Mokhtarzadeh, A.; Shanehbandi, D.; Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Baradaran, B. The dual role of alpha7 nicotinic acetylcholine receptor in inflammation-associated gastrointestinal cancers. Heliyon 2020, 6, e03611. [Google Scholar] [CrossRef]
- Wei, P.L.; Chang, Y.J.; Ho, Y.S.; Lee, C.H.; Yang, Y.Y.; An, J.; Lin, S.Y. Tobacco-specific carcinogen enhances colon cancer cell migration through alpha7-nicotinic acetylcholine receptor. Ann. Surg. 2009, 249, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Cucina, A.; Dinicola, S.; Coluccia, P.; Proietti, S.; D’Anselmi, F.; Pasqualato, A.; Bizzarri, M. Nicotine stimulates proliferation and inhibits apoptosis in colon cancer cell lines through activation of survival pathways. J. Surg. Res. 2012, 178, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Morini, V.; Coluccia, P.; Proietti, S.; D’Anselmi, F.; Pasqualato, A.; Masiello, M.G.; Palombo, A.; De Toma, G.; Bizzarri, M.; et al. Nicotine increases survival in human colon cancer cells treated with chemotherapeutic drugs. Toxicol. In Vitro 2013, 27, 2256–2263. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Clapp, R.W.; Sheahan, K. Associations between smoking status and stage of colorectal cancer at diagnosis in Massachusetts between 1982 and 1987. Cancer 1989, 64, 1372–1374. [Google Scholar] [CrossRef]
- Ho, J.W.; Lam, T.H.; Tse, C.W.; Chiu, L.K.; Lam, H.S.; Leung, P.F.; Ng, K.C.; Ho, S.Y.; Woo, J.; Leung, S.S.; et al. Smoking, drinking and colorectal cancer in Hong Kong Chinese: A case-control study. Int. J. Cancer 2004, 109, 587–597. [Google Scholar] [CrossRef]
- Shimizu, N.; Nagata, C.; Shimizu, H.; Kametani, M.; Takeyama, N.; Ohnuma, T.; Matsushita, S. Height, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: A prospective study. Br. J. Cancer 2003, 88, 1038–1043. [Google Scholar] [CrossRef]
- Slattery, M.L.; Curtin, K.; Anderson, K.; Ma, K.N.; Ballard, L.; Edwards, S.; Schaffer, D.; Potter, J.; Leppert, M.; Samowitz, W.S. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J. Natl. Cancer Inst. 2000, 92, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Dénes, M.I.; Borz, C.; Török, Á.; Kántor, T.; Nădășan, V.; Csibi, M.; Ábrám, Z. The Role of Smoking in the Development of Colorectal Cancer. Acta Marisiensis-Ser. Med. 2016, 62, 400–402. [Google Scholar] [CrossRef][Green Version]
- Tsong, W.H.; Koh, W.P.; Yuan, J.M.; Wang, R.; Sun, C.L.; Yu, M.C. Cigarettes and alcohol in relation to colorectal cancer: The Singapore Chinese Health Study. Br. J. Cancer 2007, 96, 821–827. [Google Scholar] [CrossRef]
| Total | Nonsmoking | Smoking | ||
|---|---|---|---|---|
| N = 18,816 | N = 12,544 | N = 6272 | p | |
| Age group, n (%), years | ||||
| <40 | 554 (2.94) | 377 (3.01) | 177 (2.82) | 0.8870 |
| 40–50 | 1298 (6.90) | 859 (6.85) | 439 (7.00) | |
| 50–60 | 3642 (19.36) | 2421 (19.30) | 1221 (19.47) | |
| 60–70 | 6213 (33.02) | 4163 (33.19) | 2050 (32.68) | |
| ≥70 | 7109 (37.78) | 4724 (37.66) | 2385 (38.03) | |
| Sex, n (%) | ||||
| Male | 17,019 (90.45) | 11,346 (90.45) | 5673 (90.45) | 1.0000 |
| Female | 1797 (9.55) | 1198 (9.55) | 599 (9.55) | |
| Drinking, n (%) | ||||
| No | 13,821 (73.45) | 10,817 (86.23) | 3004 (47.90) | <0.0001 |
| Yes | 4995 (26.55) | 1727 (13.77) | 3268 (52.10) | |
| Remote area, n (%) | ||||
| No | 18,477 (98.20) | 12,334 (98.33) | 6143 (97.94) | 0.0628 |
| Yes | 339 (1.80) | 210 (1.67) | 129 (2.06) | |
| Cancer subsite, n (%) | ||||
| Colon | 12,006 (63.81) | 7999 (63.77) | 4007 (63.89) | 0.8722 |
| Rectum | 6810 (36.19) | 4545 (36.23) | 2265 (36.11) | |
| Clinical stage, n (%) | ||||
| I | 4472 (23.77) | 2986 (23.80) | 1486 (23.69) | 0.9532 |
| II | 3610 (19.19) | 2398 (19.12) | 1212 (19.32) | |
| III | 7125 (37.87) | 4742 (37.80) | 2383 (37.99) | |
| IV | 3609 (19.18) | 2418 (19.28) | 1191 (18.99) | |
| Grade, n (%) | ||||
| Well-differentiated | 1221 (6.49) | 794 (6.33) | 427 (6.81) | 0.1276 |
| Moderately differentiated | 15,877 (84.38) | 10,638 (84.81) | 5239 (83.53) | |
| Poorly differentiated | 1562 (8.30) | 1015 (8.09) | 547 (8.72) | |
| Undifferentiated | 156 (0.83) | 97 (0.77) | 59 (0.94) | |
| CCI group, n (%) | ||||
| 0–1 | 14,453 (76.81) | 9644 (76.88) | 4809 (76.67) | 0.4946 |
| 2–4 | 3863 (20.53) | 2579 (20.56) | 1284 (20.47) | |
| ≥5 | 500 (2.66) | 321 (2.56) | 179 (2.85) | |
| Death, n (%) | 5384 (28.61) | 3495 (27.86) | 1889 (30.12) | 0.0012 |
| Patients | Death | % | Crude HR (95% CI) | p | Adjusted HR (95% CI) a | p | |
|---|---|---|---|---|---|---|---|
| Smoking | |||||||
| Never | 12,544 | 3495 | 27.86 | Ref. | Ref. | ||
| Quit/Current | 6272 | 1889 | 30.12 | 1.11 (1.05–1.19) | 0.0009 | 1.10 (1.03–1.18) | 0.0056 |
| Smoking count | |||||||
| 0 | 12,544 | 3495 | 27.86 | Ref. | Ref. | ||
| 1–10/day | 1757 | 518 | 2948 | 1.03 (0.93–1.15) | 0.5581 | 1.03 (0.92–1.15) | 0.6435 |
| 11–20/day | 3297 | 1008 | 30.57 | 1.17 (1.08–1.27) | 0.0001 | 1.16 (1.07–1.26) | 0.0006 |
| >20/day | 1218 | 363 | 29.80 | 1.08 (0.95–1.23) | 0.2270 | 1.07 (0.94–1.22) | 0.3144 |
| Trend test | p = 0.3686 | ||||||
| Smoking years | |||||||
| 0 | 12,544 | 3495 | 27.86 | Ref. | Ref. | ||
| 1–10 | 1248 | 338 | 27.08 | 1.01 (0.89–1.15) | 0.8435 | 1.01 (0.88–1.15) | 0.9426 |
| 11–30 | 2528 | 692 | 27.37 | 1.12 (1.02–1.23) | 0.0184 | 1.11 (1.01–1.23) | 0.0356 |
| >30 | 2496 | 859 | 34.42 | 1.15 (1.06–1.26) | 0.0014 | 1.14 (1.04–1.25) | 0.0044 |
| Trend test | p = 0.0474 |
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Death | % | Adjusted HR (95%) CI) a | p | Patients | Death | % | Adjusted HR (95%) CI) a | p | |
| Smoking | ||||||||||
| Never | 11,346 | 3213 | 28.32 | Ref. | 1198 | 282 | 23.54 | Ref. | ||
| Quit/Current | 5673 | 1753 | 30.90 | 1.09 (1.02–1.18) | 0.0156 | 599 | 136 | 22.70 | 1.26 (0.96–1.66) | 0.1032 |
| Smoking count | ||||||||||
| 0 | 11,346 | 3213 | 28.32 | Ref. | 1198 | 282 | 23.54 | Ref. | ||
| 1–10/day | 1484 | 455 | 30.66 | 1.00 (0.89–1.12) | 0.9766 | 273 | 63 | 23.08 | 1.35 (0.93–1.96) | 0.1093 |
| 11–20/day | 3027 | 951 | 31.42 | 1.16 (1.06–1.26) | 0.0011 | 270 | 57 | 21.11 | 1.17 (0.79–1.74) | 0.4235 |
| >20/day | 1162 | 347 | 29.86 | 1.06 (0.93–1.22) | 0.3752 | 56 | 16 | 28.57 | 1.20 (0.61–2.36) | 0.5938 |
| Trend test | p = 0.4186 | p = 0.6222 | ||||||||
| Smoking year | ||||||||||
| 0 | 11,346 | 3213 | 28.32 | Ref. | 1198 | 282 | 23.54 | Ref. | ||
| 1–10 | 1057 | 286 | 27.06 | 0.96 (0.83–1.10) | 0.5430 | 191 | 52 | 27.23 | 1.59 (1.03–2.45) | 0.0367 |
| 11–30 | 2242 | 645 | 28.77 | 1.11 (1.01–1.24) | 0.0390 | 286 | 47 | 16.43 | 1.04 (0.70–1.55) | 0.8407 |
| >30 | 2374 | 822 | 34.63 | 1.14 (1.04–1.25) | 0.0076 | 122 | 37 | 30.33 | 1.28 (0.79–2.09) | 0.3108 |
| Trend test | p = 0.1460 | p = 0.8621 |
| Colon | Rectum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Death | % | Adjusted HR (95% CI) a | p | Patients | Death | % | Adjusted HR (95% CI) a | p | |
| Smoking | ||||||||||
| Never | 7999 | 2229 | 27.87 | Ref. | 4545 | 1266 | 27.85 | Ref. | ||
| Quit/Current | 4007 | 1206 | 30.10 | 1.12 (1.03–1.22) | 0.0096 | 2265 | 683 | 30.15 | 1.08 (0.95–1.22) | 0.2339 |
| Smoking count | ||||||||||
| 0 | 7999 | 2229 | 27.87 | Ref. | 4545 | 1266 | 27.85 | Ref. | ||
| 1–10/day | 1134 | 325 | 28.66 | 1.02 (0.89–1.17) | 0.7660 | 623 | 193 | 30.98 | 1.03 (0.86–1.25) | 0.7316 |
| 11–20/day | 2117 | 647 | 30.56 | 1.15 (1.04–1.28) | 0.0072 | 1180 | 361 | 30.59 | 1.17 (1.01–1.35) | 0.0337 |
| >20/day | 756 | 234 | 30.95 | 1.18 (1.01–1.39) | 0.0376 | 462 | 129 | 27.92 | 0.88 (0.70–1.12) | 0.2961 |
| Trend test | p = 0.0463 | p = 0.7617 | ||||||||
| Smoking year | ||||||||||
| 0 years | 7999 | 2229 | 27.87 | Ref. | 4545 | 1266 | 27.85 | Ref. | ||
| 1–10 years | 823 | 219 | 26.61 | 0.98 (0.83–1.15) | 0.8084 | 425 | 119 | 28.00 | 1.06 (0.84–1.33) | 0.6220 |
| 11–30 years | 1636 | 453 | 27.69 | 1.13 (1.00–1.28) | 0.0447 | 892 | 239 | 26.79 | 1.08 (0.90–1.28) | 0.4154 |
| >30 years | 1548 | 534 | 34.50 | 1.18 (1.05–1.32) | 0.0046 | 948 | 325 | 34.28 | 1.09 (0.93–1.27) | 0.3092 |
| Trend test | p = 0.0889 | p = 0.0713 |
| Age ≤ 60 | Age > 60 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Death | % | Adjusted HR (95% CI) a | p | Patients | Death | % | Adjusted HR (95% CI) a | p | |
| Smoking | ||||||||||
| Never | 4117 | 829 | 20.14 | Ref. | 8427 | 2666 | 31.64 | Ref. | ||
| Quit/Current | 2055 | 445 | 21.65 | 1.07 (0.92–1.23) | 0.3971 | 4217 | 1444 | 34.24 | 1.12 (1.03–1.21) | 0.0061 |
| Smoking count | ||||||||||
| 0 | 4117 | 829 | 20.14 | Ref. | 8427 | 2666 | 31.64 | Ref. | ||
| 1–10/day | 590 | 125 | 21.19 | 0.97 (0.77–1.23) | 0.8078 | 1167 | 393 | 33.68 | 1.05 (0.92–1.19) | 0.4655 |
| 11–20/day | 1046 | 232 | 22.18 | 1.19 (0.99–1.42) | 0.0616 | 2251 | 776 | 34.47 | 1.16 (1.05–1.27) | 0.0034 |
| >20/day | 419 | 88 | 21.00 | 0.93 (0.71–1.21) | 0.5737 | 799 | 275 | 34.42 | 1.12 (0.97–1.31) | 0.1274 |
| Trend test | p = 0.9888 | p = 0.1497 | ||||||||
| Smoking year | ||||||||||
| 0 | 4117 | 829 | 20.14 | Ref. | 8427 | 2666 | 31.64 | Ref. | ||
| 1–10 | 525 | 118 | 22.48 | 0.92 (0.72–1.17) | 0.4871 | 723 | 220 | 30.43 | 1.04 (0.89–1.22) | 0.6071 |
| 11–30 | 1200 | 242 | 20.17 | 1.03 (0.86–1.24) | 0.7198 | 1328 | 450 | 33.89 | 1.15 (1.02–1.30) | 0.0190 |
| >30 | 330 | 85 | 25.76 | 1.43 (1.10–1.87) | 0.0074 | 2166 | 774 | 35.73 | 1.12 (1.02–1.24) | 0.0209 |
| Trend test | p = 0.2077 | p = 0.1265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-M.; Wei, P.-L.; Ho, C.-H.; Yeh, C.-C. Cigarette Smoking Associated with Colorectal Cancer Survival: A Nationwide, Population-Based Cohort Study. J. Clin. Med. 2022, 11, 913. https://doi.org/10.3390/jcm11040913
Huang Y-M, Wei P-L, Ho C-H, Yeh C-C. Cigarette Smoking Associated with Colorectal Cancer Survival: A Nationwide, Population-Based Cohort Study. Journal of Clinical Medicine. 2022; 11(4):913. https://doi.org/10.3390/jcm11040913
Chicago/Turabian StyleHuang, Yu-Min, Po-Li Wei, Chung-Han Ho, and Chih-Ching Yeh. 2022. "Cigarette Smoking Associated with Colorectal Cancer Survival: A Nationwide, Population-Based Cohort Study" Journal of Clinical Medicine 11, no. 4: 913. https://doi.org/10.3390/jcm11040913
APA StyleHuang, Y.-M., Wei, P.-L., Ho, C.-H., & Yeh, C.-C. (2022). Cigarette Smoking Associated with Colorectal Cancer Survival: A Nationwide, Population-Based Cohort Study. Journal of Clinical Medicine, 11(4), 913. https://doi.org/10.3390/jcm11040913







