Incremental Value of Myocardial Work over Global Longitudinal Strain in the Surveillance for Cancer-Treatment-Related Cardiac Dysfunction: A Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
- i.
- CTRCD+BP−: cardiotoxicity and no increase in afterload (defined arbitrarily as an increase in SBP > 20 mmHg;) at a follow-up examination;
- ii.
- CTRCD+BP+: cardiotoxicity with a concomitant increase in afterload at a follow-up examination;
- iii.
- CTRCD−BP+: no cardiotoxicity in the presence of an increase in afterload at a follow-up examination;
- iv.
- CTRCD−BP−: no cardiotoxicity and no increase in afterload at a follow-up examination.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, M.P.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J.; et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Hooning, M.J.; Botma, A.; Aleman, B.M.P.; Baaijens, M.H.A.; Bartelink, H.; Klijn, J.G.M.; Taylor, C.W.; Van Leeuwen, F.E. Long-Term Risk of Cardiovascular Disease in 10-Year Survivors of Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, J.J.; Neugut, A.I.; Jacobson, J.S.; Grann, V.R.; Hershman, D.L. Chemotherapy and cardiotoxicity in older breast cancer pa-tients: A population-based study. J. Clin. Oncol. 2005, 23, 8597–8605. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovas-cular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [Green Version]
- Felker, G.; Thompson, R.E.; Hare, J.M.; Hruban, R.H.; Clemetson, D.E.; Howard, D.; Baughman, K.L.; Kasper, E.K. Underlying Causes and Long-Term Survival in Patients with Initially Unexplained Cardiomyopathy. N. Engl. J. Med. 2000, 342, 1077–1084. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Al-Biltagi, M.; Abd Rab Elrasoul Tolba, O.; El-Shanshory, M.R.; El-Shitany, A.E.A.; El-Sayed El-Hawary, E. Strain echo-cardiography in early detection of Doxorubicin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. ISRN Pediatr. 2012, 2012, 870549. [Google Scholar] [CrossRef] [Green Version]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Byth, K.; Stuart, K.; Clarke, J.L.; Thomas, L. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: A comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur. J. Cancer 2013, 49, 3396–3403. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardio-toxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Fallah-Rad, N.; Walker, J.R.; Wassef, A.; Lytwyn, M.; Bohonis, S.; Fang, T.; Tian, G.; Kirkpatrick, I.D.; Singal, P.K.; Krahn, M.; et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J. Am. Coll. Cardiol. 2011, 57, 2263–2270. [Google Scholar]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation in-dices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Haluska, B.A.; Hare, J.L.; Plana, J.C.; Marwick, T.H. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur. Heart J. Cardiovasc. Imaging 2013, 15, 324–331. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; Cho, G.Y.; Popescu, B.A.; et al. Cardio-protection using Strain-Guided Management of Potentially Car-diotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar]
- Boe, E.; Russell, K.; Eek, C.; Eriksen, M.; Remme, E.W.; Smiseth, O.A.; Skulstad, H. Non-invasive myocardial work index identifies acute cor-onary occlusion in patients with non-ST-segment elevation-acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Hubert, A.; Le Rolle, V.; Leclercq, C.; Galli, E.; Samset, E.; Casset, C.; Mabo, P.; Hernandez, A.; Donal, E. Estimation of myocardial work from pressure–strain loops analysis: An experimental evaluation. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1372–1379. [Google Scholar] [CrossRef]
- Przewlocka-Kosmala, M.; Marwick, T.H.; Mysiak, A.; Kosowski, W.; Kosmala, W. Usefulness of myocardial work measurement in the assessment of left ventricular systolic reserve response to spironolactone in heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1138–1146. [Google Scholar] [CrossRef]
- Galli, E.; Leclercq, C.; Fournet, M.; Hubert, A.; Bernard, A.; Smiseth, O.A.; Mabo, P.; Samset, E.; Hernandez, A.; Donal, E. Value of Myocardial Work Estimation in the Prediction of Response to Cardiac Resyn-chronization Therapy. J. Am. Soc. Echocardiogr. 2018, 31, 220–230. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Negishi, K.; Marwick, T.H.; SUCCOUR Investigators. Rationale and Design of the Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes: The SUCCOUR Trial. JACC Cardiovasc. Imaging 2018, 11, 1098–1105. [Google Scholar] [CrossRef]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, C.; Bricknell, K.; Hanekom, L.; Marwick, T.H. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J. Am. Coll. Cardiol. 2004, 44, 878–886. [Google Scholar] [CrossRef] [Green Version]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Thavendiranathan, P.; Grant, A.D.; Negishi, T.; Plana, J.C.; Popović, Z.B.; Marwick, T.H. Reproducibility of echocardiographic tech-niques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J. Am. Coll. Cardiol. 2013, 61, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiog-raphy for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-Induced Cardiomyopathy: Clinical Relevance and Response to Pharmacologic Therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of tro-ponin I evaluation. J. Clin. Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef]
- Fallah-Rad, N.; Lytwyn, M.; Fang, T.; Kirkpatrick, I.; Jassal, D.S. Delayed contrast enhancement cardiac magnetic resonance im-aging in trastuzumab induced cardiomyopathy. J. Cardiovasc. Magn. Reson. 2008, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Telli, M.L.; Hunt, S.A.; Carlson, R.W.; Guardino, A.E. Trastuzumab-related cardiotoxicity: Calling into question the concept of re-versibility. J. Clin. Oncol. 2007, 25, 3525–3533. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudi-nal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef] [PubMed]
| Parameter | CTRCD+ BP− n = 11 (A) | CTRCD+ BP+ n = 11 (B) | CTRCD−BP+ n = 11 (C) | CTRCD−BP− n = 11 (D) | P A−B | P A−C | P A−D | P B−C | P B−D | P C−D |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 51.8 ± 10.3 | 48.4 ± 10.1 | 53.1 ± 7.6 | 50.4 ± 10.2 | 0.77 | 0.99 | 0.99 | 0.59 | 0.82 | 0.94 |
| Body Mass Index, kg/m2 | 24.8 ± 3.7 | 26.8 ± 6.8 | 24.7 ± 2.4 | 27.5 ± 9.9 | 0.89 | 0.99 | 0.81 | 0.87 | 0.99 | 0.78 |
| Hypertension, n (%) | 2 (18) | 3 (27) | 0 (0) | 3 (27) | 0.62 | 0.15 | 0.62 | 0.06 | 1.00 | 0.06 |
| Diabetes Mellitus, n (%) | 0 (0) | 3 (27) | 2 (18) | 0 (0) | 0.06 | 0.15 | 1.00 | 0.62 | 0.06 | 0.15 |
| Family History of Heart Failure, n (%) | 4 (36) | 2 (18) | 3 (27) | 3 (27) | 0.35 | 0.65 | 0.65 | 0.62 | 0.62 | 1.00 |
| Hemoglobin, g/dL | 12.4 ± 1.3 | 11.4 ± 2.2 | 11.0 ± 1.6 | 12.1 ± 1.6 | 0.54 | 0.24 | 0.99 | 0.96 | 0.61 | 0.26 |
| eGRF, ml/min/1.73 m2 | 95 ± 22 | 106 ± 29 | 104 ± 22 | 105 ± 22 | 0.85 | 0.93 | 0.91 | 0.99 | 0.99 | 0.99 |
| Systolic Blood Pressure, mmHg | 125 ± 15 | 115 ± 5 | 118 ± 13 | 127 ± 19 | 0.65 | 0.90 | 0.98 | 0.93 | 0.33 | 0.64 |
| Diastolic Blood Pressure, mmHg | 79 ± 10 | 76 ± 7 | 76 ± 10 | 77 ± 15 | 0.95 | 0.94 | 0.98 | 0.99 | 0.99 | 0.99 |
| Medications | ||||||||||
| Betablockers, n (%) | 1 (9) | 1 (9) | 0(0) | 0 (0) | 1.00 | 0.32 | 0.32 | 0.32 | 0.32 | 1.00 |
| ACEI/ARB, n (%) | 1 (9) | 1 (9) | 0 (0) | 0 (0) | 1.00 | 0.32 | 0.32 | 0.32 | 0.32 | 1.00 |
| Chemotherapy: A/A-T, n/n (%/%) | 1/10 (9/91) | 2/9 (18/82) | 2/9 (18/82) | 1/10 (9/91) | 0.54 | 0.54 | 1.00 | 1.00 | 0.54 | 0.54 |
| Parameter | CTRCD+ BP− n = 11 (A) | CTRCD+ BP+ n = 11 (B) | CTRCD−BP+ n = 11 (C) | CTRCD−BP− n = 11 (D) | P A−B | P A−C | P A−D | P B−C | P B−D | P C−D |
|---|---|---|---|---|---|---|---|---|---|---|
| LV Diastolic Dimension, mm | 47.2 ± 4.3 | 47.5 ± 4.3 | 46.6 ± 3.4 | 49.7 ± 3.3 | 0.99 | 0.98 | 0.64 | 0.95 | 0.73 | 0.47 |
| LV Mass Index, g/m2 | 79.4 ± 26.9 | 88.8 ± 17.8 | 85.5 ± 19.1 | 85.1 ± 21.9 | 0.82 | 0.95 | 0.96 | 0.99 | 0.98 | 0.99 |
| Left Atrial Volume Index, ml/m2 | 27.3 ± 6.5 | 29.0 ± 9.9 | 28.8 ± 6.9 | 28.6 ± 4.4 | 0.96 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 |
| E/A | 1.04 ± 0.42 | 1.34 ± 0.42 | 1.06 ± 0.39 | 1.10 ± 0.34 | 0.45 | 0.99 | 0.99 | 0.51 | 0.66 | 0.99 |
| e’ septal, cm/s | 7.9 ± 1.4 | 9.0 ± 1.7 | 7.8 ± 1.7 | 7.7 ± 3.1 | 0.68 | 0.99 | 0.99 | 0.63 | 0.56 | 0.99 |
| e’ lateral, cm/s | 10.0 ± 1.8 | 11.4 ± 2.0 | 10.4 ± 2.9 | 9.7 ± 3.5 | 0.66 | 0.98 | 0.99 | 0.88 | 0.56 | 0.93 |
| E/e’ | 7.7 ± 2.1 | 8.4 ± 1.4 | 7.9 ± 1.8 | 9.4 ± 3.6 | 0.93 | 0.99 | 0.55 | 0.96 | 0.86 | 0.62 |
| LV Ejection Fraction, % | 62.0 ± 3.6 | 64.0 ± 4.8 | 63.4 ± 4.4 | 62.5 ± 5.4 | 0.81 | 0.93 | 0.99 | 0.99 | 0.90 | 0.97 |
| GLS, % | 19.3 ± 3.0 | 20.6 ± 2.0 | 20.0 ± 2.6 | 19.2 ± 2.6 | 0.73 | 0.95 | 0.99 | 0.95 | 0.63 | 0.90 |
| GWI, mmHg% | 2076 ± 526 | 1846 ± 133 | 1846 ± 279 | 1834 ± 298 | 0.48 | 0.48 | 0.44 | 0.99 | 0.99 | 0.99 |
| GCW, mmHg% | 2405 ± 572 | 2146 ± 170 | 2168 ± 272 | 2205 ± 257 | 0.41 | 0.49 | 0.63 | 0.99 | 0.98 | 0.99 |
| GWW, mmHg% | 88 ± 65 | 90 ± 46 | 80 ± 45 | 96 ± 138 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.97 |
| GWE | 95.5 ± 3.0 | 94.6 ± 2.7 | 95.8 ± 1.7 | 95.5 ± 4.4 | 0.94 | 0.99 | 0.99 | 0.85 | 0.92 | 0.99 |
| Parameter | CTRCD+ BP− n = 11 (A) | CTRCD+ BP+ n = 11 (B) | CTRCD−BP+ n = 11 (C) | CTRCD−BP− n = 11 (D) | P A−B | P A−C | P A−D | P B−C | P B−D | P C−D |
|---|---|---|---|---|---|---|---|---|---|---|
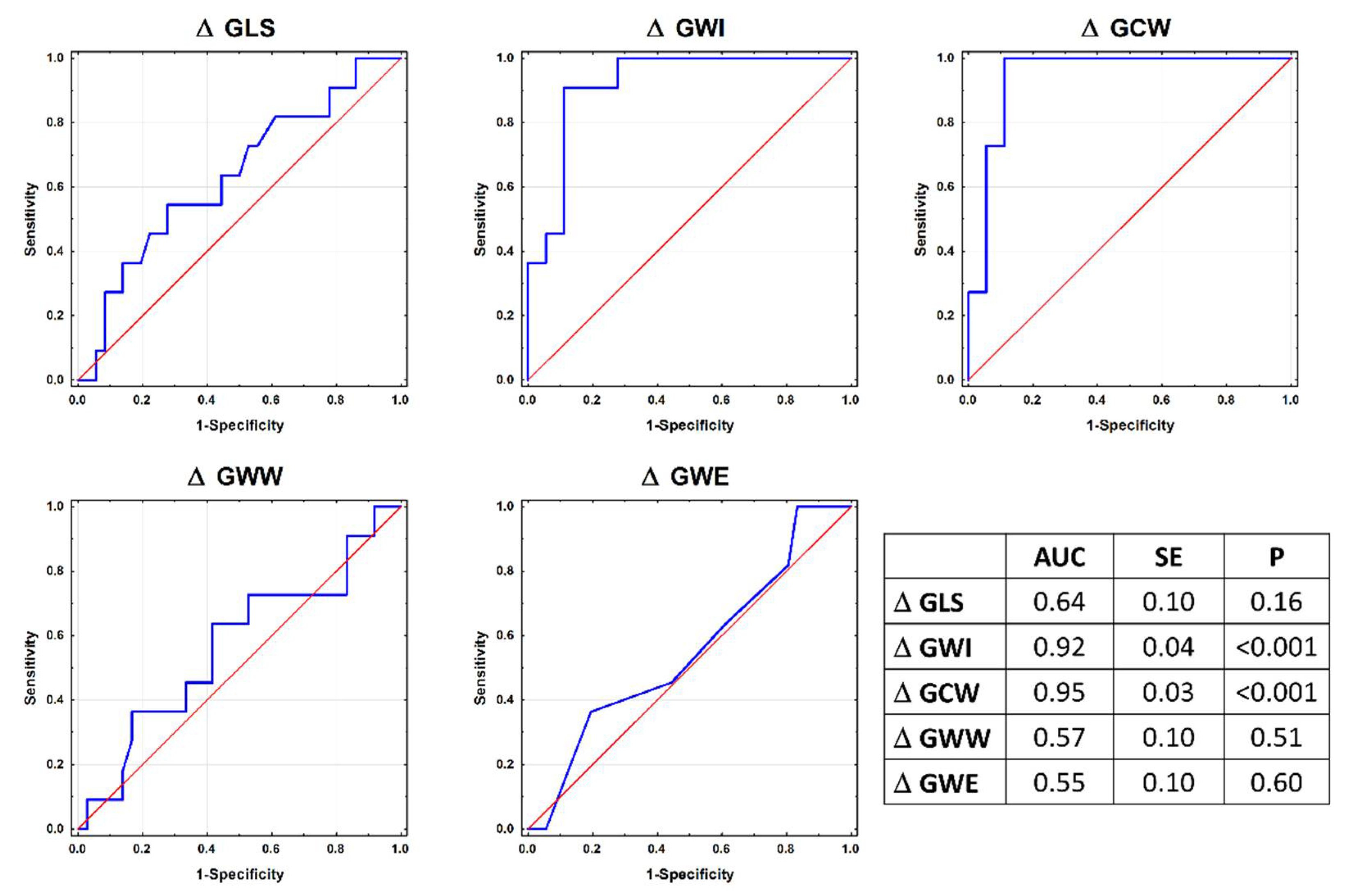
| delta GLS, % | −3.40 ± 2.42 | −5.71 ± 2.81 | −1.05 ± 2.21 | −1.01 ± 2.00 | 0.14 | 0.13 | 0.12 | <0.001 | <0.001 | 0.99 |
| delta GWI, mmHg% | −493 ± 448 | −110 ± 271 | 422 ± 292 | −45 ± 281 | 0.05 | <0.001 | 0.01 | 0.004 | 0.97 | 0.01 |
| delta GCW, mmHg% | −541 ± 515 | −114 ± 287 | 401 ± 233 | −152 ± 284 | 0.01 | <0.001 | 0.03 | 0.008 | 0.99 | 0.005 |
| delta GWW, mmHg% | 33.7 ± 63.8 | 60.8 ± 64.3 | 27.2 ± 46.2 | −16.0 ± 102.2 | 0.85 | 0.99 | 0.46 | 0.75 | 0.12 | 0.58 |
| delta GWE | −2.09 ± 3.38 | −3.27 ± 3.49 | −0.64 ± 1.91 | −0.18 ± 2.78 | 0.83 | 0.72 | 0.52 | 0.24 | 0.13 | 0.98 |
| delta EF, % | −11.6 ± 1.7 | −14.4 ± 4.6 | −0.2 ± 3.7 | −0.6 ± 2.8 | 0.33 | <0.001 | <0.001 | <0.001 | <0.001 | 0.99 |
| delta SBP, mmHg | −7.2 ± 18.4 | 25.7 ± 7.1 | 29.3 ± 5.2 | −2.8 ± 6.0 | <0.001 | <0.001 | 0.67 | 0.88 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmala, W.; Negishi, T.; Thavendiranathan, P.; Penicka, M.; De Blois, J.; Murbræch, K.; Miyazaki, S.; Shirazi, M.; Santoro, C.; Vinereanu, D.; et al. Incremental Value of Myocardial Work over Global Longitudinal Strain in the Surveillance for Cancer-Treatment-Related Cardiac Dysfunction: A Case–Control Study. J. Clin. Med. 2022, 11, 912. https://doi.org/10.3390/jcm11040912
Kosmala W, Negishi T, Thavendiranathan P, Penicka M, De Blois J, Murbræch K, Miyazaki S, Shirazi M, Santoro C, Vinereanu D, et al. Incremental Value of Myocardial Work over Global Longitudinal Strain in the Surveillance for Cancer-Treatment-Related Cardiac Dysfunction: A Case–Control Study. Journal of Clinical Medicine. 2022; 11(4):912. https://doi.org/10.3390/jcm11040912
Chicago/Turabian StyleKosmala, Wojciech, Tomoko Negishi, Paaladinesh Thavendiranathan, Martin Penicka, Jonathan De Blois, Klaus Murbræch, Sakiko Miyazaki, Mitra Shirazi, Ciro Santoro, Dragos Vinereanu, and et al. 2022. "Incremental Value of Myocardial Work over Global Longitudinal Strain in the Surveillance for Cancer-Treatment-Related Cardiac Dysfunction: A Case–Control Study" Journal of Clinical Medicine 11, no. 4: 912. https://doi.org/10.3390/jcm11040912
APA StyleKosmala, W., Negishi, T., Thavendiranathan, P., Penicka, M., De Blois, J., Murbræch, K., Miyazaki, S., Shirazi, M., Santoro, C., Vinereanu, D., Cho, G.-Y., Hristova, K., Popescu, B. A., Kurosawa, K., Izumo, M., Negishi, K., Przewlocka-Kosmala, M., & Marwick, T. H. (2022). Incremental Value of Myocardial Work over Global Longitudinal Strain in the Surveillance for Cancer-Treatment-Related Cardiac Dysfunction: A Case–Control Study. Journal of Clinical Medicine, 11(4), 912. https://doi.org/10.3390/jcm11040912









