Performance of Coronary Angiography in the Detection of Coronary Artery Disease in Patients with Systolic Left Ventricular Dysfunction and No Prior Ischemic Heart Disease
Abstract
:1. Introduction
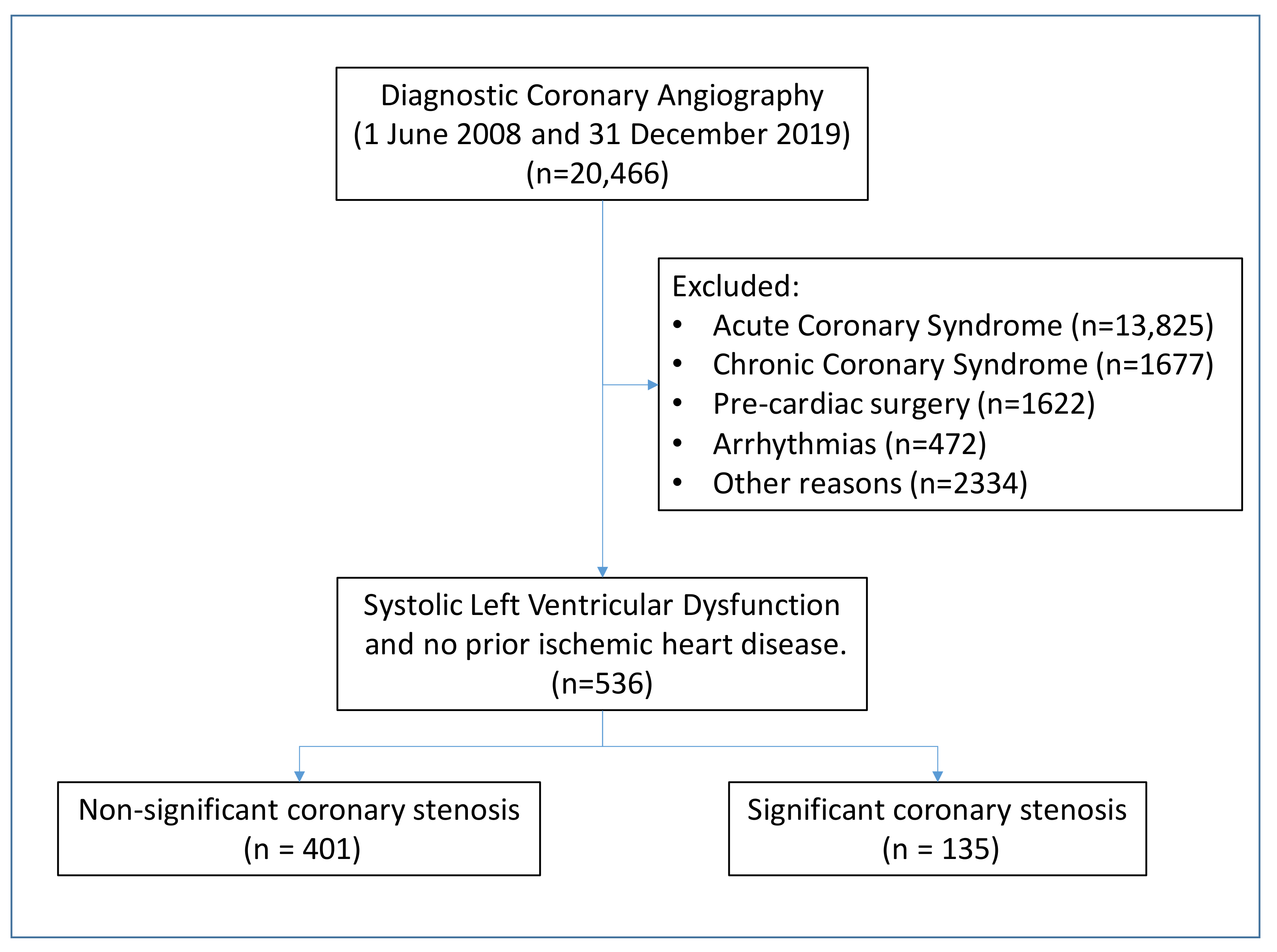
2. Materials and Methods
3. Result
3.1. Clinical Differences between Patients with and without Coronary Lesions
3.2. Characteristics of Patients with Multi-Vessel Lesion
3.3. Predictors of Coronary Heart Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Devanabanda, A.R.; Zakhem, G.; Iqbal, S.N.; Slater, W.; Coppola, J.T. Comparison of Clinical and Electrocardiographic Predictors of Ischemic and Nonischemic Cardiomyopathy during the Initial Evaluation of Patients with Reduced (≤40%) Left Ventricular Ejection Fraction. Am. J. Cardiol. 2017, 119, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Desroche, L.-M.; Milleron, O.; Safar, B.; Ou, P.; Garbarz, E.; Lavie-Badie, Y.; Abtan, J.; Millischer, D.; Pathak, A.; Durand-Zaleski, I.; et al. Cardiovascular Magnetic Resonance May Avoid Unnecessary Coronary Angiography in Patients with Unexplained Left Ventricular Systolic Dysfunction: A Retrospective Diagnostic Pilot Study. J. Card Fail. 2020, 26, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Estornell-Erill, J.; Igual-Muñoz, B.; Monmeneu-Menadas, J.V.; Soriano-Navarro, C.; Valle-Muñoz, A.; Vilar-Herrero, J.V.; Pérez-Boscá, L.; Payá-Serrano, R.; Martínez-Alzamora, N.; Ridocci-Soriano, F. Etiological diagnosis of left ventricular dysfunction: Computed tomography compared with coronary angiography and cardiac magnetic resonance. Rev. Esp. Cardiol. 2012, 65, 515–524. [Google Scholar] [CrossRef]
- Doshi, D.; Ben-Yehuda, O.; Bonafede, M.; Josephy, N.; Karmpaliotis, D.; Parikh, M.A.; Moses, J.W.; Stone, G.W.; Leon, M.B.; Schwartz, A.; et al. Underutilization of Coronary Artery Disease Testing Among Patients Hospitalized with New-Onset Heart Failure. J. Am. Coll Cardiol. 2016, 68, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.A.; Lenzo, J.; Magid, D.J.; Gurwitz, J.H.; Smith, D.H.; Hsu, G.; Sung, S.H.; Go, A.S. Hospital-level variation in use of cardiovascular testing for adults with incident heart failure: Findings from the cardiovascular research network heart failure study. JACC Cardiovasc. Imaging 2014, 7, 690–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.P.; Rossignol, P.; Demissei, B.; Sharma, A.; Girerd, N.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Hillege, H.L.; et al. Coronary angiography in worsening heart failure: Determinants, findings and prognostic implications. Heart 2018, 104, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.; White, C.J. Coronary Angiography Is the Gold Standard for Patients with Significant Left Ventricular Dysfunction. Prog. Cardiovasc. Dis. 2013, 55, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Ragosta, M. The Table of Truth: Value of Coronary Angiography in the Evaluation of Patients with Heart Failure Syndromes. Cardiovasc. Revascularization Med. 2019, 20, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitts, W.R.; Lange, R.A.; David Hillis, L.; Cigarroa, J.E. Coronary arterial anatomy in patients with left ventricular systolic dysfunction without chest pain or previous myocardial infarction. Am. J. Cardiol. 1998, 82, 1530–1531. [Google Scholar] [CrossRef]
- Silva, F.; Borges, T.; Ribeiro, A.; Mesquita, R.; Laszczynska, O.; Magalhães, D.; Silva, J.C.; Azevedo, A.; Bettencourt, P. Heart failure with reduced ejection fraction: Should we submit patients without angina to coronary angiography? Int. J. Cardiol. 2015, 190, 131–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doukky, R.; Shih, M.J.; Rahaby, M.; Alyousef, T.; Abusin, S.; Ansari, N.H.; Kelly, R.F. A simple validated clinical tool to predict the absence of coronary artery disease in patients with systolic heart failure of unclear etiology. Am. J. Cardiol. 2013, 112, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlett, J.G.; Stebbins, A.; Petrie, M.C.; Jhund, P.S.; Castelvecchio, S.; Cherniavsky, A.; Sueta, C.A.; Roy, A.; Pina, I.L.; Wurm, R.; et al. CABG Improves Outcomes in Patients with Ischemic Cardiomyopathy: 10-Year Follow-Up of the STICH Trial. JACC Hear. Fail. 2019, 7, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; MacH, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall (N = 536) | Non-Significant Coronary Stenosis (N = 401) | Significant Coronary Stenosis (N = 135) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 64.4 (56.5–71.8) | 64.3 (55.5–71.9) | 64.7 (58.0–71.5) | 0.189 |
| Age ≤ 50 years | 63 (11.8) | 57 (14.2) | 6 (4.4) | 0.002 |
| Female sex | 114 (21.3) | 97 (24.2) | 17 (12.6) | 0.004 |
| Cardiovascular risk factors | ||||
| Past or current smoker | 335 (62.5) | 235 (58.6) | 100 (74.1) | 0.001 |
| Hypertension | 377 (70.5) | 273 (68.1) | 104 (77.6) | 0.036 |
| Diabetes mellitus | 207 (38.6) | 127 (31.7) | 80 (59.3) | <0.001 |
| Hypercholesterolemia | 244 (45.5) | 161 (40.2) | 83 (61.5) | <0.001 |
| Obesity (BMI ≥ 30 kg/m2) | 159 (33.1) | 120 (33.2) | 39 (33.1) | 0.984 |
| Medical history | ||||
| Heart failure | 106 (19.9) | 73 (18.3) | 33 (24.6) | 0.109 |
| ECG | ||||
| Atrial fibrillation/flutter | 110 (20.5) | 95 (23.7) | 15 (11.1) | 0.002 |
| Left bundle branch block | 171 (32.4) | 135 (34.1) | 36 (27.3) | 0.147 |
| Right bundle branch block | 36 (6.8) | 25 (6.3) | 11 (8.3) | 0.425 |
| Presence of Q wave | 46 (8.7) | 20 (5.0) | 26 (19.7) | <0.001 |
| Laboratory findings | ||||
| eGFR (mL/min per 1.73 m2) | 79.4 (59.7–93.9) | 81.1 (60.1–95.7) | 76.8 (56.5–87.6) | 0.011 |
| Echocardiography | ||||
| Left ventricular ejection fraction | 31 (25–37) | 31 (25–37) | 30 (25–37) | 0.547 |
| Segmentary alterations | 136 (26.5) | 78 (20.3) | 58 (45.0) | <0.001 |
| Variable | Overall (N = 135) | 1-Vessel Disease (N = 54) | 2- or 3-Vessel Disease (N = 81) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 64.7 (58.0–71.5) | 64.6 (56.7–70.4) | 64.9 (60.7–72.6) | 0.305 |
| Age ≤ 50 years | 6 (4.4) | 3 (5.6) | 3 (3.7) | 0.683 |
| Female sex | 17 (12.6) | 10 (18.5) | 7 (8.6) | 0.090 |
| Cardiovascular risk factors | ||||
| Past or current smoker | 100 (74.1) | 36 (66.7) | 64 (79.0) | 0.109 |
| Hypertension | 104 (77.6) | 43 (79.6) | 61 (76.3) | 0.645 |
| Diabetes mellitus | 80 (59.3) | 32 (59.3) | 48 (59.3) | 1.000 |
| Hypercholesterolemia | 83 (61.5) | 38 (70.4) | 45 (55.6) | 0.083 |
| Obesity (BMI ≥30 kg/m2) | 39 (33.1) | 15 (31.3) | 24 (34.3) | 0.731 |
| Medical history | ||||
| Heart failure | 33 (24.6) | 13 (24.1) | 20 (25.0) | 0.903 |
| ECG | ||||
| Atrial fibrillation/flutter | 15 (11.1) | 4 (7.4) | 11 (13.6) | 0.264 |
| Left bundle branch block | 36 (27.3) | 16 (30.2) | 20 (25.3) | 0.538 |
| Right bundle branch block | 11 (8.3) | 5 (9.4) | 6 (7.6) | 0.755 |
| Presence of Q wave | 26 (19.7) | 9 (17.0) | 17 (21.5) | 0.520 |
| Laboratory findings | ||||
| eGFR (mL/min per 1.73 m2) | 76.8 (56.5–87.6) | 74.1 (52.9–84.1) | 77.6 (59.9–90.2) | 0.329 |
| Echocardiography | ||||
| Left ventricular ejection fraction | 30 (25–37) | 30 (25–38) | 30 (27–36) | 0.652 |
| Segmentary alterations | 58 (45.0) | 20 (37.7) | 38 (50.0) | 0.168 |
| Angiography | ||||
| Significant coronary stenosis in left main coronary artery | 13 (9.6) | 3 (5.6) | 10 (12.4) | 0.190 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age ≤ 50 years | 0.28 (0.12–0.67) | 0.004 | 0.38 (0.15–0.98) | 0.045 |
| Female sex | 0.45 (0.26–0.79) | 0.005 | 0.40 (0.22–0.75) | 0.004 |
| Past or current smoker | 2.02 (1.31–3.11) | 0.001 | - | - |
| Hypertension | 1.63 (1.03–2.57) | 0.037 | - | - |
| Diabetes mellitus | 3.14 (2.10–4.69) | <0.001 | 2.33 (1.46–3.72) | <0.001 |
| Hypercholesterolemia | 2.38 (1.59–3.55) | <0.001 | 2.22 (1.38–3.56) | 0.001 |
| Atrial fibrillation/flutter | 0.40 (0.22–0.72) | 0.002 | 0.31 (0.16–0.59) | <0.001 |
| Presence of Q wave | 4.62 (2.48–8.61) | <0.001 | 4.85 (2.30–10.22) | <0.001 |
| eGFR ≤ 30 mL/min per 1.73 m2 | 3.09 (1.14–8.41) | 0.027 | - | - |
| Segmentary alterations | 3.22 (2.10–4.93) | <0.001 | 2.47 (1.52–4.00) | <0.001 |
| Variable | Overall (N = 536) | Low Risk (N = 89) | Intermediate Risk (N = 395) | High Risk (N = 52) | p Value |
|---|---|---|---|---|---|
| Age ≤ 50 years | 63 (11.8) | 37 (41.6) | 26 (6.6) | 0 (0.0) | <0.001 |
| Atrial fibrillation/flutter | 110 (20.5) | 38 (42.7) | 69 (17.5) | 3 (5.8) | <0.001 |
| Female sex | 114 (21.3) | 41 (46.1) | 70 (17.7) | 3 (5.8) | <0.001 |
| Hypercholesterolemia | 244 (45.5) | 6 (6.7) | 194 (49.1) | 44 (84.6) | <0.001 |
| Diabetes mellitus | 207 (38.6) | 2 (2.3) | 160 (40.5) | 45 (86.5) | <0.001 |
| Segmentary alterations | 136 (25.4) | 3 (3.4) | 89 (22.5) | 44 (84.6) | <0.001 |
| Presence of Q wave | 46 (8.6) | 0 (0.0) | 20 (5.1) | 26 (50.0) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peiró, Ó.M.; Ferrero, M.; Romeu, A.; Carrasquer, A.; Bonet, G.; Mohandes, M.; Pernigotti, A.; Bardají, A. Performance of Coronary Angiography in the Detection of Coronary Artery Disease in Patients with Systolic Left Ventricular Dysfunction and No Prior Ischemic Heart Disease. J. Clin. Med. 2022, 11, 1097. https://doi.org/10.3390/jcm11041097
Peiró ÓM, Ferrero M, Romeu A, Carrasquer A, Bonet G, Mohandes M, Pernigotti A, Bardají A. Performance of Coronary Angiography in the Detection of Coronary Artery Disease in Patients with Systolic Left Ventricular Dysfunction and No Prior Ischemic Heart Disease. Journal of Clinical Medicine. 2022; 11(4):1097. https://doi.org/10.3390/jcm11041097
Chicago/Turabian StylePeiró, Óscar M., Maria Ferrero, Alba Romeu, Anna Carrasquer, Gil Bonet, Mohsen Mohandes, Alberto Pernigotti, and Alfredo Bardají. 2022. "Performance of Coronary Angiography in the Detection of Coronary Artery Disease in Patients with Systolic Left Ventricular Dysfunction and No Prior Ischemic Heart Disease" Journal of Clinical Medicine 11, no. 4: 1097. https://doi.org/10.3390/jcm11041097
APA StylePeiró, Ó. M., Ferrero, M., Romeu, A., Carrasquer, A., Bonet, G., Mohandes, M., Pernigotti, A., & Bardají, A. (2022). Performance of Coronary Angiography in the Detection of Coronary Artery Disease in Patients with Systolic Left Ventricular Dysfunction and No Prior Ischemic Heart Disease. Journal of Clinical Medicine, 11(4), 1097. https://doi.org/10.3390/jcm11041097






