Abnormal Ultrasonographic Findings of Acellular Dermal Matrix in Implant-Based Breast Reconstruction: Correlations with Histopathology
Abstract
:1. Introduction
2. Methods
2.1. Patients and Clinical Data Collection
2.2. Ultrasound Examinations and Analysis
2.3. Tissue Sample Collection
2.4. Statistical Analyses
3. Results
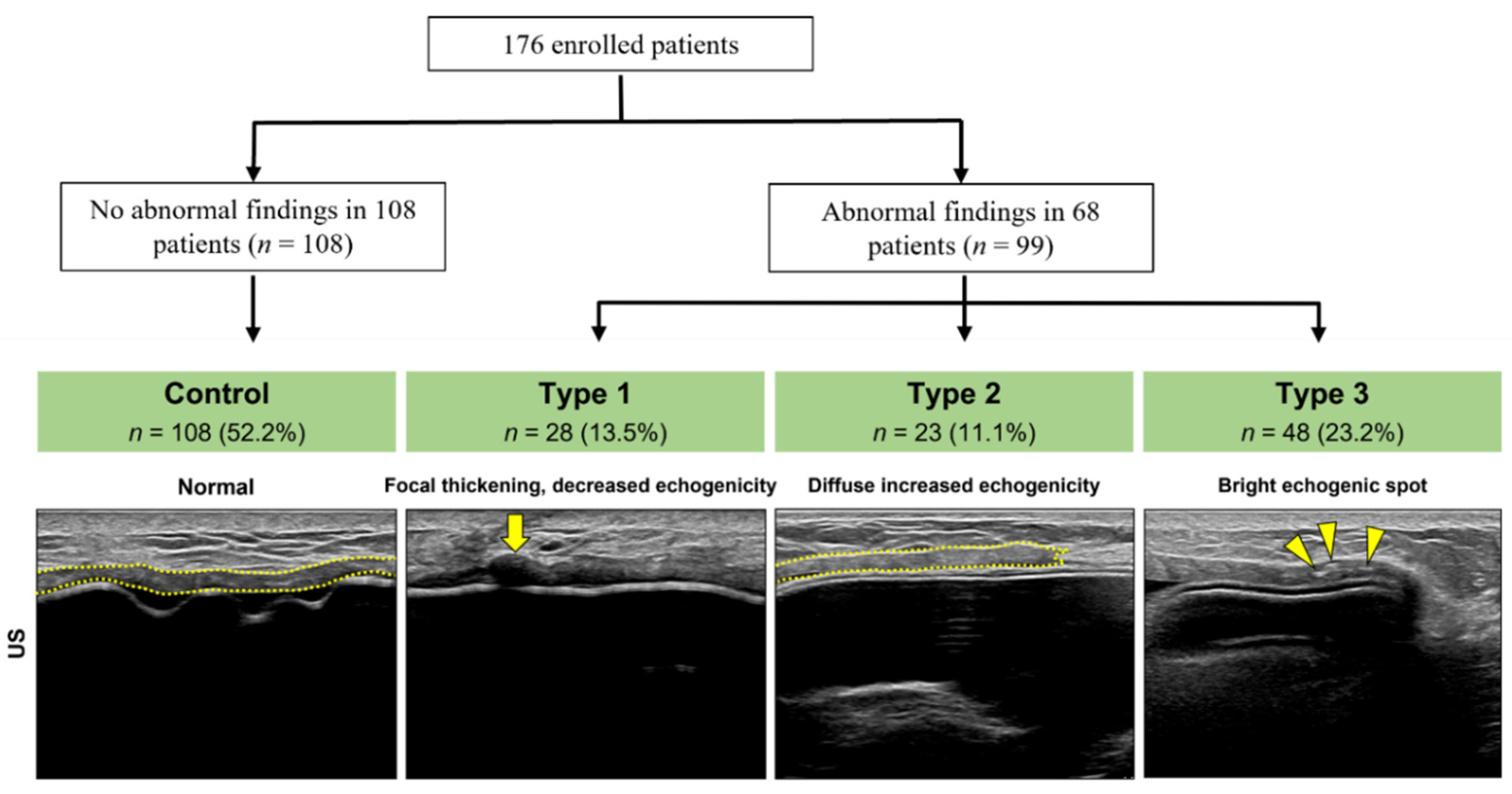
3.1. Abnormal US Findings Classification
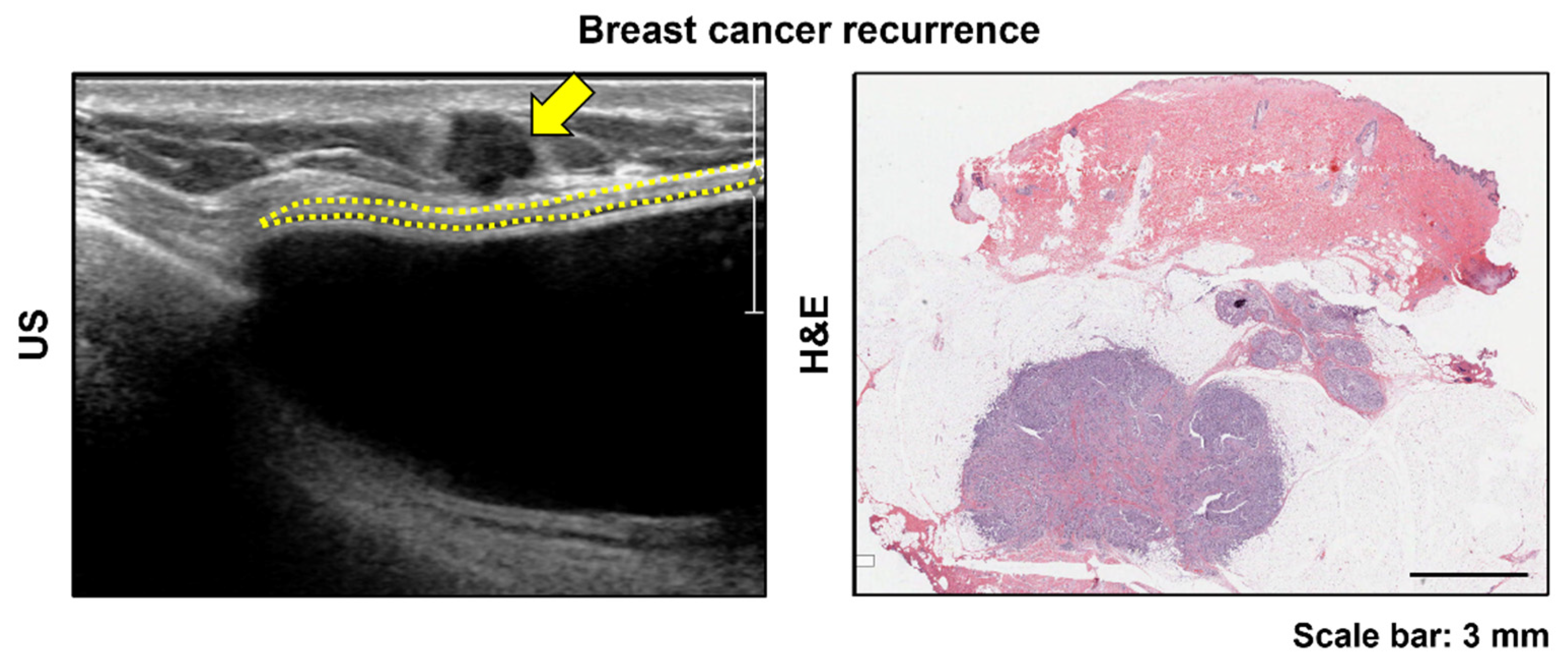
3.2. Histologic Findings by Type
3.3. Patient Characteristics and Clinical Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albornoz, C.R.; Bach, P.B.; Mehrara, B.J.; Disa, J.J.; Pusic, A.L.; McCarthy, C.M.; Cordeiro, P.G.; Matros, E. A paradigm shift in U.S. Breast reconstruction: Increasing implant rates. Plast. Reconstr. Surg. 2013, 131, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zenn, M.R.; Salzberg, C.A. A Direct Comparison of Alloderm-Ready to Use (RTU) and DermACELL in Immediate Breast Implant Reconstruction. Eplasty 2016, 16, e23. [Google Scholar] [PubMed]
- Rebowe, R.E.; Allred, L.J.; Nahabedian, M.Y. The Evolution from Subcutaneous to Prepectoral Prosthetic Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1797. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.U.; Bobr, A.; Torres-Mora, J. Radiologic-Pathologic Correlation: Acellular Dermal Matrix (Alloderm®) Used in Breast Reconstructive Surgery. J. Clin. Imaging Sci. 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Vidya, R.; Iqbal, F.M. A Guide to Prepectoral Breast Reconstruction: A New Dimension to Implant-based Breast Reconstruction. Clin. Breast Cancer 2017, 17, 266–271. [Google Scholar] [CrossRef]
- Gandhi, A.; Barr, L.; Johnson, R. Bioprosthetics: Changing the landscape for breast reconstruction? Eur. J. Surg. Oncol. 2013, 39, 24–25. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Koolen, P.G.; Ashraf, A.A.; Kim, K.; Mureau, M.A.; Lee, B.T.; Lin, S.J. Acellular Dermal Matrix in Reconstructive Breast Surgery: Survey of Current Practice among Plastic Surgeons. Plast. Reconstr. Surg. Glob. Open 2015, 3, e381. [Google Scholar] [CrossRef]
- Macadam, S.A.; Lennox, P.A. Acellular dermal matrices: Economic considerations in reconstructive and aesthetic breast surgery. Clin. Plast. Surg. 2012, 39, 187–216. [Google Scholar] [CrossRef]
- Nguyen, T.J.; Carey, J.N.; Wong, A.K. Use of human acellular dermal matrix in implant- based breast reconstruction: Evaluating the evidence. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Park, S.O.; Chang, H.; Jin, U.S. Inhibition Mechanism of Acellular Dermal Matrix on Capsule Formation in Expander-Implant Breast Reconstruction After Postmastectomy Radiotherapy. Ann. Surg. Oncol. 2018, 25, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Breuing, K.H.; Warren, S.M. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann. Plast. Surg. 2005, 55, 232–239. [Google Scholar] [CrossRef]
- Nahabedian, M.Y. Acellular dermal matrices in primary breast reconstruction: Principles, concepts, and indications. Plast. Reconstr. Surg. 2012, 130, 44s–53s. [Google Scholar] [CrossRef]
- Domenici, L.; Caputo, G.G.; Losco, L.; Di Taranto, G.; Lo Torto, F.; Pierazzi, D.M.; Governa, M.; Benedetti Panici, P.; Ribuffo, D.; Cigna, E. Muscle-Sparing Skin-Reducing Breast Reconstruction with Pre-Pectoral Implants in Breast Cancer Patients: Long-Term Assessment of Patients’ Satisfaction and Quality of Life. J. Investig. Surg. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Sigalove, S.; Sigalove, N.M.; Storm-Dickerson, T.L.; Rice, J.; Pope, N.; Maxwell, G.P. Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthetic Surg. J. 2018, 38, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.M.; Koolen, P.G.; Ganor, O.; Markarian, M.K.; Tobias, A.M.; Lee, B.T.; Lin, S.J.; Mureau, M.A. Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction? Aesthetic Plast. Surg. 2015, 39, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, C.G.; Kelly, D.A.; Wood, B.C.; Mastrangelo, S.L.; DeFranzo, A.J.; Thompson, J.T.; David, L.R.; Marks, M.W. Aesthetic outcomes of acellular dermal matrix in tissue expander/implant-based breast reconstruction. Ann. Plast. Surg. 2014, 72, S116–S120. [Google Scholar] [CrossRef]
- Laura, B.; Alice, C.; Silvia, G.; Cristiana, B.; Giuseppe, D.T.; Antonio, A.; Giuseppina, O.M. Postsurgical Ultrasound Evaluation of Patients with Prosthesis in Acellular Dermal Matrix: Results from Monocentric Experience. Int. J. Surg. Oncol. 2019, 2019, 7437324. [Google Scholar] [CrossRef]
- Lee, C.U.; Clapp, A.J.; Jacobson, S.R. Imaging Features of AlloDerm® Used in Postmastectomy Breast Reconstructions. J. Clin. Imaging Sci. 2014, 4, 19. [Google Scholar] [CrossRef]
- Seon Kim, Y. Ultrasonography Findings of AlloDerm® Used in Postmastectomy Alloplastic Breast Reconstruction: A Case Report and Literature Review. Iran. J. Radiol. 2016, 13, e38252. [Google Scholar] [CrossRef] [PubMed]
- Hangiandreou, N.J. AAPM/RSNA physics tutorial for residents. Topics in US: B-mode US: Basic concepts and new technology. Radiographics 2003, 23, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Kuba, M.G.; Giess, C.S.; Wieczorek, T.J.; Lester, S.C. Hyperechoic malignancies of the breast: Underlying pathologic features correlating with this unusual appearance on ultrasound. Breast J. 2020, 26, 643–652. [Google Scholar] [CrossRef]
- Hodgson, R.J.; O’Connor, P.J.; Grainger, A.J. Tendon and ligament imaging. Br. J. Radiol. 2012, 85, 1157–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Control (%) | Type 1 (%) | Type 2 (%) | Type 3 (%) | OR | 95% CI | p Value | |
|---|---|---|---|---|---|---|---|
| No. | 108 | 28 | 23 | 48 | |||
| Age at breast reconstruction, years | |||||||
| Mean ± SD | 45.5 ± 7.36 | 48.5 ± 9.14 | 51.6 ± 8.01 | 48.9 ± 7.74 | 1.074 | 1.022–1.129 | 0.005 * |
| BMI | |||||||
| Mean ± SD | 21.9 ± 2.87 | 23.1 ± 2.68 | 23.2 ± 3.06 | 23.5 ± 2.96 | 1.062 | 0.901–1.252 | 0.475 |
| Chemotherapy | 40 (37.0) | 14 (50.0) | 8 (34.8) | 23 (47.9) | 2.792 | 1.272–6.127 | 0.010 * |
| Radiotherapy | 13 (12.0) | 2 (7.1) | 2 (8.7) | 8 (16.7) | 0.692 | 0.236–2.026 | 0.501 |
| Implant volume, cc | |||||||
| Mean ± SD | 224.6 ± 83.58 | 255.9 ± 108.84 | 250.7 ± 113.25 | 260.4 ± 103.49 | 1.002 | 0.997–1.007 | 0.423 |
| Implant surface | |||||||
| Macrotextured | 91 (84.3) | 15 (53.6) | 18 (78.3) | 30 (62.5) | Reference | ||
| Microtextured | 3 (2.8) | 7 (25.0) | 3 (13.0) | 10 (20.8) | 5.403 | 1.284–22.736 | 0.021 * |
| Smooth | 14 (13.0) | 6 (21.4) | 2 (8.7) | 8 (16.7) | 2.302 | 0.844–6.277 | 0.103 |
| ADM type | |||||||
| Megaderm | 69 (63.9) | 7 (25.0) | 7 (30.4) | 6 (12.5) | Reference | ||
| CGCryoderm | 29 (26.9) | 20 (71.4) | 15 (65.2) | 38 (79.2) | 8.167 | 3.722–17.922 | <0.005 * |
| Alloderm | 10 (9.3) | 1 (3.6) | 1 (4.3) | 2 (4.2) | 1.659 | 0.421–6.546 | 0.470 |
| DermaCELL | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.2) | N/A | 0–0 | 1.000 |
| Complication | |||||||
| Capsular contracture | 19 (17.6) | 4 (14.3) | 5 (21.7) | 7 (14.6) | 0.799 | 0.31–2.062 | 0.643 |
| Infection | 2 (1.9) | 0 (0.0) | 1 (4.3) | 1 (2.1) | 5.410 | 0.528–55.421 | 0.155 |
| Necrosis | 2 (1.9) | 0 (0.0) | 1 (4.3) | 1 (2.1) | 0.285 | 0.021–3.947 | 0.349 |
| Wound dehiscence | 6 (5.6) | 1 (3.6) | 0 (0.0) | 2 (4.2) | 0.542 | 0.069–4.233 | 0.559 |
| Seroma | 0 (0.0) | 1 (3.6) | 2 (8.7) | 2 (4.2) | N/A | 0–0 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.S.; Lee, W.S.; Park, B.-Y.; Choi, M.; Lee, J.H.; Bae, Y.K.; Kim, I.-K. Abnormal Ultrasonographic Findings of Acellular Dermal Matrix in Implant-Based Breast Reconstruction: Correlations with Histopathology. J. Clin. Med. 2022, 11, 1057. https://doi.org/10.3390/jcm11041057
Kim YS, Lee WS, Park B-Y, Choi M, Lee JH, Bae YK, Kim I-K. Abnormal Ultrasonographic Findings of Acellular Dermal Matrix in Implant-Based Breast Reconstruction: Correlations with Histopathology. Journal of Clinical Medicine. 2022; 11(4):1057. https://doi.org/10.3390/jcm11041057
Chicago/Turabian StyleKim, Young Seon, Won Seob Lee, Bo-Yoon Park, Manki Choi, Jun Ho Lee, Young Kyung Bae, and Il-Kug Kim. 2022. "Abnormal Ultrasonographic Findings of Acellular Dermal Matrix in Implant-Based Breast Reconstruction: Correlations with Histopathology" Journal of Clinical Medicine 11, no. 4: 1057. https://doi.org/10.3390/jcm11041057
APA StyleKim, Y. S., Lee, W. S., Park, B.-Y., Choi, M., Lee, J. H., Bae, Y. K., & Kim, I.-K. (2022). Abnormal Ultrasonographic Findings of Acellular Dermal Matrix in Implant-Based Breast Reconstruction: Correlations with Histopathology. Journal of Clinical Medicine, 11(4), 1057. https://doi.org/10.3390/jcm11041057






