Management of Childhood Glaucoma Following Cataract Surgery
Abstract
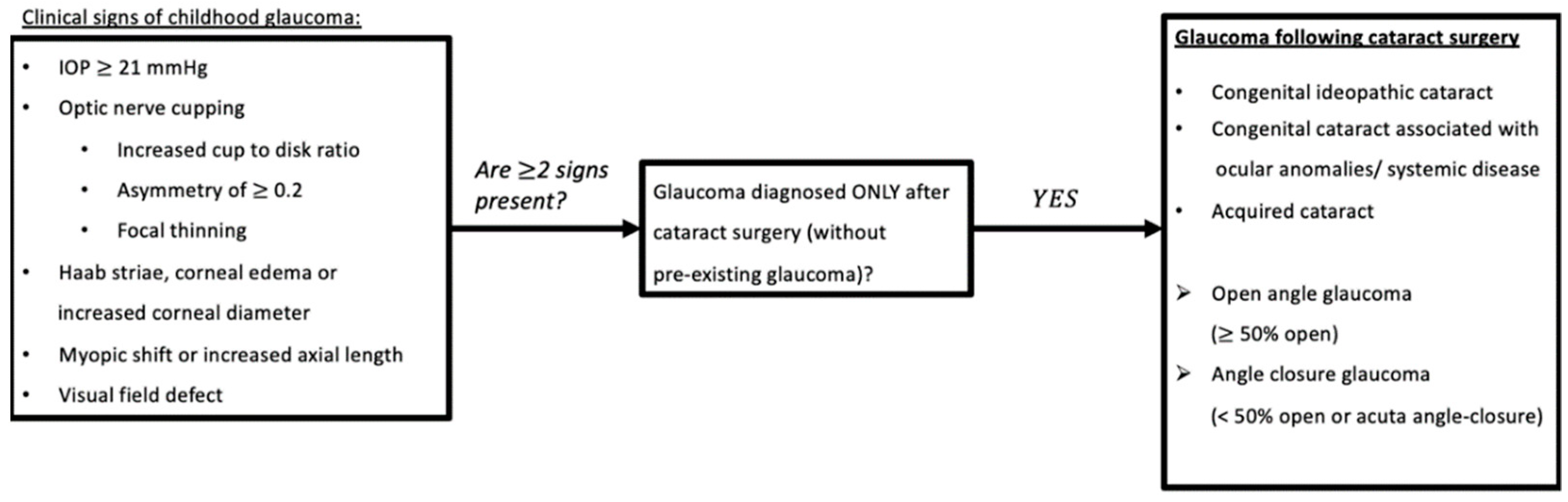
1. Introduction
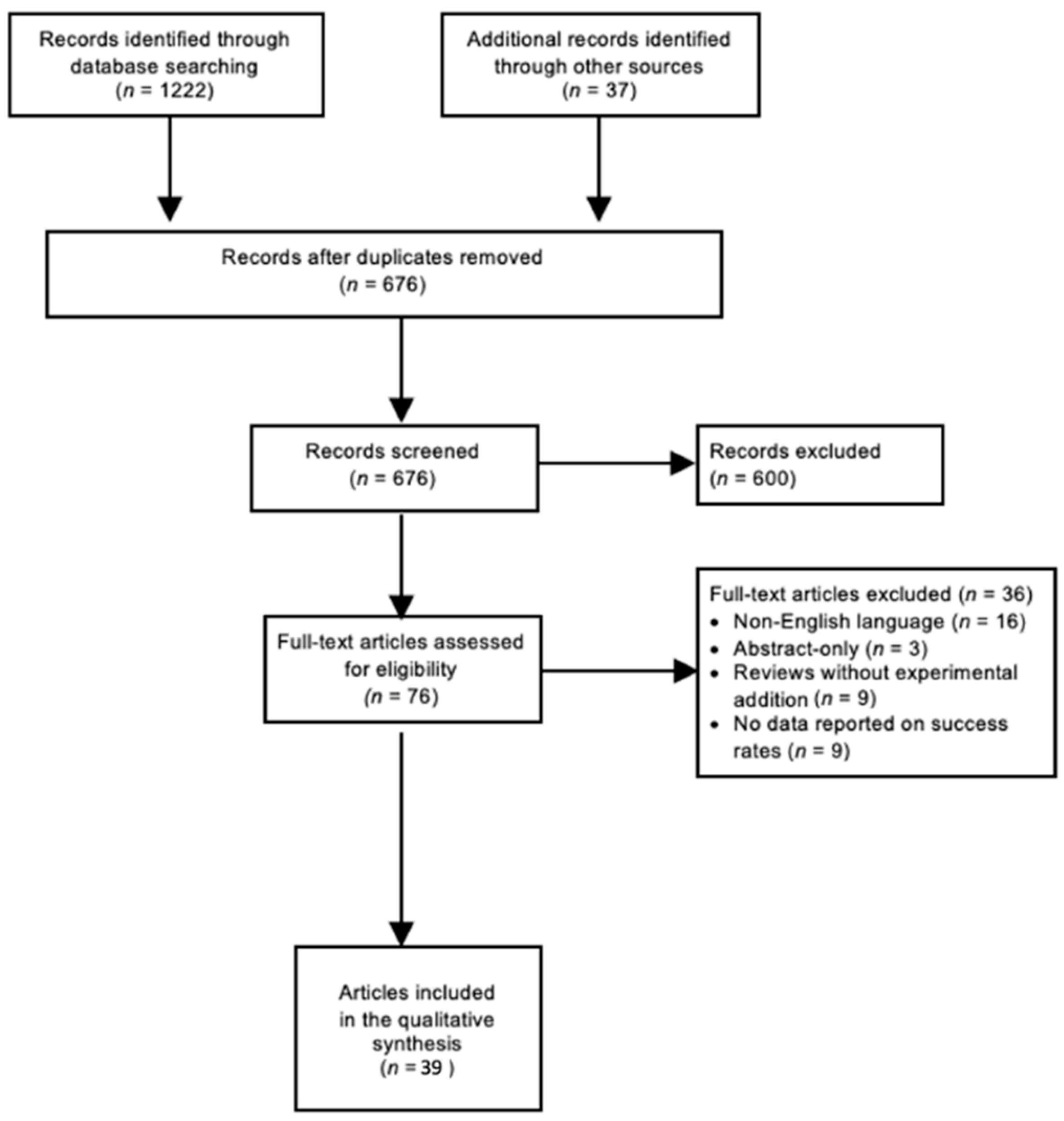
2. Materials and Methods
3. Results
3.1. Medical Treatment
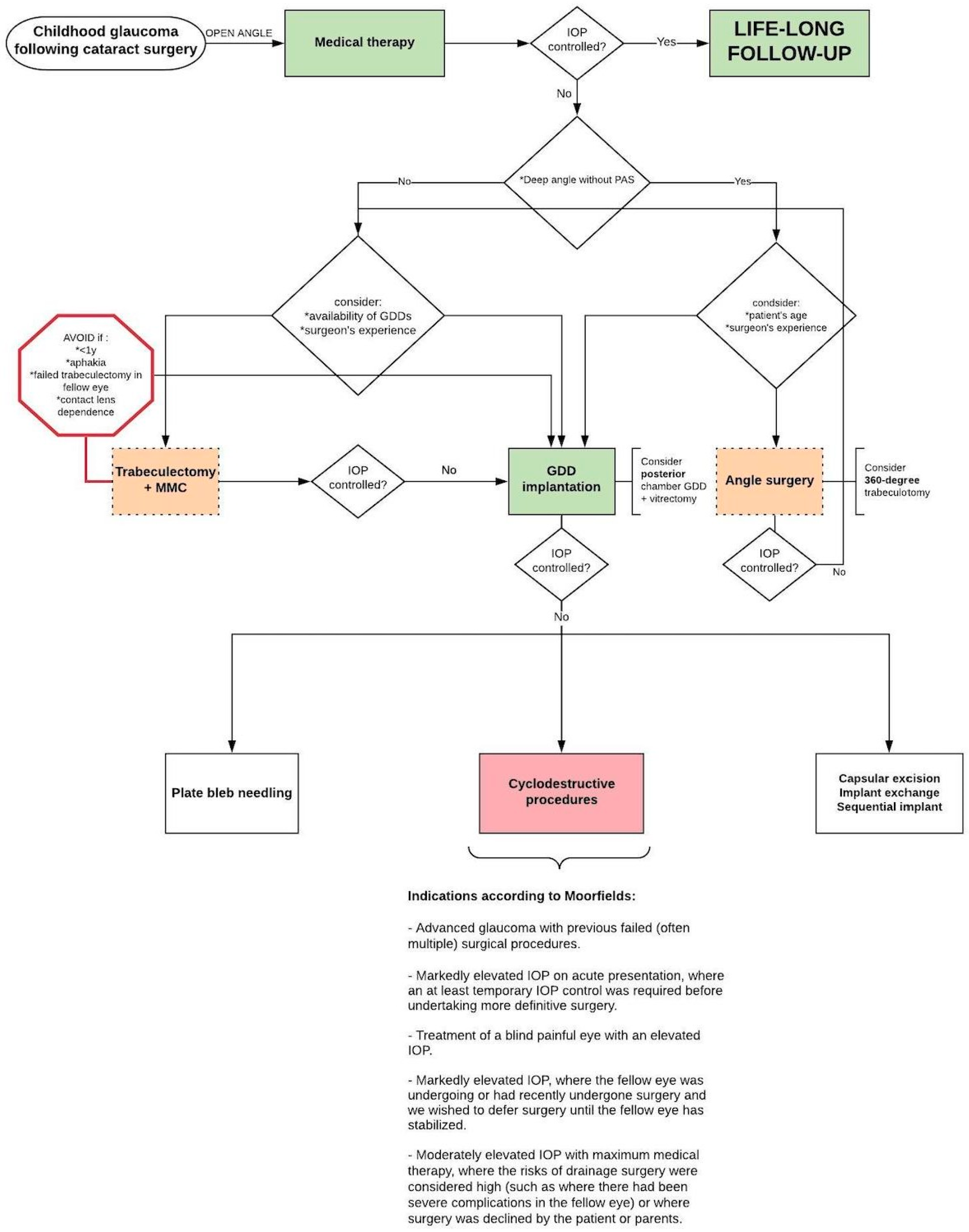
3.2. Surgical Treatment
3.2.1. Angle Surgery
3.2.2. Trabeculectomy (+Antimetabolites)
3.2.3. Glaucoma Drainage Device Implantation
3.2.4. Cyclodestructive Procedures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Roy, F.H. Comprehensive Developmental Glaucoma Classification. Ann. Ophthalmol. 2005, 37, 237–244. [Google Scholar] [CrossRef]
- Yeung, H.H.; Walton, D.S. Clinical Classification of Childhood Glaucomas. Arch. Ophthalmol. 2010, 128, 680–684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thau, A.; Lloyd, M.; Freedman, S.; Beck, A.; Grajewski, A.; Levin, A.V. New classification system for pediatric glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hoguet, A.; Grajewski, A.; Hodapp, E.; Chang, T.C.P. A retrospective survey of childhood glaucoma prevalence according to Childhood Glaucoma Research Network classification. Indian J. Ophthalmol. 2016, 64, 118–123. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Grajewski, A.L.; Papadopoulos, M. Definition, classification, differential diagnosis. In Childhood Glaucoma: The 9th Consensus Report of the World Glaucoma Association; Kugler Publications: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Rabiah, P.K. Frequency and predictors of glaucoma after pediatric cataract surgery. Am. J. Ophthalmol. 2004, 137, 30–37. [Google Scholar] [CrossRef]
- Chen, T.C.; Chen, P.P.; Francis, B.A.; Junk, A.K.; Smith, S.D.; Singh, K.; Lin, S.C. Pediatric Glaucoma Surgery. Ophthalmology 2014, 121, 2107–2115. [Google Scholar] [CrossRef]
- Swamy, B.N.; Billson, F.; Martin, F.; Donaldson, C.; Hing, S.; Jamieson, R.; Grigg, J.; Smith, J.E.H. Secondary glaucoma after paediatric cataract surgery. Br. J. Ophthalmol. 2007, 91, 1627–1630. [Google Scholar] [CrossRef]
- Freedman, S.F.; Beck, A.D.; Nizam, A.; Vanderveen, D.K.; Plager, D.A.; Morrison, D.G.; Drews-Botsch, C.D.; Lambert, S.R.; Infant Aphakia Treatment Study Group. Glaucoma-Related Adverse Events at 10 Years in the Infant Aphakia Treatment Study: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 165–173. [Google Scholar] [CrossRef]
- Walton, D.S.; Chen, T.C.; Bhatia, L.S. Aphakic Glaucoma After Congenital Cataract Surgery. Int. Ophthalmol. Clin. 2008, 48, 87–94. [Google Scholar] [CrossRef]
- Mandal, A.K.; Netland, P.A. Glaucomas in aphakia and pseudophakia after congenital cataract surgery. Indian J. Ophthalmol. 2006, 52, 93–102. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15510457 (accessed on 9 April 2019).
- Asrani, S.; Freedman, S.; Hasselblad, V.; Buckley, E.G.; Egbert, J.; Dahan, E.; Parks, M.; Johnson, D.; Maselli, E.; Gimbel, H.; et al. Does primary intraocular lens implantation prevent ‘aphakic’ glaucoma in children? J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2000, 4, 33–39. [Google Scholar] [CrossRef]
- Michael, I.; Shmoish, M.; Walton, D.S.; Levenberg, S. Interactions between Trabecular Meshwork Cells and Lens Epithelial Cells: A Possible Mechanism in Infantile Aphakic Glaucoma. Investig. Opthalmol. Vis. Sci. 2008, 49, 3981–3987. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, C.; O’Keefe, M. Paediatric aphakic glaucoma. Acta Ophthalmol. Scand. 2006, 84, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.S.C.; Fan, D.S.P.; Ng, J.S.K.; Yu, C.B.O.; Wong, C.Y.; Cheung, A.Y.K. Ocular hypertensive and anti-inflammatory responses to different dosages of topical dexamethasone in children: A randomized trial. Clin. Exp. Ophthalmol. 2005, 33, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Fenerty, C.; Grigg, J.; Freedman, S. Glaucoma Following Cataract Surgery. In Childhood Glaucoma: The 9th Consensus Report of the World Glaucoma Association; Kugler Publications: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mataftsi, A.; Haidich, A.-B.; Kokkali, S.; Rabiah, P.K.; Birch, E.; Stager, D.R.; Cheong-Leen, R.; Singh, V.; Egbert, J.E.; Astle, W.F.; et al. Postoperative Glaucoma Following Infantile Cataract Surgery: An individual patient data meta-analysis. JAMA Ophthalmol. 2014, 132, 1059–1067. [Google Scholar] [CrossRef]
- Freedman, S.F.; Lynn, M.J.; Beck, A.D.; Bothun, E.D.; Örge, F.H.; Lambert, S.R. Glaucoma-Related Adverse Events in the First 5 Years After Unilateral Cataract Removal in the Infant Aphakia Treatment Study. JAMA Ophthalmol. 2015, 133, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Solebo, A.; Cumberland, P.; Rahi, J.S. 5-year outcomes after primary intraocular lens implantation in children aged 2 years or younger with congenital or infantile cataract: Findings from the IoLunder2 prospective inception cohort study. Lancet Child Adolesc. Health 2018, 2, 863–871. [Google Scholar] [CrossRef]
- Dosunmu, E.; Freedman, S. Aphakic/pseudophakic glaucoma. In Practical Management of Pediatric Ocular Disorders and Strabismus: A Case-Based Approach; Springer: New York, NY, USA, 2016; pp. 459–470. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Philips, B. Oxford Centre for Evidence-Based Medicine–Levels of Evidence; Centre for Evidence Based Medicine: Oxford, UK, 2014. [Google Scholar]
- Baris, M.; Biler, E.D.; Yilmaz, S.G.; Ates, H.; Uretmen, O.; Kose, S. Treatment results in aphakic patients with glaucoma following congenital cataract surgery. Int. Ophthalmol. 2017, 39, 11–19. [Google Scholar] [CrossRef]
- Bhola, R.; Keech, R.V.; Olson, R.; Petersen, D.B. Long-Term Outcome of Pediatric Aphakic Glaucoma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2006, 10, 243–248. [Google Scholar] [CrossRef]
- Comer, R.M.; Kim, P.; Cline, R.; Lyons, C.J. Cataract surgery in the first year of life: Aphakic glaucoma and visual outcomes. Can. J. Ophthalmol. 2011, 46, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Spiess, K.; Calvo, J.P. Clinical Characteristics and Treatment of Secondary Glaucoma After Pediatric Congenital Cataract Surgery in a Tertiary Referral Hospital in Spain. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 57, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.L.; Trivedi, R.H.; Wilson, M.E. Intraocular pressure control with echothiophate iodide in children’s eyes with glaucoma after cataract extraction. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2015, 19, 116–118.e1. [Google Scholar] [CrossRef] [PubMed]
- Bothun, E.D.; Guo, Y.; Christiansen, S.P.; Summers, C.G.; Anderson, J.S.; Wright, M.M.; Kramarevsky, N.Y.; Lawrence, M.G. Outcome of angle surgery in children with aphakic glaucoma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2010, 14, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.D.; Lynn, M.J.; Crandall, J.; Mobin-Uddin, O. Surgical outcomes with 360-degree suture trabeculotomy in poor-prognosis primary congenital glaucoma and glaucoma associated with congenital anomalies or cataract surgery. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2011, 15, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Dao, J.B.; Sarkisian, S.R.; Freedman, S.F. Illuminated Microcatheter–facilitated 360-Degree Trabeculotomy for Refractory Aphakic and Juvenile Open-angle Glaucoma. J. Glaucoma 2014, 23, 449–454. [Google Scholar] [CrossRef]
- El Sayed, Y.M.; Elhusseiny, A.M.; Gawdat, G.I.; Elhilali, H.M. One-year results of two-site trabeculotomy in paediatric glaucoma following cataract surgery. Eye 2020, 35, 1637–1643. [Google Scholar] [CrossRef]
- Lim, M.E.; Dao, J.B.; Freedman, S.F. 360-Degree Trabeculotomy for Medically Refractory Glaucoma Following Cataract Surgery and Juvenile Open-Angle Glaucoma. Am. J. Ophthalmol. 2017, 175, 1–7. [Google Scholar] [CrossRef]
- Rojas, C.; Bohnsack, B.L. Rate of Complete Catheterization of Schlemm’s Canal and Trabeculotomy Success in Primary and Secondary Childhood Glaucomas. Am. J. Ophthalmol. 2019, 212, 69–78. [Google Scholar] [CrossRef]
- Azuara-Blanco, A.; Wilson, R.P.; Spaeth, G.L.; Schmidt, C.M.; Augsburger, J.J. Filtration procedures supplemented with mitomycin C in the management of childhood glaucoma. Br. J. Ophthalmol. 1999, 83, 151–156. [Google Scholar] [CrossRef]
- Beck, A.D.; Wilson, W.R.; Lynch, M.G.; Lynn, M.J.; Noe, R. Trabeculectomy with adjunctive mitomycin C in pediatric glaucoma. Am. J. Ophthalmol. 1998, 126, 648–657. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9822228 (accessed on 1 June 2019). [CrossRef]
- Freedman, S.F.; Mccormick, K.; Cox, T.A. Mitomycin C-augumented trabeculectomy with Postoperative Wound Modulation in Pediatric Glaucoma. J. Am. Assoc. Ped. Ophthalmol. Strabismus 1999, 3, 117–124. [Google Scholar] [CrossRef]
- Mandal, A.K.; Bagga, H.; Nutheti, R.; Gothwal, V.K.; Nanda, A.K. Trabeculectomy with or without mitomycin-C for paediatric glaucoma in aphakia and pseudophakia following congenital cataract surgery. Eye 2003, 17, 53–62. [Google Scholar] [CrossRef]
- Pakravan, M.; Homayoon, N.; Shahin, Y.; Reza, B.R.A. Trabeculectomy With Mitomycin C Versus Ahmed Glaucoma Implant With Mitomycin C for Treatment of Pediatric Aphakic Glaucoma. J. Glaucoma 2007, 16, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.K.; Plager, D.A.; Snyder, S.K.; Raiesdana, A.; Helveston, E.M.; Ellis, F.D. Surgical results of secondary glaucomas in childhood. Ophthalmology 1998, 105, 101–111. [Google Scholar] [CrossRef]
- Balekudaru, S.; Vadalkar, J.; George, R.; Vijaya, L. The use of Ahmed glaucoma valve in the management of pediatric glaucoma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2014, 18, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Banitt, M.R.; Sidoti, P.A.; Gentile, R.C.; Tello, C.; Liebmann, J.M.; Rodriguez, N.; Dhar, S. Pars Plana Baerveldt Implantation for Refractory Childhood Glaucomas. J. Glaucoma 2009, 18, 412–417. [Google Scholar] [CrossRef]
- Chen, T.C.; Bhatia, L.S.; Walton, D.S. Ahmed valve surgery for refractory pediatric glaucoma: A report of 52 eyes. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2005, 42, 274–283. [Google Scholar] [CrossRef]
- Donahue, S.P.; Keech, R.V.; Munden, P.; Scott, W.E. Baerveldt implant surgery in the treatment of advanced childhood glaucoma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 1997, 1, 41–45. [Google Scholar] [CrossRef]
- Elshatory, Y.M.; Gauger, E.H.; Kwon, Y.; Alward, W.L.M.; Boldt, H.C.; Russell, S.; Mahajan, V. Management of Pediatric Aphakic Glaucoma With Vitrectomy and Tube Shunts. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2016, 53, 339–343. [Google Scholar] [CrossRef]
- Englert, J.A.; Freedman, S.; Cox, T.A. The Ahmed Valve in refractory pediatric glaucoma. Am. J. Ophthalmol. 1999, 127, 34–42. [Google Scholar] [CrossRef]
- Kirwan, C.; O’Keefe, M.; Lanigan, B.; Mahmood, U. Ahmed valve drainage implant surgery in the management of paediatric aphakic glaucoma. Br. J. Ophthalmol. 2005, 89, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.P.; Reynolds, A.; Emond, M.J.; Barlow, W.E.; Leen, M.M. Long-term Survival of Molteno Glaucoma Drainage Devices. Ophthalmology 1996, 103, 299–305. [Google Scholar] [CrossRef]
- O’Malley Schotthoefer, E.; Yanovitch, T.L.; Freedman, S.F. Aqueous drainage device surgery in refractory pediatric glaucomas: I. Long-term outcomes. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2008, 12, 33–39. [Google Scholar] [CrossRef]
- Pakravan, M.; Esfandiari, H.; Yazdani, S.; Doozandeh, A.; Dastborhan, Z.; Gerami, E.; Kheiri, B.; Pakravan, P.; Yaseri, M.; Hassanpour, K. Clinical outcomes of Ahmed glaucoma valve implantation in pediatric glaucoma. Eur. J. Ophthalmol. 2018, 29, 44–51. [Google Scholar] [CrossRef]
- Rotsos, T.; Tsioga, A.; Andreanos, K.; Diagourtas, A.; Petrou, P.; Georgalas, I.; Papaconstantinou, D. Managing high risk glaucoma with the Ahmed valve implant: 20 years of experience. Int. J. Ophthalmol. 2018, 11, 240–244. [Google Scholar] [CrossRef]
- Spiess, K.; Calvo, J.P. Outcomes of Ahmed glaucoma valve in paediatric glaucoma following congenital cataract surgery in persistent foetal vasculature. Eur. J. Ophthalmol. 2020, 31, 1070–1078. [Google Scholar] [CrossRef]
- Geyer, O.; Segal, A.; Melamud, A.; Wolf, A. Clinical Outcomes After Ahmed Glaucoma Valve Implantation for Pediatric Glaucoma After Congenital Cataract Surgery. J. Glaucoma 2020, 30, 78–82. [Google Scholar] [CrossRef]
- Beck, A.D.; Freedman, S.; Kammer, J.; Jin, J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am. J. Ophthalmol. 2003, 136, 994–1000. [Google Scholar] [CrossRef]
- Autrata, R.; Lokaj, M. Trans-scleral diode laser cyclophotocoagulation in children with refractory glaucoma. Long-term outcomes. Scripta Med. Fac. Med. Univ. Brun. Masaryk. 2003, 76, 67–78. [Google Scholar]
- Cantor, A.J.; Wang, J.; Li, S.; Neely, D.E.; Plager, D.A. Long-term efficacy of endoscopic cyclophotocoagulation in the management of glaucoma following cataract surgery in children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.C.; Plager, D.A.; Neely, D.E.; Sprunger, D.T.; Sondhi, N.; Roberts, G.J. Endoscopic diode laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2006, 11, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.S.; Mulvihill, M.S.; Freedman, S.F. Endoscopic cyclophotocoagulation (ECP) for childhood glaucoma: A large single-center cohort experience. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2019, 23, 84.e1–84.e7. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.F.; Shah, P.; Khaw, P.T. Diode laser cyclophotocoagulation: Role in the management of refractory pediatric glaucomas. Ophthalmology 2002, 109, 316–323. [Google Scholar] [CrossRef]
- Neely, D.E.; Plager, D.A. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2001, 5, 221–229. [Google Scholar] [CrossRef]
- Schlote, T.; Grüb, M.; Kynigopoulos, M. Long-term results after transscleral diode laser cyclophotocoagulation in refractory posttraumatic glaucoma and glaucoma in aphakia. Glaucoma 2007, 246, 405–410. [Google Scholar] [CrossRef]
- Hirabayashi, M.T.; Lee, D.; King, J.T.; Thomsen, S.; An, J.A. Comparison of Surgical Outcomes of 360° Circumferential Trabeculotomy Versus Sectoral Excisional Goniotomy with the Kahook Dual Blade At 6 Months. Clin. Ophthalmol. 2019, 13, 2017–2024. [Google Scholar] [CrossRef]
- Laroche, D.; Rickford, K.; Sakkari, S. Case report: Cataract extraction/lensectomy, excisional goniotomy and transscleral cyclophotocoagulation: Affordable combination MIGS for plateau iris glaucoma. J. Natl. Med. Assoc. 2022. [Google Scholar] [CrossRef]
- Laroche, D.; Nkrumah, G.; Ng, C. Combination microinvasive glaucoma surgery: 23-gauge cystotome goniotomy and intra-scleral ciliary sulcus suprachoroidal microtube surgery in refractory and severe glaucoma: A case series. Indian J. Ophthalmol. 2020, 68, 2557–2561. [Google Scholar] [CrossRef]
- Laroche, D.; Okaka, Y.; Ng, C. A Novel Low Cost Effective Technique in Using a 23 Gauge Straight Cystotome to Perform Goniotomy: Making Micro-invasive Glaucoma Surgery (MIGS) Accessible to the Africans and the Diaspora. J. Natl. Med. Assoc. 2019, 111, 193–197. [Google Scholar] [CrossRef]
- Tanito, M.; Sano, I.; Ikeda, Y.; Fujihara, E. Short-term results of microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery in Japanese eyes: Initial case series. Acta Ophthalmol. 2017, 95, e354–e360. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery. Clin. Ophthalmol. 2018, 12, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.P.; Palmberg, P.F. Needling Revision of Glaucoma Drainage Device Filtering Blebs. Ophthalmology 1997, 104, 1004–1010. [Google Scholar] [CrossRef]
- Zuo, W.; Lesk, M.R. Surgical Outcome of Replacing a Failed Ahmed Glaucoma Valve by a Baerveldt Glaucoma Implant in the Same Quadrant in Refractory Glaucoma. J. Glaucoma 2018, 27, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Tello, C.; Sidoti, P.A.; Ritch, R.; Liebmann, J.M. Sequential Glaucoma Implants in Refractory Glaucoma. Am. J. Ophthalmol. 2010, 149, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Adams, G.G.W.; Dahlmann-Noor, A. Medical Management of Children with Congenital/Infantile Cataract Associated with Microphthalmia, Microcornea, or Persistent Fetal Vasculature. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2019, 56, 43–49. [Google Scholar] [CrossRef]
| Author, Year, Study Design (LOE) Reference | Inclusion and Exclusion Criteria | Mean Pre-Treatment IOP ± SD (mmHg) | Mean Age at GDx ± SD (Years) | n Eyes (a-p) | Success Criteria | Success Rate with Medications Alone (%) Number of Drugs (% of Cases) | Need for Surgery (%) Number of Operations (% of Cases) | Mean (*) Follow-Up ± SD (Years) |
|---|---|---|---|---|---|---|---|---|
| Bhola et al. (2006), retrospective cohort study (2b) [24] | Inclusion:
Exclusion:
| 32 ± 6 | 7 | 55 (55-0) | IOP ≤ 25 mmHg | 73 1–2 (36) 3 (33) ≥4 (31) Β-blockers, Cholinergic agents, Adrenergic agonists, Carbonic anhydrase inhibitors, Prostaglandin analogues. | 27 1 (40) 2–3 (53) 4–6 (7) Goniotomy, trabeculotomy, trabeculectomy ± MMC, GDD implantation, cyclodestruction. | 18.7 ± 8 |
| Comer et al. (2011), retrospective cohort study (2b) [25] | Inclusion:
| 28.6 ± 5.9 | 2.6 | 18 (18-0) | IOP ≤ 20 mmHg | 17 Not further specified | 83 ≥ 2 (61) GDD implantation, trabeculectomy + MMC, goniotomy, cyclodestruction. | 6.5 |
| Kraus et al. (2015), retrospective case series (4) [27] | Inclusion:
| 32.1 | 3.2 | 32 (27-5) | IOP-lowering effect of:
| 41 37 EI | 12.5 rabeculotomy, GDD implantation | 7.88 |
| Baris et al. (2019), retrospective cohort study (2b) [23] | Inclusion:
| 29.8 ± 14.8 | 1 ± 2.1 | 40 (40-0) | IOP < 21 mmHg | 50 1 (75) 2 (20) 3 (5) First choice: dorzolamide-timolol combination; Second choice: prostaglandin analogues. | 50 1 (30) 2 (20) ≥3 (50) First choice: trabeculectomy +MMC (0.2 mg/mL for 4 min); Second choice: GDD implantation; Third choice: cyclodestruction | 6.6 ± 2.6 |
| Spiess et al (2020), retrospective cohort study (2b) [26] | Inclusion:
| 29.1 ± 5.6 | - | 58 (47-9) | IOP < 21 mmHg with or without medication | 41 40% monotherapy 60% combination therapy The most frequently prescribed drugs were beta-blockers (82%), followed by carbonic anhydrase inhibitors, prostaglandins, and alpha-2 adrenergic agonists. | 59 70% tube implantation 24% trabeculectomy 6% peripheral iridotomy | 4.6 * |
| Author, Year, Study Design (LOE) Reference | Inclusion and Exclusion Criteria | Mean Pre-Treatment IOP ± SD (mmHg) | Mean Age at GDx ± SD (y) | Mean (* Median) Age at Glaucoma Surgery ± SD (y) | n Eyes (a-p) | Procedure | Success Criteria | Success Rate (%) | Mean (*) Follow-Up ± SD (y) | Factors Affecting Treatment Outcomes % of Eyes That Had Prior Glaucoma Surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2004), retrospective cohort study (2b) [10] | Inclusion:
| - | - | - | 24 (24-0) | Goniotomy and rigid-probe Trabeculectomy Ab externo | IOP ≤ 21 mmHg with and without medications and with no need for further surgery. | 16 | 8.6 ± 7.6 | - Not specified |
| Bothun et al. (2010), retrospective cohort study (2b) [28] | Inclusion:
| 35 ± 10 | - | 3.1 | 14 (14-0) | Goniotomy and/or rigid-probe trabeculotomy (lateral 180° initially, repeat nasal 180°) Ab externo | IOP ≤ 24 mmHg with or without topical medication; a lack of sight-threatening complication: and avoidance of trabeculectomy or GDD. | 57 (after a mean of 1.4 angle surgeries per eye) 43 (after a single procedure) | 4.7 | Eyes with initial trabeculotomy required fewer procedures than those with an initial goniotomy. 0% |
| Beck et al. (2011), retrospective case series (4) [29] | Inclusion:
Exclusion:
| 33.0 ± 7.2 | - | * 5.0 | 4 (4-0) | 360-degree suture trabeculotomy Ab externo | IOP < 22 mmHg with and without medication. | 75 | * 1.6 | - 0% |
| Dao et al. (2014), retrospective case series (4) [30] | Inclusion:
Exclusion:
| 35.4 ± 4.7 | - | 3.1 | 13 (10-3) All open angle | 360° microcatheter trabeculotomy Ab externo | IOP ≤ 22 mmHg with 30% reduction, without disease progression, oral glaucoma medications or additional glaucoma surgery. | 62 | 1.4 | - 0% |
| Lim et al. (2017), retrospective case series (2b) [32] | Inclusion:
Exclusion:
| 31.5 ± 7.5 | 3.3 ± 3.9 | 5.6 ± 5.6 | 25 (19-6) | 360° microcatheter trabeculotomy Ab externo | IOP ≤ 22 mmHg and 20% reduction without additional glaucoma surgery or devastating complication. | 72 | 2.7 ± 2.2 | Lens status (p = 0.88) 0% |
| El Sayed et al. (2020), prospective cohort study (2b) [31] | Inclusion:
Exclusion:
| 26.8 ± 8.2 | - | 5.73 ± 1.79 | 29 (16-13) | Two-site rigid probe trabeculotomy 180–360° Ab externo | IOP < 23 mmHg or 30% IOP reduction, on the same or fewer number of medications at 1 year, without the need for another glaucoma procedure | 89.6 (51.7% without medications) | 1.4 | No significant difference in the final IOP of aphakic and pseudophakic eyes. 0% |
| Rojas et al. (2020), retrospective case series [33] | Inclusion:
| 27.1 ± 7 | - | 7.8 ± 5.8 | 15 (12-3) | 360° microcatheter trabeculotomy Ab externo | 5 < IOP < 20 without additional surgery | 93 | 3.3 ± 2.4 | - 0% |
| Author, Year, Study Design (LOE) Reference | Inclusion and Exclusion Criteria | Mean Pre-Treatment IOP ± SD (mmHg) | Mean Age at GDx ± SD (y) | Mean (*) Age at Glaucoma Surgery ± SD (y) | n eyes (a-p) | Antimetabolites | Success Criteria | Success Rate (%) | Mean (*) Follow-Up ± sd (y) | Factors Affecting Treatment Outcomes % of Eyes That Had Prior Glaucoma Surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| Beck et al. (1998), retrospective case series (4) [35] | Inclusion:
| 35.8 ± 8.0 | - | 7.6 | 9 (7-2) | MMC 0.25 mg/mL for 5 min | IOP ≤ 22 mmHg with and without medication, no evidence of glaucoma progression, no further need of glaucoma surgery. | 78 | 2.5 ± 1.3 | Age < 1 y (p = 0.0005) Aphakia (p = 0.0364) Anterior segment dysgenesis/aniridia (p = 0.49) Not specified |
| Wallace et al. (1998) retrospective cohort study (2b) [39] | Inclusion:
PCG. | 35.9 | 6.1 | 8.7 | 13 (13-0) | MMC 0.2 to 0.4 mg/mL for 4 min | IOP ≤ 25 mmHg without medications and IOP ≤ 21 mmHg with medications. | 62 | 4.2 | - Not specified |
| Azuara-Blanco et al. (1999), retrospective case series (4) [34] | Inclusion:
| 35.7 ± 10.5 | - | 5.7 ± 5.0 | 8 (8-0) | MMC 0.4 mg/mL for 1–5 min | Absolute success: IOP < 21 mmHg with no antiglaucoma medications, with apparently stable glaucoma and absence of severe complications. Relative success: No performance of or recommendation for further glaucoma surgery and absence of severe complications. | 0 33 | 1.6 ± 1.2 | Phakic cases (PCG) seemed to have a better outcome than aphakic cases. 12.5% |
| Freedman et al. (1999), retrospective case series (4) [36] | Inclusion:
Exclusion: - | 35.6 | - | 7.2 | 7 (7-0) | MMC 0.4 mg/mL for 3–5 min and postoperative 5-fluorouracil, laser suture or both | 4 mmHg < IOP < 16 mmHG without further glaucoma surgery or devastating complication. | 29 | 1.9 | Age < 1 y and aphakia (vs. phakic status in PCG and JOAG), taken together. (p = 0.013) The addition of postoperative 5-fluorouracil and suture lysis did not provide improvement and may have increased complication rate. 42.8% |
| Mandal et al. (2003), retrospective case series (4) [37] | Inclusion:
Exclusion: - | 34.2 ± 8.9 | 9.6 | 9.9 ± 9.0 | 23 (21-2) | MMC 0.4 mg/mL for 3 min | Complete success: 6 mmHg < IOP < 21 mmHg without medication. Qualified success: 6 mmHg < IOP < 21 mmHg, with or without 1 topical medication, no further need of glaucoma surgery, and no visually devastating complication. | 37 58 | 2.0 ± 1.5 | - Not specified |
| Chen et al. (2004), retrospective cohort study (2b) [10] | Inclusion:
| - | - | - | 61 (61-0) | MMC (n = 43) 5-fluorouracil (n = 17) None (n = 1) | IOP ≤ 21 mmHg with and without medications and no need for further surgery. | 25 | 8.6 ± 7.6 | - Not specified |
| Pakravan et al. (2007), prospective randomized clinical trial (1) [37] | Inclusion:
| 31 ± 10.7 | - | 9.1 ± 4.1 | 15 (15-0) | MMC 0.02% for 2 min | Absolute success: 5 mmHg ≤ IOP < 21 without medications. Qualified success: 5 mmHg ≤ IOP < 21 with ≤2 medications. Overall success: absolute + qualified success. mmHg ≤ IOP < 21 | 33 40 73 | 1.2 ± 0.9 | - 0% |
| Baris et al. (2019), retrospective cohort study (2b) [23] | Inclusion:
Exclusion:
| 29.8 ± 14.8 | 1 ± 2.1 | - | 20 | MMC 0.2 mg/mL for 4 min | Complete success: IOP < 21 mmHg without medication. Qualified success: IOP < 21 mmHg with and without medication. | 5 30 | 6.6 ± 2.6 | - 0% |
| Author, Year, Study Design (LOE) Reference | Inclusion and Exclusion Criteria | Mean Pre-Treatment IOP ± SD (mmHg) | Mean Age at GDx ± SD (y) | Mean (*) Age at Glaucoma Surgery ± SD (y) | Number of Eyes (a-p) | Device ± Antimetabolites | Success Criteria | Success Rate (%) | Mean (*) Follow-Up ± SD (y) | Factors Affecting Treatment Outcomes % of Eyes That Had Prior Glaucoma Surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| Donahue et al. (1997), retrospective cohort study (2b) [43] | Inclusion:
Exclusion: - | 33 | - | - | 10 (9-1) | Baerveldt 350 mm | Complete success: No further reoperation, no decrease in vision, and IOP at last follow-up < 21 mmHg, without complication not associated with tube failure. Qualified success: With and without medication necessary to bring IOP < 21 mmHg, with or without complication not associated with tube failure. | 40 70 | 1.6 | It appeared that the aphakic patients who has had multiple previous procedures were at higher risk for shunt failure. 40% |
| Wallace et al. (1998), retrospective cohort study (2b) [39] | Inclusion:
PCG. | 35.9 | 6.1 | 8.7 | 9 | Molteno | IOP ≤ 25 mmHg without medication and IOP ≤ 21 mmHg with medication. | 67 at 6 m 33 at 1 y | 4.2 | - Not specified |
| Englert et al. (1999), retrospective case series (4) [45] | Inclusion:
Exclusion: - | 32.8 ± 7.5 | - | - | 7 (7-0) | Ahmed S-2 model in the superotemporal quadrant | IOP ≤ 21 mmHg without medication without further surgery without visually devastating complication | 86 | 1.0 ± 0.7 | Previous cycloablation was not a significant risk factor for failure. 14.2% |
| Chen et al. (2004), retrospective cohort study (2b) [10] | Inclusion:
Exclusion:
| - | - | - | 34 | Ahmed (32 eyes) Molteno (2 eyes) | IOP ≤ 21 mmHg with and without medications and no need for further surgery. | 44 | 8.6 | - Not specified |
| Chen et al. (2005), retrospective case series (4) [42] | Inclusion:
Exclusion: - | 38.1 ± 6.4 | - | 4.9 ± 6.5 | 19 | Ahmed S-2 model | IOP ≤ 22 mmHg with or without medications, without further surgery, without visually devastating complications | 68 (75 if GDD implantation was the initial surgery) | 2.2 ± 1.8 | - 57.9% |
| Kirwan et al. (2005), retrospective case series (4) [46] | Inclusion:
Exclusion: - | 31.1 | - | 8 | 19 | Ahmed S-2 model In 10 eyes: +MMC 0.5 mg/mL for 3 min | IOP ≤ 15 with and without medical therapy. | 95 | 2.7 | - 47.4% |
| Pakravan et al. (2007), prospective randomized control trial (1) [38] | Inclusion:
| 31 ± 7.5 | - | 10.9 ± 5.1 | 15 (15-0) | Ahmed + MMC 0.2 mg/mL for 2 min | Absolute success: 5 mmHg ≤ IOP < 21 mmHg without medications. Qualified success: 5 mmHg ≤ IOP < 21 mmHg with ≤ 2 medications. Overall success: absolute + qualified success. | 20 67 87 | 1.1 ± 0.8 | - 0% |
| O’Malley Schotthoefer et al. (2008), retrospective cohort study (2b) [48] | Inclusion:
Exclusion: - | 36 | - | 4.3 * | 41 (38-3) | Ahmed S-2 or FP-7 (n = 16) Baerveldt (n = 22) Molteno (n = 3) All in superotemporal quadrant. PP + V in 5 eyes | IOP ≤ 21 mmHg without medication, without further surgery, without visually devastating complications. | 90 at 1 y 82 at 2 y 55 at 10 y | 0.5 * | Better reported outcomes with Ahmed valve implantation in aphakic glaucoma than refractory PCG, no statistically significant difference in Kaplan Meier. 0% |
| Banitt et al. (2009), retrospective cohort study (2b) [41] | Inclusion:
Exclusion: Prior aqueous shunt surgery with anterior tube insertion. | 32.9 ± 7.9 | - | 6.9 ± 5.0 | 30 (24-6) | Baerveldt PP + V in all eyes | 5 mmHg ≤ IOP < 21 mmHg, with and without medications, and without visually devastating complication or further surgery. | 85 at 1 y 81 at 2 y 72 at 3 y | 2.5 ± 2.2 | Lens status (aphakia vs. pseudophakia) had comparable IOP results (p = 0.77). Not specified |
| Balekudaru et al. (2014), retrospective cohort study (2b) [40] | Inclusion:
Exclusion: - | 35.86 ± 9.57 | - | - | 47 | Ahmed S-2 or FP-7 model in the superior-temporal quadrant | Complete success: 6 mmHg ≤ IOP ≤ 18 mmHg with and without medication. Qualified success: 6 mmHg ≤ IOP ≤ 18 mmHg, without visually devastating complication. | 95 at 1 y 86 at 2 y | - | No significant differences in outcomes between the two Ahmed valve models. 76,6% |
| Elshatory et al. (2016), retrospective case series (4) [44] | Inclusion:
Exclusion: Follow-up < 6 m. | 33.9 ± 10.9 | - | 9.2 ± 5.7 | 14 | Ahmed (36%) Baerveldt (64%) PP+V in all eyes | Improved postoperative IOP control without any intra- or postoperative complications. | Average decrease in IOP of 51% | 1.0 | - 0% |
| Pakravan et al. (2019), retrospective case series (4) [49] | Inclusion:
Exclusion:
| 28.9 ± 6.1 | - | 9.9 ± 5.6 | 33 | Ahmed FP-7 | 5 mmHg < IOP < 21 mmHg with or without medication. | 90 at 1 y 72 at 5 y | 4.1 ± 3.4 | Better reported outcomes with Ahmed valve implantation in aphakic glaucoma than refractory PCG. 0% |
| Geyer et al. (2021) Retrospective case series (4) [52] | Inclusion:
Exclusion: - | 35.8 ± 7.4 | - | 6.6 * | 41 | Ahmed | IOP ≤ 22 mmHg without glaucoma reoperations and without significant complications | 95 at 1 y 90 at 2 y 83 at 5 y 73 at 7 y | 5 * | - 17% |
| Spiess et al. (2021), retrospective cohort study (2b) [51] | Inclusion:
| 32.66 ± 6.73 | Median 2.9y after cataract surgery | 2 | 29 (23 aphakic, 6 pseudophakic) 41% PHPV 59% non-PHPV | Ahmed: model FP7, model S2, and model FP8 | IOP < 21 mmHg with or without medication. PHPV Non-PHPV | 37.5 at 1 y, 28.1 at 5 y 88.2 at 1 y, 71.9 at 5 y | 7.5 | Eyes with PHPV and GFCS followed by AGV implantation had a higher number of complications and a decreased probability of success compared to the nonpersistent foetal vasculature group. Both groups achieved a significant decrease in intraocular pressure. Not specified |
| Author, Year, Study Design (LOE) Reference | Inclusion and Exclusion Criteria | Mean Pre-Treatment IOP ± SD (mmHg) | Mean Age at GFCS Diagnosis ± SD (y) | Mean (* Median) Age at Glaucoma Surgery ± SD (y) | Number of Eyes (a-p) | Procedure | Success Criteria | Success Rate (%) | Mean (* Median) Follow-Up ± SD (y) | Factors Affecting Treatment Outcomes % of Eyes That Had Prior Glaucoma Surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| Wallace et al. (1998), retrospective cohort study (2b) [39] | Inclusion:
PCG. | 35.9 | 6.1 | 8.7 | 4 | ECP | IOP ≤ 25 mmHg without medications and IOP ≤ 21 mmHg with medications. | 50 | 4.2 | - Not specified |
| Neely and Plager (2001), retrospective cohort study (2b) [59] | Inclusion:
Note: In addition to the 19 eyes with aphakic glaucoma after removal of congenital cataracts, 3 additional ones | 35.06 ± 8.55 | - | 4.90 ± 4.17 | 22 aphakic eyes (19 GFCS, 3 PCG) | ECP | IOP ≤ 21 mmHg, with and without antiglaucoma medications. | 50 | 1.6 ± 1.6 | Aphakic patients may have an increased risk of significant postoperative complications, such as retinal detachment. Not specified |
| Kirwan et al. (2002), retrospective cohort study (2b) [58] | Inclusion:
Exclusion: Follow-up < 1 y. | 32.0 ± 6.4 | - | 7.4 | 34 | TDLC (300°) | IOP < 22 mmHg or reduction by 30%, with and without antiglaucoma medications. | 42 at 1 y | 1.8 | Aphakic eyes had a more sustained IOP control than phakic eyes (PCG, aniridia, anterior segment dysgenesis, uveitic glaucoma, Sturge-Weber, silicone-oil-associated glaucoma, naevus- or Ota-associated glaucoma, secondary angle-closure glaucoma). Aphakic patients had a 42% IOP control at one year versus 14% in phakic eyes. (p < 0.001 log rank test). Success rate is lower than in adults, and younger eyes may recover from treatment more rapidly. Not specified |
| Autrata and Lokaj (2003), retrospective cohort study (2b) [54] | Inclusion:
Exclusion:
| 34.08 ± 7.13 | - | 6.1 | 26 | TDLC (300°) | IOP ≤ 21 mmHg, with and without adjunctive antiglaucoma medications. | 47 at 1 y | 5.6 ± 2.8 | Aphakic patients had a more sustained IOP-lowering response after their first treatment session. Of aphakic eyes, 47% had IOP control at one year versus 19% of the phakic eyes (PCG, uveitic glaucoma, secondary angle closure, Sturge-Weber, aniridia). The data suggest that multiple repeated cyclodiode treatments may still have an IOP-lowering effect. Not specified. |
| Chen et al. (2004), retrospective cohort study (2b) [10] | Inclusion:
| - | - | - | 21 (21-0) | Cyclocryotherapy, TDLC, contact Nd:YAG laser cyclotherapy | IOP ≤ 21 mmHg with and without medications and no need for further surgery. | 14 | 8.6 ± 7.6 | - Not specified |
| Carter et al. (2007), Retrospective case series (4) [56] | Inclusion:
Exclusion:
| 32.6 | 3.3 | 4.2 | 34 (32-2) | ECP (180°–270°) | IOP ≤ 24 mmHg and IOP decrease of more than 15% despite the addition of glaucoma medications, without sight-threatening complications. | 53 | 3.7 | Retreatment of eyes increased the overall success rate. 18% |
| Schlote et al. (2008), retrospective cohort study (2b) [60] | Inclusion:
Exclusion: Follow-up < 1 y. | 31.1 ± 8.8 | - | 53.1 ± 23.6 | 21 | TDLC | 5 ≤IOP ≤ 21 mmHg with and without medication. | 19 after 1 TDLC 48 after repeated TDLC | 3.5 ± 2.4 | Translimbal or pars-plana-modified GDD may be associated with a better long-term prognosis, and should be used prior to TDLC to avoid the increasing risk of hypotonia using a filtering procedure after cyclodestruction. 42.9% |
| Cantor et al. (2018), Retrospective cohort study (2b) [55] | Inclusion:
| 34.1 ± 8.3 | 4.0 ± 2.5 | 6.0 ± 3.8 | 35 (27-8) | ECP (average 230° for first ECP, average of 151° for repeat ECP) | IOP ≤ 24 mmHg, no alternative glaucoma procedure following ECP, or occurrence of devastating complications With and without medications. | 54 48 in a 75 in p Successful eyes had 1.1 ± 0.2 ECP treatments (average). | 7.2 ± 3.6 | The failure rate was not increased in pseudophakic patients relative to aphakic patients. 0% |
| Glaser et al. (2019), retrospective cohort study (2b) [57] | Inclusion:
Exclusion: - | 30.8 ± 7.9 | - | 9.5 ± 6.0 (for all eyes) | 48 (60% of all eyes) | ECP | IOP ≤ 24 mmHg with and without medications, without any additional glaucoma surgery, without devastating complications, without progression to NLP visual acuity. | 64 at 1 y 36 at 3 y 16 at 5 y (after single ECP) (for all eyes) | * 2.2 | In multivariable analysis, of many risk factors considered, only a preoperative IOP < 32 mmHg was significantly associated with treatment success. Not specified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simons, A.-S.; Casteels, I.; Grigg, J.; Stalmans, I.; Vandewalle, E.; Lemmens, S. Management of Childhood Glaucoma Following Cataract Surgery. J. Clin. Med. 2022, 11, 1041. https://doi.org/10.3390/jcm11041041
Simons A-S, Casteels I, Grigg J, Stalmans I, Vandewalle E, Lemmens S. Management of Childhood Glaucoma Following Cataract Surgery. Journal of Clinical Medicine. 2022; 11(4):1041. https://doi.org/10.3390/jcm11041041
Chicago/Turabian StyleSimons, Anne-Sophie, Ingele Casteels, John Grigg, Ingeborg Stalmans, Evelien Vandewalle, and Sophie Lemmens. 2022. "Management of Childhood Glaucoma Following Cataract Surgery" Journal of Clinical Medicine 11, no. 4: 1041. https://doi.org/10.3390/jcm11041041
APA StyleSimons, A.-S., Casteels, I., Grigg, J., Stalmans, I., Vandewalle, E., & Lemmens, S. (2022). Management of Childhood Glaucoma Following Cataract Surgery. Journal of Clinical Medicine, 11(4), 1041. https://doi.org/10.3390/jcm11041041







