The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma
Abstract
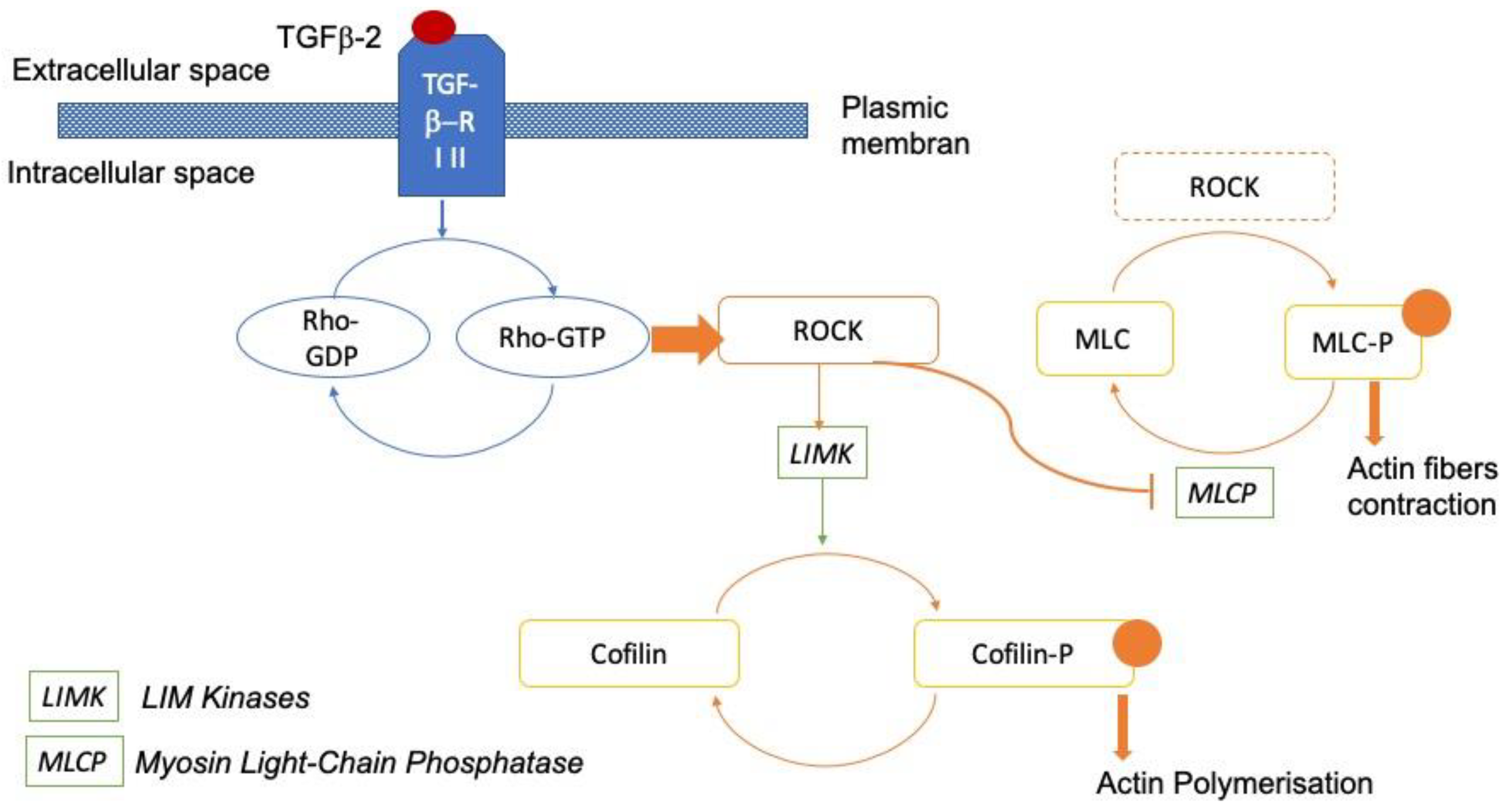
1. Introduction
2. Materials and Methods
2.1. Primary Human Trabecular Meshwork Cell Isolation and Culture
2.2. Trabecular Meshwork Cell Characterization
2.3. Exposure to TGF-β2 and Therapeutic Molecules
2.4. Immunocytochemistry
2.5. Protein Extraction and Western Blot Analysis
2.6. Three-Dimensional (3D) Trabecular Meshwork Cell Culture
2.7. Statistical Analysis
3. Results
3.1. Trabecular Meshwork Cell Characterization
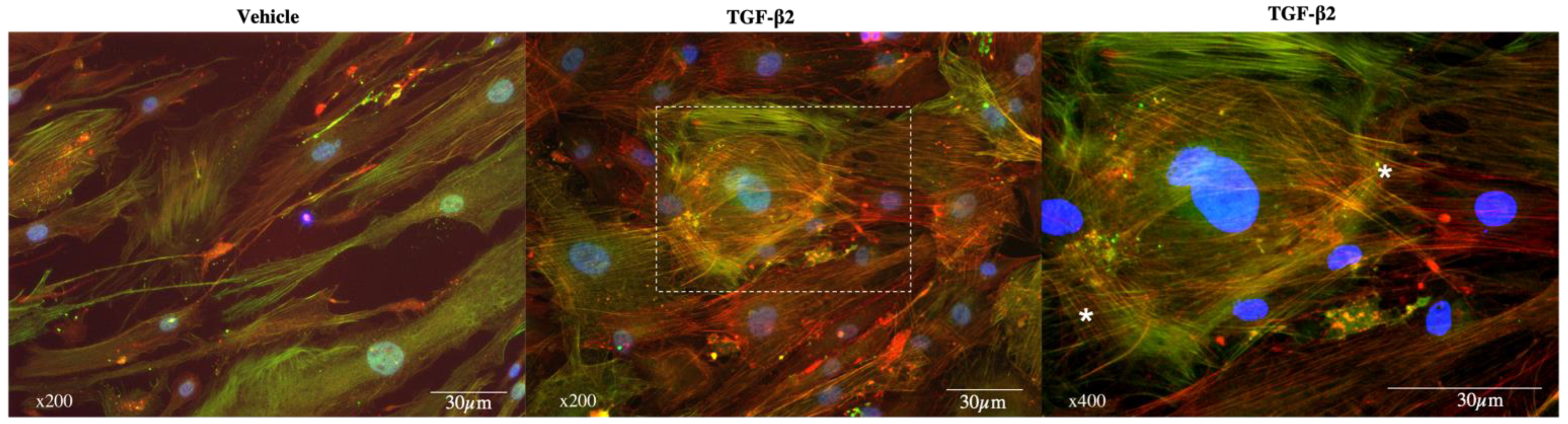
3.2. Exposure to TGF-β2
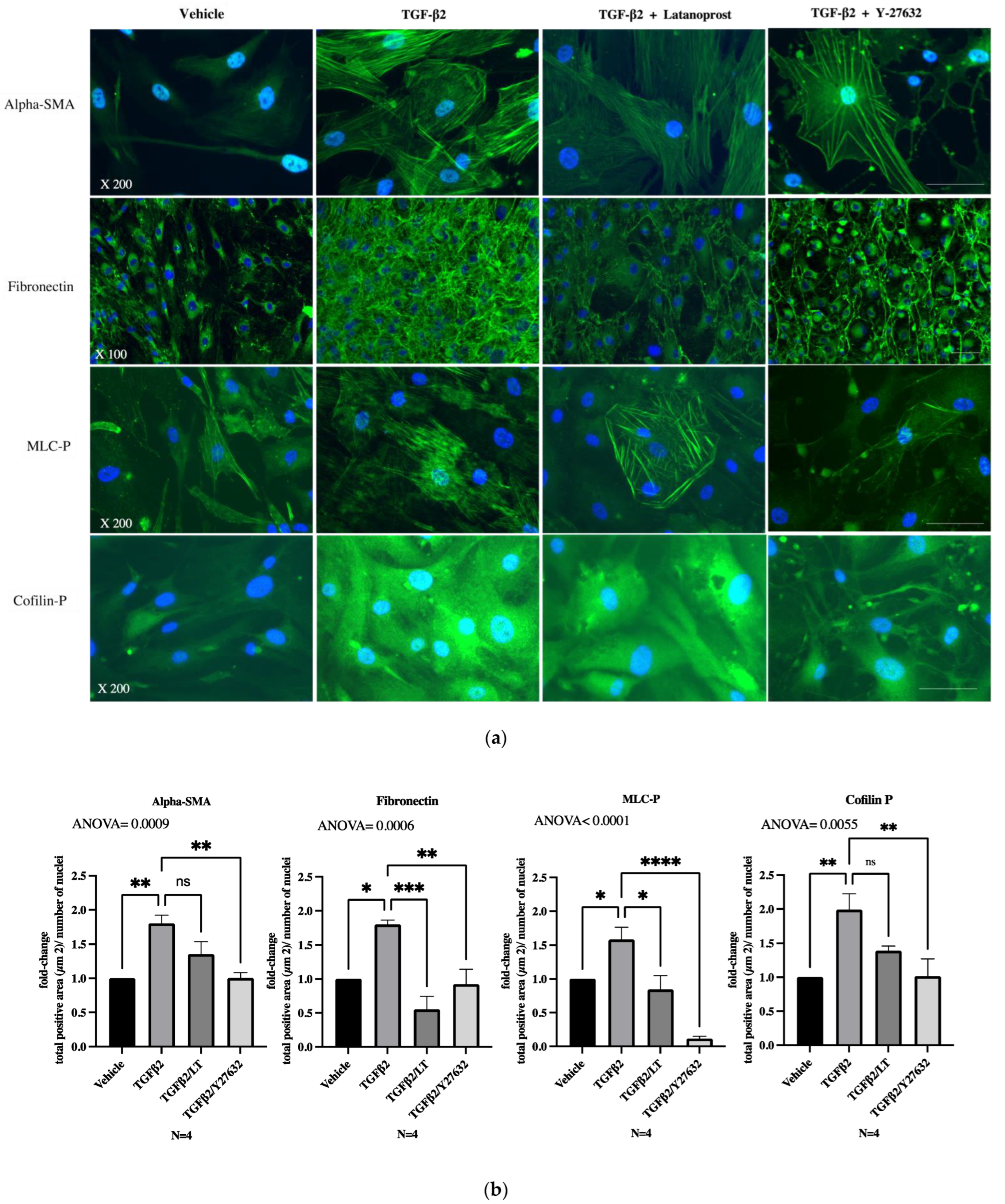
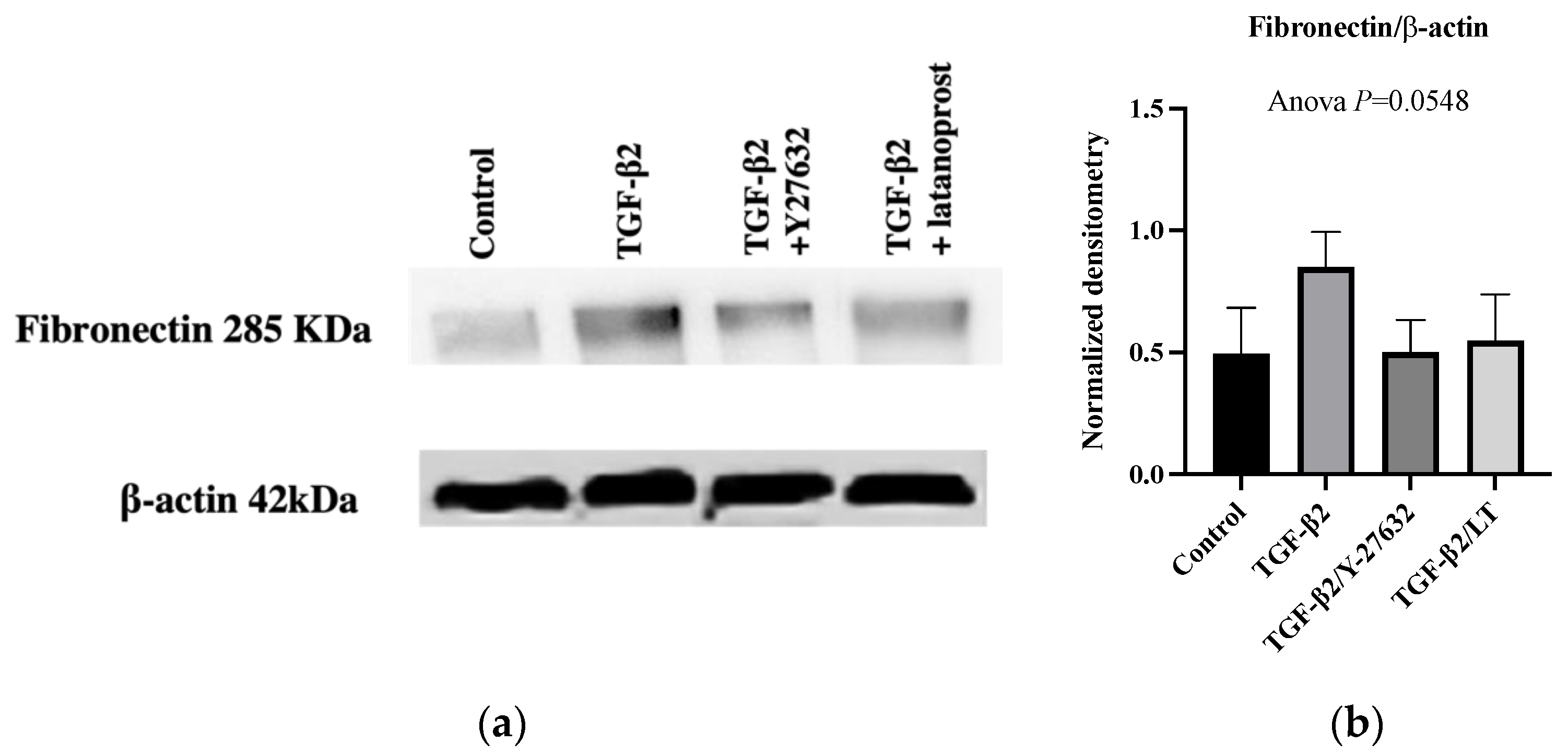
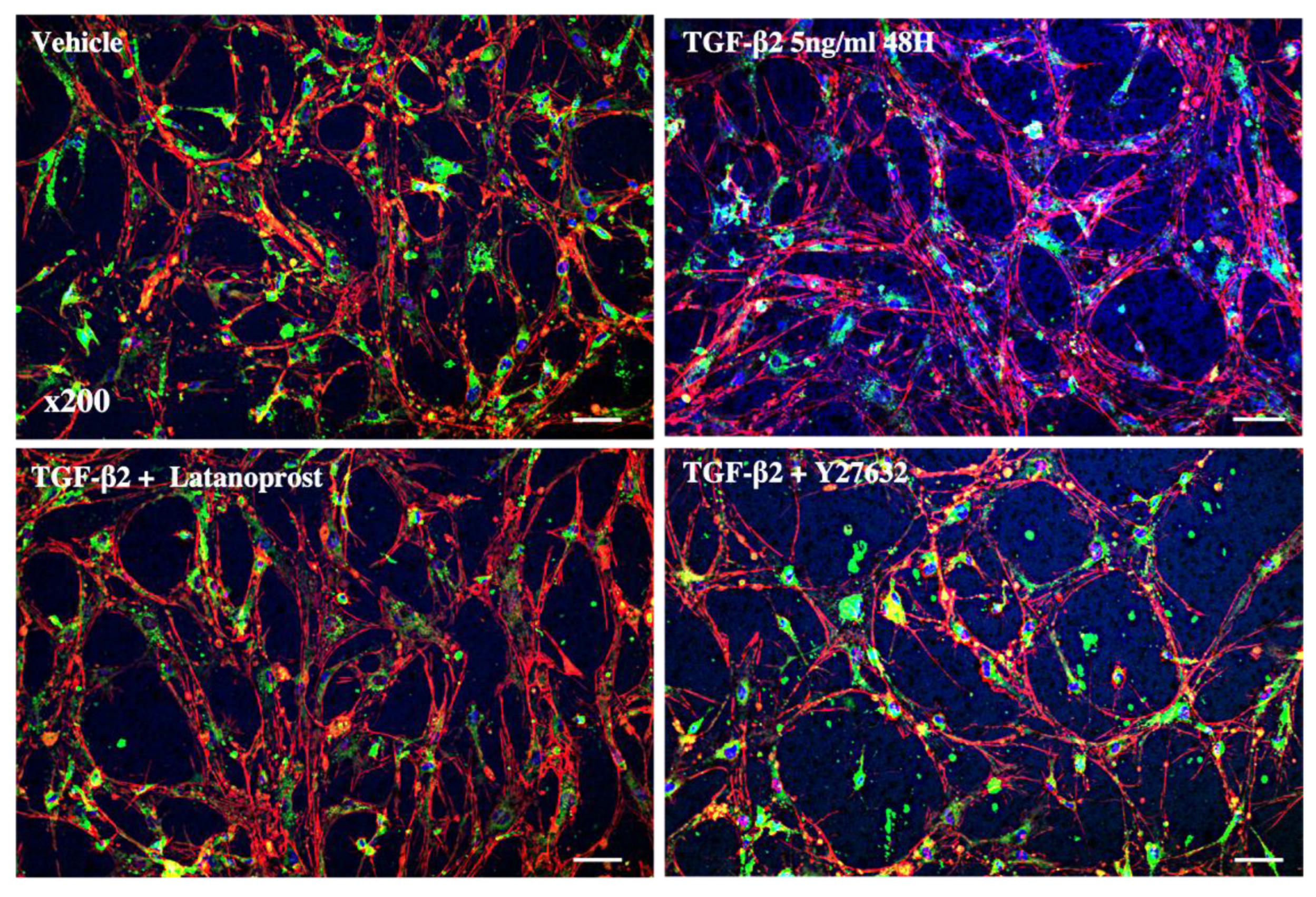
3.3. Effects of Therapeutic Molecules on TGF-β2-Induced Pathological Trabecular Meshwork Model
3.4. Three-Dimensional Trabecular Meshwork Cell Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, N.; Wang, J.; Li, Y.; Jiang, B. Prevalence of Primary Open Angle Glaucoma in the Last 20 Years: A Meta-Analysis and Systematic Review. Sci. Rep. 2021, 11, 13762. [Google Scholar] [CrossRef]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A Randomized Trial Determines That Topical Ocular Hypotensive Medication Delays or Prevents the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2002, 120, 701–713; discussion 829–830. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The Many Faces of the Trabecular Meshwork Cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Tektas, O.-Y.; Lütjen-Drecoll, E. Structural Changes of the Trabecular Meshwork in Different Kinds of Glaucoma. Exp. Eye Res. 2009, 88, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Aga, M.; Bradley, J.M.; Kelley, M.J.; Acott, T.S. Extracellular Matrix Turnover and Outflow Resistance. Exp. Eye Res. 2009, 88, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.R. The Trabecular Meshwork Outflow Pathways: Structural and Functional Aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef]
- Liton, P.B.; Challa, P.; Stinnett, S.; Luna, C.; Epstein, D.L.; Gonzalez, P. Cellular Senescence in the Glaucomatous Outflow Pathway. Exp. Gerontol. 2005, 40, 745–748. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Li, J.; Chan, W.F.; Tripathi, B.J. Aqueous Humor in Glaucomatous Eyes Contains an Increased Level of TGF-Beta 2. Exp. Eye Res. 1994, 59, 723–727. [Google Scholar] [CrossRef]
- Gottanka, J.; Chan, D.; Eichhorn, M.; Lütjen-Drecoll, E.; Ethier, C.R. Effects of TGF-Β2 in Perfused Human Eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 153–158. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Kodati, B.; Nagarajan, B.; Yacoub, S. Astragaloside IV Attenuates Ocular Hypertension in a Mouse Model of TGFβ2 Induced Primary Open Angle Glaucoma. Int. J. Mol. Sci. 2021, 22, 12508. [Google Scholar] [CrossRef]
- Wang, J.; Harris, A.; Prendes, M.A.; Alshawa, L.; Gross, J.C.; Wentz, S.M.; Rao, A.B.; Kim, N.J.; Synder, A.; Siesky, B. Targeting Transforming Growth Factor-β Signaling in Primary Open-Angle Glaucoma. J. Glaucoma 2017, 26, 390–395. [Google Scholar] [CrossRef]
- Pattabiraman, P.P.; Rao, P.V. Mechanistic Basis of Rho GTPase-Induced Extracellular Matrix Synthesis in Trabecular Meshwork Cells. Am. J. Physiol. Cell Physiol. 2010, 298, C749–C763. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Winkler, N.S.; Fautsch, M.P. Effects of Prostaglandin Analogues on Aqueous Humor Outflow Pathways. J. Ocul. Pharmacol. Ther. 2014, 30, 102–109. [Google Scholar] [CrossRef]
- Bahler, C.K.; Howell, K.G.; Hann, C.R.; Fautsch, M.P.; Johnson, D.H. Prostaglandins Increase Trabecular Meshwork Outflow Facility in Cultured Human Anterior Segments. Am. J. Ophthalmol. 2008, 145, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Pattabiraman, P.P.; Kopczynski, C. Role of the Rho GTPase/Rho Kinase Signaling Pathway in Pathogenesis and Treatment of Glaucoma: Bench to Bedside Research. Exp. Eye Res. 2017, 158, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-Kinase/ROCK: A Key Regulator of the Cytoskeleton and Cell Polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Prunier, C.; Prudent, R.; Kapur, R.; Sadoul, K.; Lafanechère, L. LIM Kinases: Cofilin and Beyond. Oncotarget 2017, 8, 41749. [Google Scholar] [CrossRef]
- Liao, J.K.; Seto, M.; Noma, K. Rho Kinase (ROCK) Inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-Associated Kinase Pathway in Glaucoma (Review). Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M.; K-115 Clinical Study Group. Phase 1 Clinical Trials of a Selective Rho Kinase Inhibitor, K-115. JAMA Ophthalmol. 2013, 131, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Serle, J.B.; Katz, L.J.; McLaurin, E.; Heah, T.; Ramirez-Davis, N.; Usner, D.W.; Novack, G.D.; Kopczynski, C.C.; ROCKET-1 and ROCKET-2 Study Groups. Two Phase 3 Clinical Trials Comparing the Safety and Efficacy of Netarsudil to Timolol in Patients With Elevated Intraocular Pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am. J. Ophthalmol. 2018, 186, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M.; K-115 Clinical Study Group. Intra-Ocular Pressure-Lowering Effects of a Rho Kinase Inhibitor, Ripasudil (K-115), over 24 Hours in Primary Open-Angle Glaucoma and Ocular Hypertension: A Randomized, Open-Label, Crossover Study. Acta Ophthalmol. 2015, 93, e254–e260. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M.; K-115 Clinical Study Group. Phase 2 Randomized Clinical Study of a Rho Kinase Inhibitor, K-115, in Primary Open-Angle Glaucoma and Ocular Hypertension. Am. J. Ophthalmol. 2013, 156, 731–736. [Google Scholar] [CrossRef]
- Bacharach, J.; Dubiner, H.B.; Levy, B.; Kopczynski, C.C.; Novack, G.D.; AR-13324-CS202 Study Group. Double-Masked, Randomized, Dose-Response Study of AR-13324 versus Latanoprost in Patients with Elevated Intraocular Pressure. Ophthalmology 2015, 122, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Fukushima, A.; Suganami, H.; Araie, M.; K-115 Clinical Study Group. One-Year Clinical Evaluation of 0.4% Ripasudil (K-115) in Patients with Open-Angle Glaucoma and Ocular Hypertension. Acta Ophthalmol. 2016, 94, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Chang, R.T. An Emerging Treatment Option for Glaucoma: Rho Kinase Inhibitors. Clin. Ophthalmol. 2014, 8, 883–890. [Google Scholar] [CrossRef][Green Version]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus Recommendations for Trabecular Meshwork Cell Isolation, Characterization and Culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Bouchemi, M.; Roubeix, C.; Kessal, K.; Riancho, L.; Raveu, A.-L.; Soualmia, H.; Baudouin, C.; Brignole-Baudouin, F. Effect of Benzalkonium Chloride on Trabecular Meshwork Cells in a New in Vitro 3D Trabecular Meshwork Model for Glaucoma. Toxicol. Vitr. 2017, 41, 21–29. [Google Scholar] [CrossRef]
- Fujimoto, T.; Inoue, T.; Inoue-Mochita, M.; Tanihara, H. Live Cell Imaging of Actin Dynamics in Dexamethasone-Treated Porcine Trabecular Meshwork Cells. Exp. Eye Res. 2016, 145, 393–400. [Google Scholar] [CrossRef]
- Hamard, P.; Blondin, C.; Debbasch, C.; Warnet, J.-M.; Baudouin, C.; Brignole, F. In Vitro Effects of Preserved and Unpreserved Antiglaucoma Drugs on Apoptotic Marker Expression by Human Trabecular Cells. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From Discovery and ECM Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Tibullo, D.; Saccone, S.; Distefano, G.; Basile, M.S.; Di Raimondo, F.; Malaguarnera, L. CHI3L1 Nuclear Localization in Monocyte Derived Dendritic Cells. Immunobiology 2016, 221, 347–356. [Google Scholar] [CrossRef]
- Kottler, U.B.; Jünemann, A.G.M.; Aigner, T.; Zenkel, M.; Rummelt, C.; Schlötzer-Schrehardt, U. Comparative Effects of TGF-Beta 1 and TGF-Beta 2 on Extracellular Matrix Production, Proliferation, Migration, and Collagen Contraction of Human Tenon’s Capsule Fibroblasts in Pseudoexfoliation and Primary Open-Angle Glaucoma. Exp. Eye Res. 2005, 80, 121–134. [Google Scholar] [CrossRef]
- Connor, T.B.; Roberts, A.B.; Sporn, M.B.; Danielpour, D.; Dart, L.L.; Michels, R.G.; de Bustros, S.; Enger, C.; Kato, H.; Lansing, M. Correlation of Fibrosis and Transforming Growth Factor-Beta Type 2 Levels in the Eye. J. Clin. Investig. 1989, 83, 1661–1666. [Google Scholar] [CrossRef]
- Torrejon, K.Y.; Papke, E.L.; Halman, J.R.; Bergkvist, M.; Danias, J.; Sharfstein, S.T.; Xie, Y. TGFβ2-Induced Outflow Alterations in a Bioengineered Trabecular Meshwork Are Offset by a Rho-Associated Kinase Inhibitor. Sci. Rep. 2016, 6, 38319. [Google Scholar] [CrossRef] [PubMed]
- Ota, C.; Ida, Y.; Ohguro, H.; Hikage, F. ROCK Inhibitors Beneficially Alter the Spatial Configuration of TGFβ2-Treated 3D Organoids from a Human Trabecular Meshwork (HTM). Sci. Rep. 2020, 10, 20292. [Google Scholar] [CrossRef]
- Li, G.; Lee, C.; Read, A.T.; Wang, K.; Ha, J.; Kuhn, M.; Navarro, I.; Cui, J.; Young, K.; Gorijavolu, R.; et al. Anti-Fibrotic Activity of a Rho-Kinase Inhibitor Restores Outflow Function and Intraocular Pressure Homeostasis. Elife 2021, 10, e60831. [Google Scholar] [CrossRef]
- Kalouche, G.; Beguier, F.; Bakria, M.; Melik-Parsadaniantz, S.; Leriche, C.; Debeir, T.; Rostène, W.; Baudouin, C.; Vigé, X. Activation of Prostaglandin FP and EP2 Receptors Differently Modulates Myofibroblast Transition in a Model of Adult Primary Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1816–1825. [Google Scholar] [CrossRef][Green Version]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular Meshwork Stiffness in Glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, V.; Jernström, S.; Fey, V.; Mpindi, J.-P.; Kleivi Sahlberg, K.; Kallioniemi, O.; Perälä, M. High-Throughput 3D Screening Reveals Differences in Drug Sensitivities between Culture Models of JIMT1 Breast Cancer Cells. PLoS ONE 2013, 8, e77232. [Google Scholar] [CrossRef]
- Ramachandran, C.; Patil, R.V.; Combrink, K.; Sharif, N.A.; Srinivas, S.P. Rho-Rho Kinase Pathway in the Actomyosin Contraction and Cell-Matrix Adhesion in Immortalized Human Trabecular Meshwork Cells. Mol. Vis. 2011, 17, 1877–1890. [Google Scholar]
- Saha, B.C.; Kumari, R.; Kushumesh, R.; Ambasta, A.; Sinha, B.P. Status of Rho Kinase Inhibitors in Glaucoma Therapeutics—An Overview. Int. Ophthalmol. 2021, 42, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Deng, P.F.; Kumar, J.; Epstein, D.L. Modulation of Aqueous Humor Outflow Facility by the Rho Kinase-Specific Inhibitor Y-27632. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1029–1037. [Google Scholar]
- Murphy, K.C.; Morgan, J.T.; Wood, J.A.; Sadeli, A.; Murphy, C.J.; Russell, P. The Formation of Cortical Actin Arrays in Human Trabecular Meshwork Cells in Response to Cytoskeletal Disruption. Exp. Cell Res. 2014, 328, 164–171. [Google Scholar] [CrossRef]
- Heo, J.Y.; Ooi, Y.H.; Rhee, D.J. Effect of Prostaglandin Analogs: Latanoprost, Bimatoprost, and Unoprostone on Matrix Metalloproteinases and Their Inhibitors in Human Trabecular Meshwork Endothelial Cells. Exp. Eye Res. 2020, 194, 108019. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Ohguro, H.; Ota, C.; Hikage, F. Diverse Effects of Pan-ROCK and ROCK2 Inhibitors on 2 D and 3D Cultured Human Trabecular Meshwork (HTM) Cells Treated with TGFβ2. Sci. Rep. 2021, 11, 15286. [Google Scholar] [CrossRef]
- US Department of Health and Human Services, Food and Drug Administration Rhopressa Approval Letter 208254. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208254Orig1s000TOC.cfm (accessed on 10 February 2022).
- Tanna, A.P.; Johnson, M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology 2018, 125, 1741–1756. [Google Scholar] [CrossRef]
- Lewis, R.A.; Levy, B.; Ramirez, N.; Kopczynski, C.C.; Usner, D.W.; Novack, G.D.; PG324-CS201 Study Group. Fixed-Dose Combination of AR-13324 and Latanoprost: A Double-Masked, 28-Day, Randomised, Controlled Study in Patients with Open-Angle Glaucoma or Ocular Hypertension. Br. J. Ophthalmol. 2016, 100, 339–344. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M.; K-115 Clinical Study Group. Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined With Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2015, 133, 755–761. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Dilution | Host | Supplier | Reference |
|---|---|---|---|---|
| Alpha-SMA | 1/100 | Rabbit polyclonal | Abcam | ab 5694 |
| CD44 | 1/125 | Rabbit monoclonal | Abcam | ab 189524 |
| Aquaporin 1 (AQP1) | 1/100 | Mouse monoclonal | Santa Cruz | sc 25287 |
| Chitinase-3like 1 (CHI3L1) | 1/125 | Rabbit polyclonal | Thermo Fisher | PAS-43746 |
| Antibody | Dilution | Host | Supplier | Reference |
|---|---|---|---|---|
| Alpha-SMA | 1/100 | Rabbit | Abcam | ab5694 |
| Fibronectin | 1/100 | Rabbit | Abcam | ab2413 |
| Phospho-Myosin Light Chain 2 (Ser19) | 1/100 | Rabbit | Cell signaling | 3671S |
| Phospho-Cofilin (Ser3) | 1/100 | Rabbit | Cell signaling | 3313S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buffault, J.; Brignole-Baudouin, F.; Reboussin, É.; Kessal, K.; Labbé, A.; Mélik Parsadaniantz, S.; Baudouin, C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. J. Clin. Med. 2022, 11, 1001. https://doi.org/10.3390/jcm11041001
Buffault J, Brignole-Baudouin F, Reboussin É, Kessal K, Labbé A, Mélik Parsadaniantz S, Baudouin C. The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. Journal of Clinical Medicine. 2022; 11(4):1001. https://doi.org/10.3390/jcm11041001
Chicago/Turabian StyleBuffault, Juliette, Françoise Brignole-Baudouin, Élodie Reboussin, Karima Kessal, Antoine Labbé, Stéphane Mélik Parsadaniantz, and Christophe Baudouin. 2022. "The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma" Journal of Clinical Medicine 11, no. 4: 1001. https://doi.org/10.3390/jcm11041001
APA StyleBuffault, J., Brignole-Baudouin, F., Reboussin, É., Kessal, K., Labbé, A., Mélik Parsadaniantz, S., & Baudouin, C. (2022). The Dual Effect of Rho-Kinase Inhibition on Trabecular Meshwork Cells Cytoskeleton and Extracellular Matrix in an In Vitro Model of Glaucoma. Journal of Clinical Medicine, 11(4), 1001. https://doi.org/10.3390/jcm11041001







