Additional Lymphaticovenular Anastomosis on the Posterior Side for Treatment of Primary Lower Extremity Lymphedema
Abstract
1. Introduction
2. Materials and Methods
2.1. ICG Lymphography
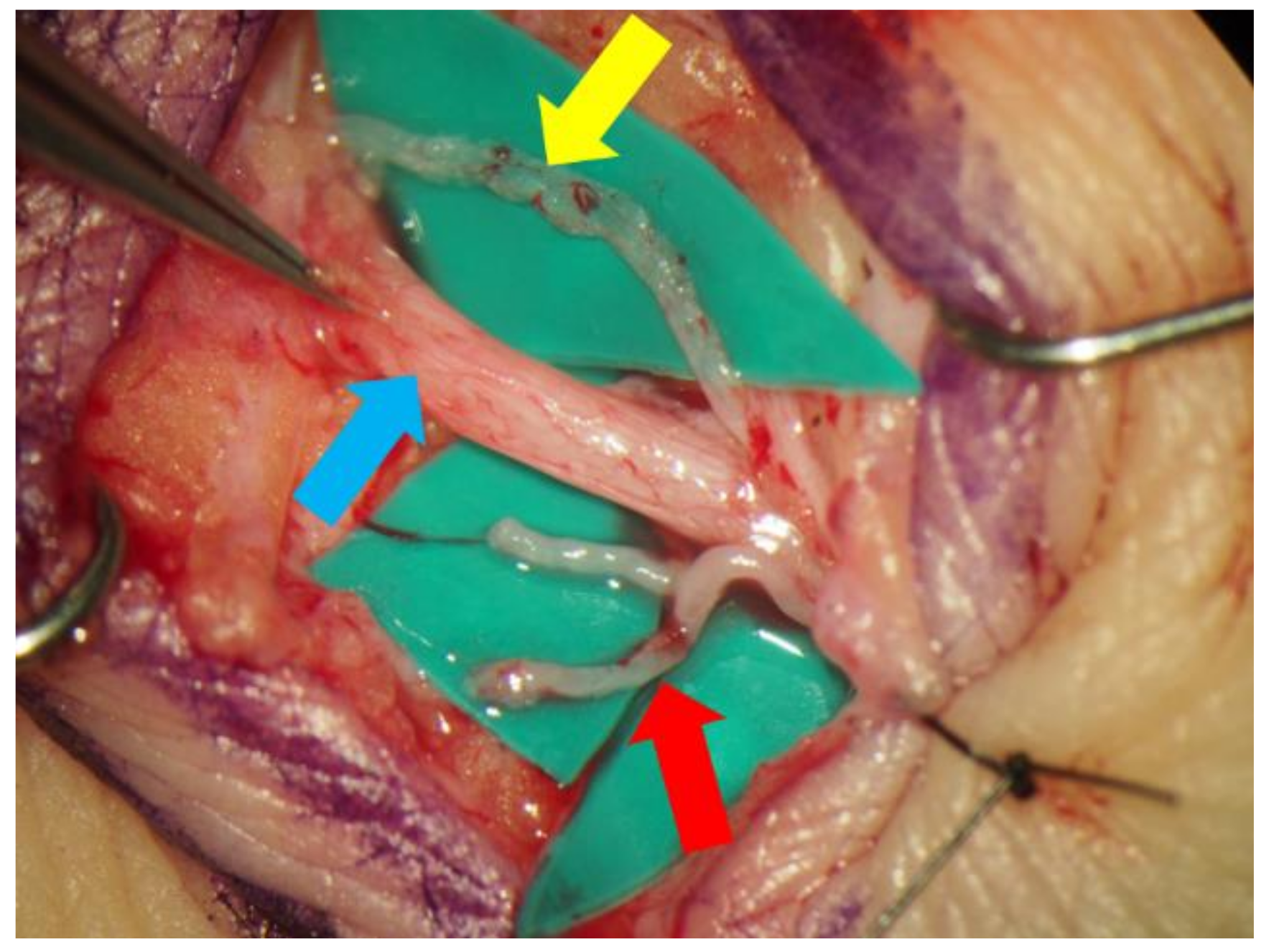
2.2. Lymphaticovenular Anastomosis
2.3. Effectiveness of Additional Lymphaticovenular Anastomosis on the Posterior Side
2.4. Statistical Analysis
3. Results
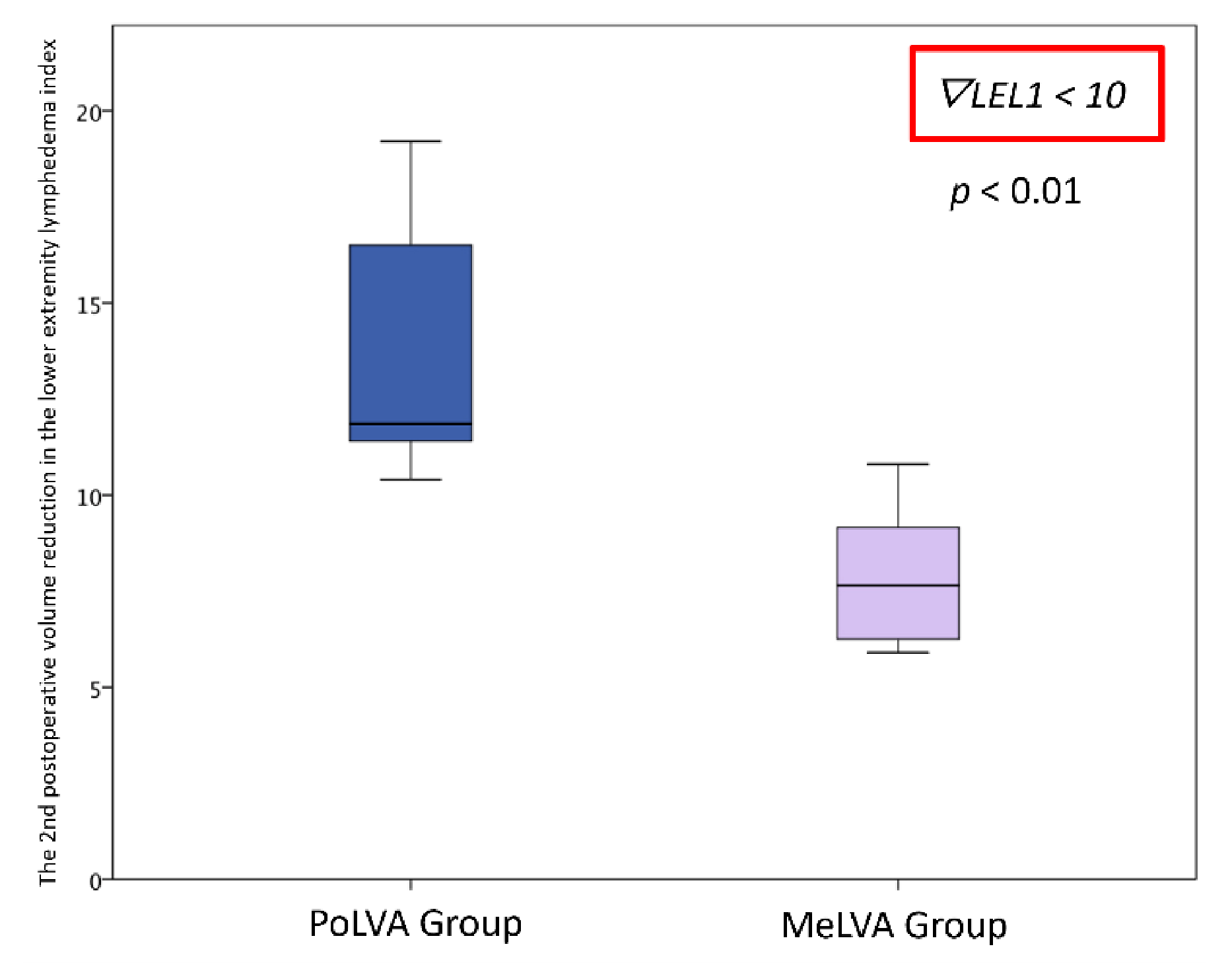
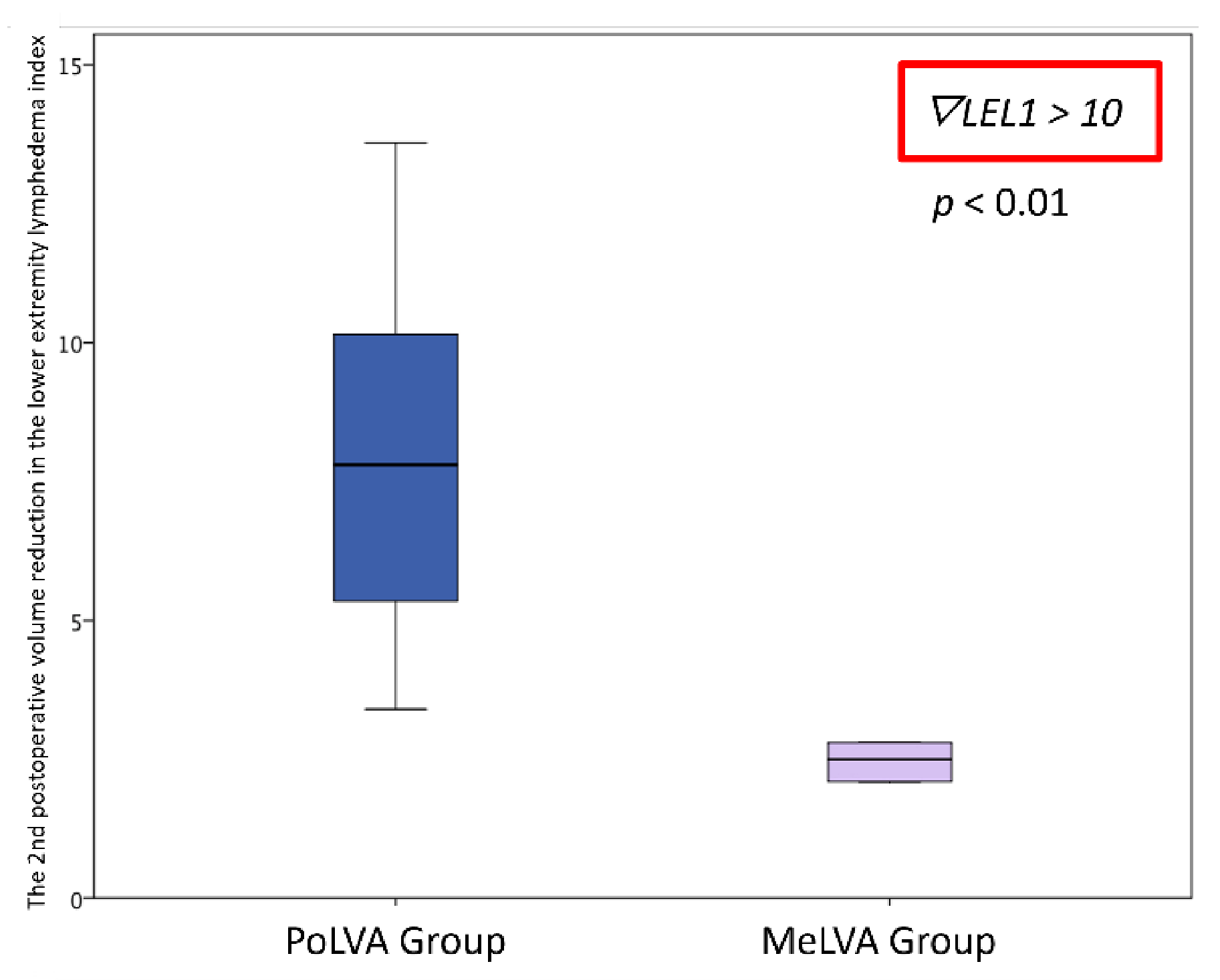
Effectiveness of Additional Lymphaticovenular Anastomosis on the Posterior Side
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koshima, I.; Inagawa, K.; Urushibara, K.; Moriguchi, T. Supermicrosurgical LVA for the treatment of lymphedema in the upper extremities. J. Reconstr. Microsurg. 2000, 16, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, K.; Fleischer, A.; Yosipovitch, G. Lower extremity lymphedema update: Pathophysiology, diagnosis, and treatment guidelines. J. Am. Acad. Dermatol. 2008, 59, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Beesley, V.; Janda, M.; Eakin, E.; Obermair, A.; Battistutta, D. Lymphedema after gynecological cancer treatment: Prevalence, correlates, and supportive care needs. Cancer 2007, 109, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Villa, G.; Bogliolo, S.; Ferrero, S.; Murelli, F.; Campisi, C. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann. Surg. Oncol. 2009, 16, 703–708. [Google Scholar] [CrossRef]
- Moseley, A.L.; Carati, C.J.; Piller, N.B. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann. Oncol. 2007, 18, 639–646. [Google Scholar] [CrossRef]
- Deltombe, T.; Jamart, J.; Recloux, S.; Legrand, C.; Vandenbroeck, N.; Theys, S.; Hanson, P. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology 2007, 40, 26–34. [Google Scholar]
- Szolnoky, G.; Lakatos, B.; Keskeny, T.; Varga, E.; Varga, M.; Dobozy, A.; Kemény, L. Intermittent pneumatic compression acts synergistically with manual lymphatic drainage in complex decongestive physiotherapy for breast cancer treatment-related lymphedema. Lymphology 2009, 42, 188–194. [Google Scholar]
- Kwan, M.L.; Darbinian, J.; Schmitz, K.H.; Citron, R.; Partee, P.; Kutner, S.E.; Kushi, L.H. Risk factors for lymphedema in a prospective breast cancer survivorship study: The Pathways Study. Arch. Surg. 2010, 145, 1055–1063. [Google Scholar] [CrossRef]
- Lee, B.B.; Andrade, M.; Antignani, P.L.; Boccardo, F.; Bunke, N.; Campisi, C.; Damstra, R.; Flour, M.; Forner-Cordero, I.; Gloviczki, P.; et al. Diagnosis and treatment of primary lymphedema: Consensus document of the International Union of Phlebology (IUP)-2013. Int. Angiol. 2013, 32, 541–574. [Google Scholar]
- Blatt, J.; Powell, C.M.; Burkhart, C.N.; Stavas, J.; Aylsworth, A.S. Genetics of hemangiomas, vascular malformations, and primary lymphedema. J. Pediatr. Hematol. Oncol. 2014, 36, 587–593. [Google Scholar] [CrossRef]
- Rustgi, A.K.; Rotolo, F.S.; Peete, W.P.; Vollmer, R.T.; Meyers, W.C. Successful management of late-onset primary lymphatic hypoplasia. Surgery 1985, 97, 714–720. [Google Scholar]
- Sapountzis, S.; Ciudad, P.; Lim, S.Y.; Chilgar, R.M.; Kiranantawat, K.; Nicoli, F.; Constantinides, J.; Wei, M.Y.; Sönmez, T.T.; Singhal, D.; et al. Modified Charles procedure and lymph node flap transfer for advanced lower extremity lymphedema. Microsurgery 2014, 34, 439–447. [Google Scholar] [CrossRef]
- Zeltzer, A.A.; Hamdi, M. Operative treatment of peripheral lymphedema: A systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplant. Plast. Reconstr. Surg. 2014, 134, 491e–492e. [Google Scholar] [CrossRef][Green Version]
- Koshima, I.; Nanba, Y.; Tsutsui, T.; Takahashi, Y.; Itoh, S. Long-term followup after LVA for lymphedema in the leg. J. Reconstr. Microsurg. 2003, 19, 209–215. [Google Scholar]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Seki, Y.; Yamamoto, N.; Oka, A.; Hara, H.; Koshima, I. Minimally invasive lymphatic supermicrosurgery (MILS): ICG lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann. Plast. Surg. 2014, 72, 67–70. [Google Scholar] [CrossRef]
- O’Brien, B.M.; Mellow, C.G.; Khazanchi, R.K.; Dvir, E.; Kumar, V.; Pederson, W.C. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast. Reconstr. Surg. 1990, 85, 562–572. [Google Scholar] [CrossRef]
- Demirtas, Y.; Ozturk, N.; Yapici, O.; Topalan, M. Comparison of primary and secondary lower-extremity lymphedema treated with supermicrosurgical lymphaticovenous anastomosis and lymphaticovenous implantation. J. Reconstr. Microsurg. 2010, 26, 137–143. [Google Scholar] [CrossRef]
- Hara, H.; Mihara, M.; Ohtsu, H.; Narushima, M.; Iida, T.; Koshima, I. Indication of Lymphaticovenous Anastomosis for Lower Limb Primary Lymphedema. Plast. Reconstr. Surg. 2015, 136, 883–893. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimatsu, H.; Narusima, M.; Yamamoto, N.; Hayashi, A.; Koshima, I. Indocyanine Green Lymphography Findings in Primary Leg Lymphedema. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 95–102. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsuda, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: The modified dermal backflow stage and concept of subclinical lymphedema. Plast. Reconstr. Surg. 2011, 128, 314e–321e. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yoshimatsu, H.; Yamamoto, N.; Narushima, M.; Iida, T.; Koshima, I. Side-to-end Lymphaticovenular anastomosis through temporary lymphatic expansion. PLoS ONE 2013, 8, e59523. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.R.; Wang, D.G.; Levy, S.M.; Sci, B.M.; Chen, Y. Superficial lymphatic drainage of the lower extremity: Anatomical study and clinical implications. Plast. Reconstr. Surg. 2013, 132, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Matsuda, N.; Todokoro, T.; Yoshimatsu, H.; Narushima, M.; Mihara, M.; Uchida, G.; Koshima, I. Lower extremity lymphedema index: A simple method for severity evaluation of lower extremity lymphedema. Ann. Plast. Surg. 2011, 67, 637–640. [Google Scholar] [CrossRef]
- Campisi, C.C.; Ryan, M.; Boccardo, F.; Campisi, C. Fibro-Lipo-Lymph-Aspiration with a Lymph Vessel Sparing Procedure to Treat Advanced Lymphedema After Multiple Lymphatic-Venous Anastomoses: The Complete Treatment Protocol. Ann. Plast. Surg. 2017, 78, 184–190. [Google Scholar] [CrossRef]
- Bolletta, A.; Taranto, G.; Losco, L.; Elia, R.; Sert, G.; Ribuffo, D.; Cigna, E.; Chen, H.-C. Combined lymph node transfer and suction-assisted lipectomy in lymphedema treatment: A prospective study. Microsurgery 2022. (ahead of print). [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: The generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: A novel severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef]
- Ogata, F.; Narushima, M.; Mihara, M.; Azuma, R.; Morimoto, Y.; Koshima, I. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann. Plast. Surg. 2007, 59, 180–184. [Google Scholar] [CrossRef]
- Hayashi, A.; Hayashi, N.; Yoshimatsu, H.; Yamamoto, T. Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J. Surg. Oncol. 2018, 117, 290–298. [Google Scholar] [CrossRef]
- Hayashi, A.; Yamamoto, T.; Yoshimatsu, H.; Hayashi, N.; Furuya, M.; Harima, M.; Narushima, M.; Koshima, I. Ultrasound visualization of the lymphatic vessels in the lower leg. Microsurgery 2016, 36, 397–401. [Google Scholar] [CrossRef]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef]
- Visconti, G.; Bianchi, A.; Hayashi, A.; Salgarello, M. Ultra-high frequency ultrasound preoperative planning of the rerouting method for lymphaticovenular anastomosis in incisions devoid of vein. Microsurgery 2020, 40, 717–718. [Google Scholar] [CrossRef]


| Stage | Description |
|---|---|
| 0 | No dermal backflow pattern and a clear linear pattern |
| 1 | Splash pattern around the groin region |
| 2 | Stardust pattern extended from proximal side of lower limb to thigh |
| 3 | Stardust pattern extended from proximal side of lower limb to lower leg |
| 4 | Stardust pattern extended to the whole limb |
| 5 | Existence of a diffuse pattern with stardust pattern in the background |
| NB | No dermal backflow pattern and some regions without even linear pattern |
| DB | Stardust pattern or diffuse pattern only in the distal lower limb |
| Exclusion Criteria | No. of Patients |
|---|---|
| Including Other Procedure | |
| -Lymph node transfer | 7 |
| -Excision | 2 |
| Postoperative stepped-up compression therapy | 3 |
| Less than 6 months of postoperative follow-up | 5 |
| Developing lymphedema before 11 years of age | 2 |
| Total | 19 |
| 33 Legs of 26 Lower Limb Primary Lymphedema Patients | |
| Age, years | 16-82 (44.2) |
| Sex * | |
| Male | 4 (15.4%) |
| Female | 22 (84.6%) |
| ISL classification * | |
| 1 | 3 (9.1%) |
| 2a | 15 (45.5%) |
| 2b | 14 (42.4%) |
| 3 | 1 (3.0%) |
| LDB stage * | |
| Stage 1 | 0 (0%) |
| Stage 2 | 2 (6.1%) |
| Stage 3 | 7 (21.2%) |
| Stage 4 | 5 (15.2%) |
| Stage 5 | 7 (21.2%) |
| NB | 4 (12.1%) |
| DB | 8 (24.2%) |
| Edema site * | |
| Bilateral | 6 (23.1%) |
| Right | 9 (34.6%) |
| Left | 11 (42.3%) |
| Duration of edema, years | 0.8-29 (8.6) |
| 227 LVAs in 26 Primary Lymphedema Patients in Two Surgeries | |
|---|---|
| No. of total LVAs per patient | 5–12 (8.7) |
| No. of LVAs on posterior side in PoLVA group | 2–5 (3.5) |
| Operative time, min | 283–725 (406) |
| Follow-up period, day | 205–1089 (526) |
| Total postoperative reduction in LEL index | 5.3–32.9 (18.1) |
| PoLVA Group (n = 13) | MeLVA Group (n = 13) | p | |
|---|---|---|---|
| Age, years | 42.1 ± 11.7 | 46.6 ± 13.8 | 0.586 |
| Body Mass Index, kg/m2 | 24.2 ± 2.5 | 23.4 ± 3.2 | 0.512 |
| Duration of edema, years | 9.1 ± 3.8 | 8.0 ± 3.3 | 0.316 |
| No. of LVAs in the first operation | 4.4 ± 1.5 | 4.7 ± 1.2 | 0.487 |
| No. of LVAs in the second operation | 3.5 ± 0.6 | 4.6 ± 1.0 | 0.038 * |
| Diameter of lymphatic vessels, mm | 0.48 ± 0.16 | 0.51 ± 0.19 | 0.629 |
| Required time for dissecting lymphatic vessels and veins, min | 15.8 ± 2.9 | 12.7 ± 2.4 | 0.093 |
| Length of skin incision, cm | 3.7 ± 1.1 | 2.9 ± 0.6 | 0.042 * |
| The first postoperative volume reduction in LEL index | 9.3 ± 4.5 | 10.7 ± 5.0 | 0.453 |
| The second postoperative volume reduction in LEL index | 10.5 ± 4.5 | 5.5 ± 3.6 | 0.008 * |
| Total postoperative volume reduction in LEL index | 19.8 ± 8.6 | 16.2 ± 8.0 | 0.282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, A.; Visconti, G.; Yang, C.-S.; Hayashi, N.; Yoshimatsu, H. Additional Lymphaticovenular Anastomosis on the Posterior Side for Treatment of Primary Lower Extremity Lymphedema. J. Clin. Med. 2022, 11, 867. https://doi.org/10.3390/jcm11030867
Hayashi A, Visconti G, Yang C-S, Hayashi N, Yoshimatsu H. Additional Lymphaticovenular Anastomosis on the Posterior Side for Treatment of Primary Lower Extremity Lymphedema. Journal of Clinical Medicine. 2022; 11(3):867. https://doi.org/10.3390/jcm11030867
Chicago/Turabian StyleHayashi, Akitatsu, Giuseppe Visconti, Chia-Shen (Johnson) Yang, Nobuko Hayashi, and Hidehiko Yoshimatsu. 2022. "Additional Lymphaticovenular Anastomosis on the Posterior Side for Treatment of Primary Lower Extremity Lymphedema" Journal of Clinical Medicine 11, no. 3: 867. https://doi.org/10.3390/jcm11030867
APA StyleHayashi, A., Visconti, G., Yang, C.-S., Hayashi, N., & Yoshimatsu, H. (2022). Additional Lymphaticovenular Anastomosis on the Posterior Side for Treatment of Primary Lower Extremity Lymphedema. Journal of Clinical Medicine, 11(3), 867. https://doi.org/10.3390/jcm11030867







