Abstract
The efficacy of lymphaticovenular anastomosis (LVA) for the treatment of primary lymphedema has been reported. Previous research suggested the efficacy of LVA on the anterior side of the lower limb, but no research has yet underlined the effectiveness of LVA on the posterior side. In the present study, we aimed to investigate the efficacy of LVA on the posterior side of the lower leg for treatment of primary lymphedema, i.e., whether further improvement of primary lower extremity lymphedema could be expected by performing LVA on the posterior side of the lower limb in addition to the LVA on the anterior side, which is usually performed. Forty-five patients with primary lower extremity lymphedema who underwent LVA twice between March 2018 and September 2020 were retrospectively investigated. Patients were classified into two groups: those who underwent LVA on the posterior side in the second operation (PoLVA group) and those who underwent LVA on the medial and anterior sides again in the second operation (MeLVA group). All patients underwent LVA on the medial and anterior sides in the first operation, but no sufficient improvement was observed. The following factors in the second operation were compared between the two groups: skin incision length, the number of anastomoses, the diameters of the lymphatic vessels, the time required for the dissection of the lymphatic vessels and veins and the reduction in volume. LVA resulted in 227 anastomoses (106 anastomoses in the PoLVA group and 121 anastomoses in the MeLVA group) in 26 patients with primary lymphedema of the lower extremities in two surgeries. The reduction in lower extremity lymphedema index was significantly greater in the PoLVA group than that in the MeLVA group (10.5 ± 4.5 vs. 5.5 ± 3.6; p = 0.008), and the number of anastomoses in the PoLVA group was significantly lower than that in the MeLVA group (3.5 ± 0.6 vs. 4.6 ± 1.0; p = 0.038). LVA on the posterior side subsequent to LVA on the medial and anterior sides resulted in the further improvement of primary lower extremity lymphedema with fewer numbers of anastomoses.
1. Introduction
Lymphedema is classified into primary and secondary types, depending on the cause. The etiology of secondary lymphedema includes parasitic infection, injury, cancer radiation therapy and lymphadenectomy following gynecologic, urologic and breast cancer [1,2,3,4,5,6,7,8]. Primary lymphedema may be caused by genetic abnormalities, and its prevalence has been reported to be 1 in 6000 to 1 in 10,000 [9]. Some genes have been linked to primary lymphedema, but most cases of primary lymphedema are idiopathic [9,10].
Management options for primary lymphedema include both conservative methods, such as compression therapy, manual lymph drainage and skincare and surgical treatment. Even though a variety of procedures are reported to be effective for the treatment of primary lymphedema, there is not sufficient evidence to determine which surgical procedure is the most appropriate [9,11,12,13]. Among the surgical procedures, lymphaticovenular anastomosis (LVA) is a minimally invasive treatment, requiring only local infiltration anesthesia and small skin incisions [1,14,15,16].
The efficacy of LVA for primary lymphedema has been reported [17,18,19]. Previous research suggested the efficacy of LVA on the medial and anterior sides of lower limbs with patients in the supine position, but no research has yet underlined the effectiveness of LVA on the posterior side with patients in the prone position. We often perform LVA more than once in a patient, including on the posterior side, for primary intractable lymphedema.
In the present study, we aimed to investigate the efficacy of LVA on the posterior side for the treatment of primary lymphedema. We conducted a retrospective observational study to evaluate the effectiveness of this technique and assessed whether LVA on the posterior side subsequent to LVA on the medial and anterior sides resulted in the further improvement of primary lower extremity lymphedema.
2. Materials and Methods
We retrospectively investigated patients with primary lower extremity lymphedema who underwent LVA twice between March 2018 and September 2020. The diagnosis of primary lymphedema was made based on the results of indocyanine green (ICG) lymphography and by excluding other edematous diseases [20].
All patients included in this study had received compression therapy using elastic stockings for at least 3 months, which resulted in no clinical improvement. The exclusion criteria included concurrent lymphedema surgical procedures, postoperative compression therapy, less than 6 months of postoperative follow-up and developing lymphedema before 11 years of age. The follow-up period was defined as the time between the first operation and the last outpatient clinic visit after the second operation.
Patients were classified into two groups: those who underwent LVA on the posterior side in the second operation (PoLVA group) and those who underwent LVA on the medial and anterior sides in the second operation (MeLVA group). All patients underwent LVA on the medial and anterior sides in the first operation, but its effect was insufficient. Patients who still complained of edema in the posterior side of the leg after the first operation underwent LVA on the posterior side in the second operation. Those who still had strong complaints of edema on the medial and anterior sides of the leg underwent LVA on the medial and anterior sides again in the second operation. When the first LVA resulted in no improvement of lymphedema, patients underwent combined surgical procedures including LVA, vascularized lymph node transfer and reductive procedure; these patients were excluded from the study.
This study was conducted under the institutional ethical review board. All patients provided written informed consent for participation in this retrospective observational study.
2.1. ICG Lymphography
We performed preoperative ICG lymphography for patients who were not allergic to iodine to determine lymphedema severity and to locate the lymphatic vessels [21]. ICG lymphography was performed as follows: 0.2 mL of ICG (Diagnogreen 0.125%; Daiichi Pharmaceutical, Tokyo, Japan) was injected subcutaneously into the first web spaces of the feet and into both edges of the Achilles tendon on the day before surgery. Fluorescent images of lymphatic drainage channels were obtained using a photodynamic eye infrared camera system (PDE; Hamamatsu Photonics K.K., Hamamatsu, Japan) before the operation.
We used lymphedema staging for primary lymphedema described by Hara et al. In their staging system, “no backflow” and “distal backflow” are added to the leg dermal backflow classification described by Yamamoto et al., as the leg dermal backflow stages do not apply to some primary lymphedema patients (Table 1) [19,21].

Table 1.
Leg dermal backflow stage chart for primary lymphedema.
2.2. Lymphaticovenular Anastomosis
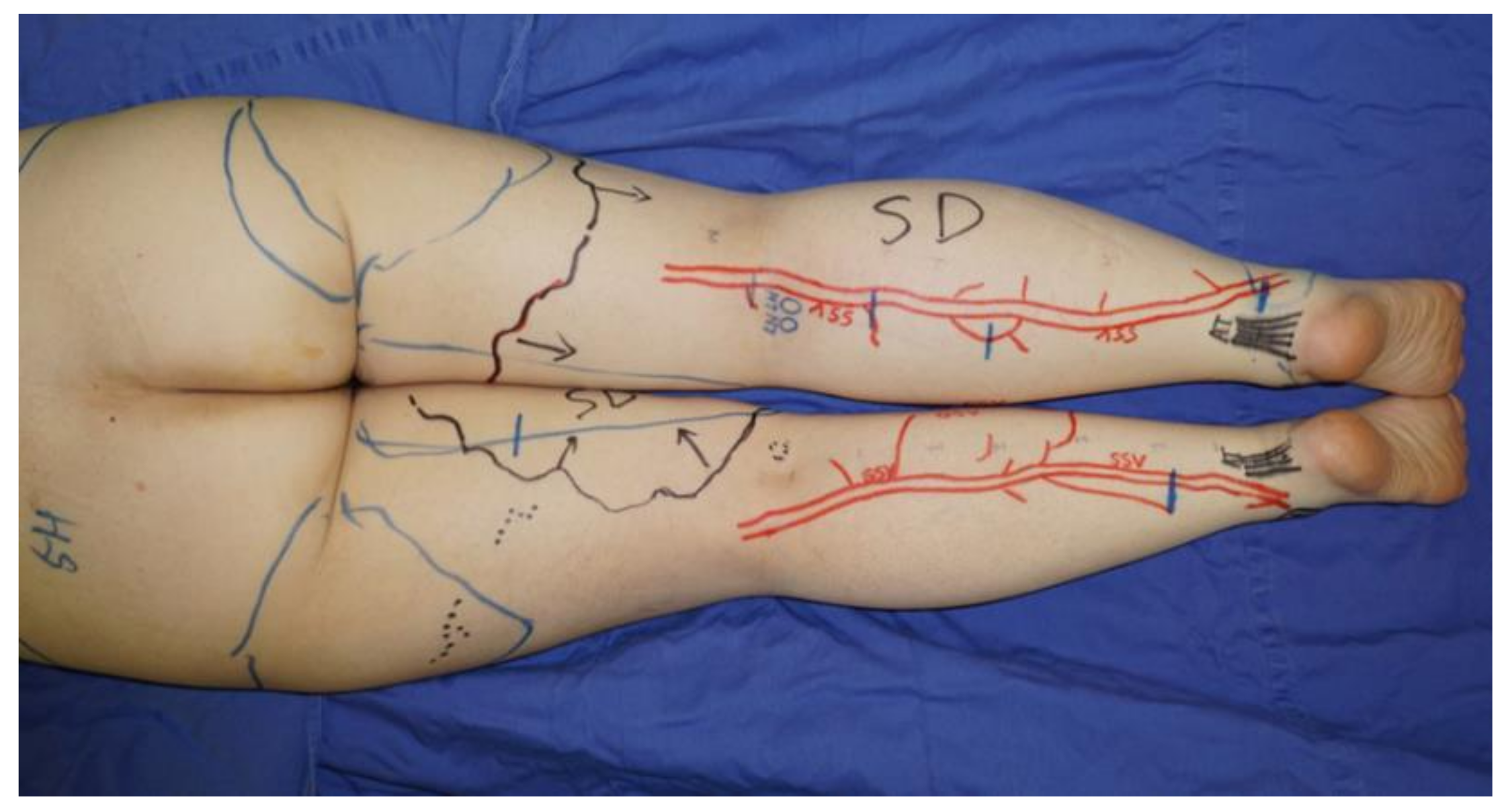
LVA was performed using previously documented procedures under local infiltrative anesthesia [22]. When a linear ICG lymphography pattern was observed, approximately 3 cm skin incisions were made on the line. If a linear pattern was not seen, the incisions were made in the region along the great saphenous vein on the medial and anterior sides or along the small saphenous vein on the posterior side, where suitable collecting lymphatic vessels are reported to be present [23]. The great saphenous vein and the small saphenous vein and their branches were preoperatively identified with ultrasound (Figure 1).
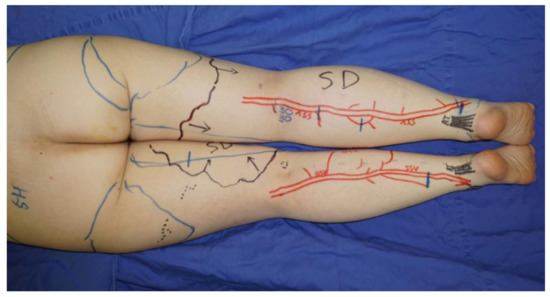
Figure 1.
If a linear pattern was not identified with indocyanine green lymphography, the incisions for lymphaticovenular anastomosis were made in the region along the small saphenous vein on the posterior side. The small saphenous vein and their branches were preoperatively identified and marked using ultrasonography.
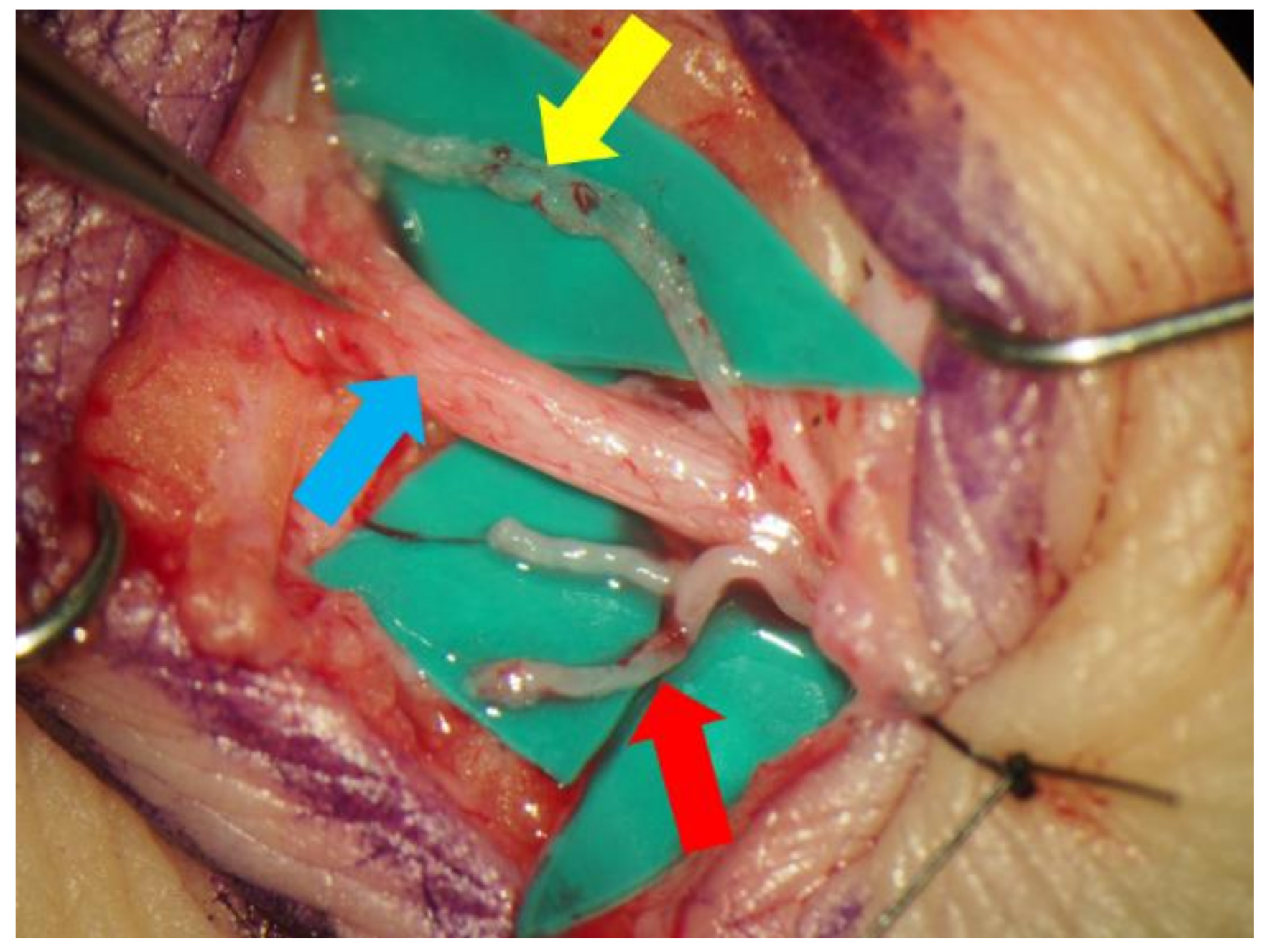
Lidocaine (1% or 0.5%) with epinephrine was injected intradermally or subcutaneously in the target region, and skin incisions were made to identify the subcutaneous veins or branches of the small saphenous vein and collecting lymphatic vessels (Figure 2). An elongation of skin incision was performed when the lymphatic vessels or veins could not be found through the initial incision. The veins and the lymphatic vessels were anastomosed in either an end-to-end or end-to-side fashion using 11-0 or 12-0 nylon micro-sutures. The patency of LVA was confirmed by observation of the anastomosis site under a surgical microscope. Preoperative conservative therapy was resumed immediately after the surgery.
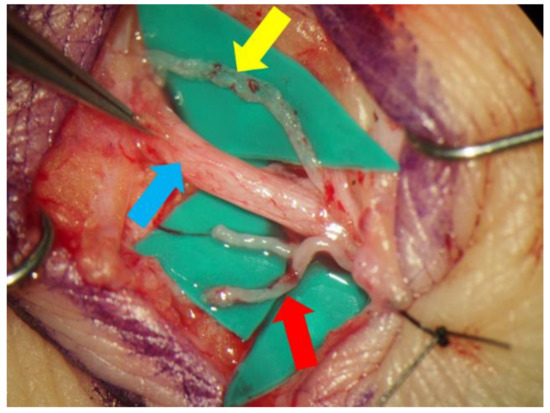
Figure 2.
The small saphenous vein (blue arrow) and the lymphatic vessel (yellow arrow) were dissected out via a small incision made in posterior side of the lower leg. In this case, the lymphatic vessel ran along the small saphenous vein. The branches of the saphenous vein (red arrow), which seemed to be suitable for lymphaticovenular anastomosis, were also dissected.
2.3. Effectiveness of Additional Lymphaticovenular Anastomosis on the Posterior Side
A comparison was made between two groups on the following aspects in the second surgery: patient characteristics (age, body mass index and duration of edema), the number of anastomoses per patient, the diameter of the lymphatic vessels, the required time for dissecting the lymphatic vessels and the veins and skin incision length. Lymphedematous volume was evaluated using a lower extremity lymphedema (LEL) index determined before the second surgery and more than 6 months after the second surgery [24]. The reduction in volume was compared between the two groups.
2.4. Statistical Analysis
Analysis was carried out using SPSS (SPSS, Inc., Chicago, IL, USA). All values were reported as mean ± SD. Differences in the means between groups were analyzed by the t test. All p values were two-sided, and statistical significance was accepted as p < 0.05.
3. Results
In total, 45 primary lower limb lymphedema patients underwent LVA twice between March 2018 and September 2020. Of the 45 patients, 19 were excluded based on the exclusion criteria (Table 2), whereas the remaining 26 (33 lower limbs) were included in the analysis of the efficacy of LVA.

Table 2.
Exclusion criteria for evaluation of the efficacy of lymphaticovenous anastomosis and the number of excluded patients.
The characteristics of the included patients (4 men and 22 women) are shown in Table 3. The average age was 44.2 years (range, 16 to 82 years), and the median age of lymphedema onset was 26.3 years (range, 14 to 76 years). The average lymphedema duration was 8.6 years (range, 10 months to 29 years). According to the preoperative leg dermal backflow stage criteria, we classified two limbs as stage 2, seven limbs as stage 3, five limbs as stage 4, seven limbs as stage 5, four limbs as no backflow and eight limbs as distal backflow (Table 3).

Table 3.
Patient characteristics.
Patients were classified into two groups, 13 (1 man and 12 women, 18 limbs) in the PoLVA group and 13 (3 men and 10 women, 15 limbs) in the MeLVA group. The LVA resulted in a total of 227 anastomoses (106 anastomoses in the PoLVA group and 121 anastomoses in the MeLVA group) in 26 patients with primary lymphedema of the lower extremities; the average total number of anastomoses in two surgeries per case was 8.7 (range, 5–12); the average number of anastomoses on the posterior side in the PoLVA group was 3.5 (range, 2–5); the average total operation time of the two surgeries was 406 min (range, 283–725 min); the average total follow-up period was 526 days (range, 205–1089 days); and the average total postoperative reduction in the lower extremity lymphedema index was 18.1 (range, 5.3 to 32.9) (Table 4).

Table 4.
Operation summary.
Effectiveness of Additional Lymphaticovenular Anastomosis on the Posterior Side
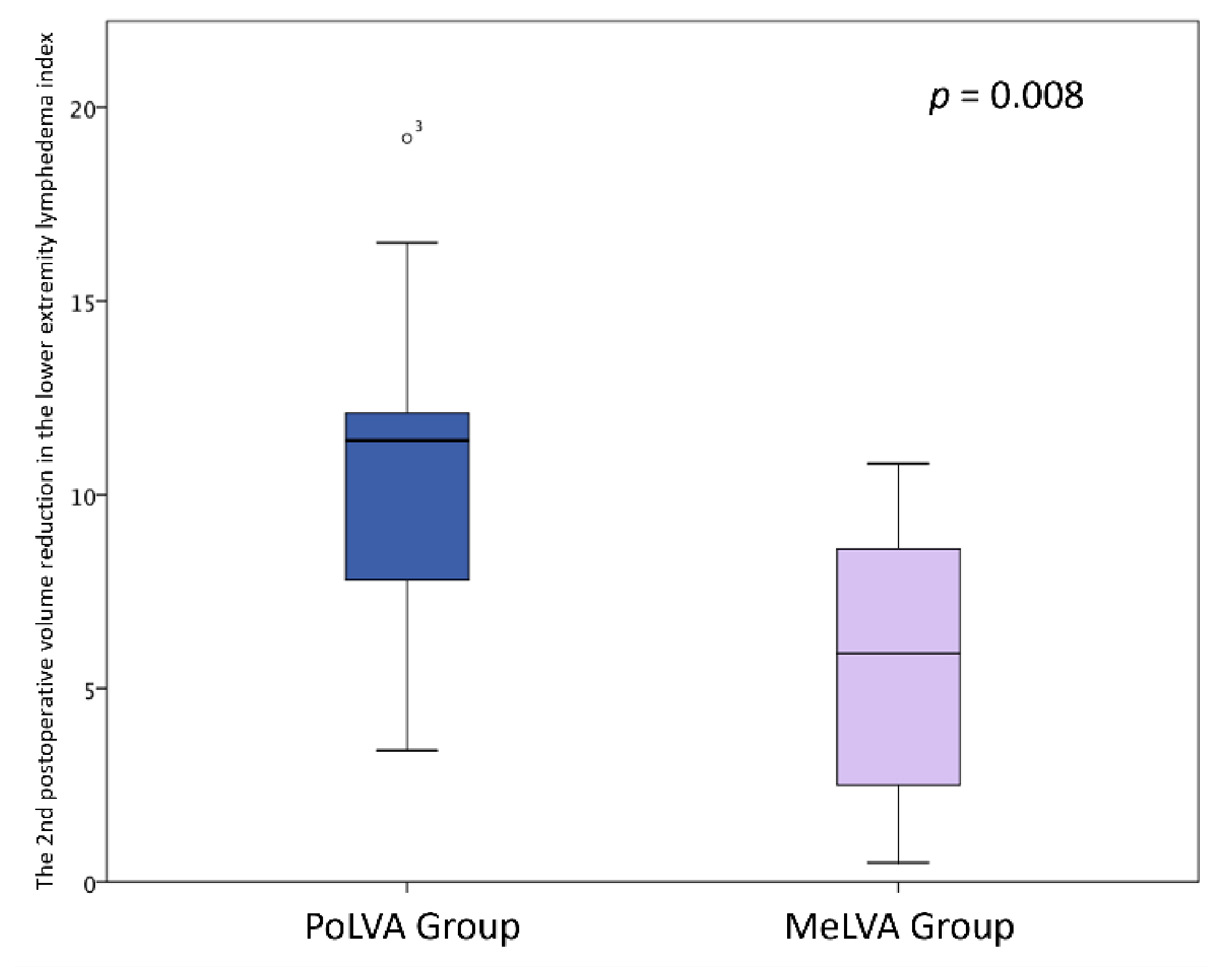
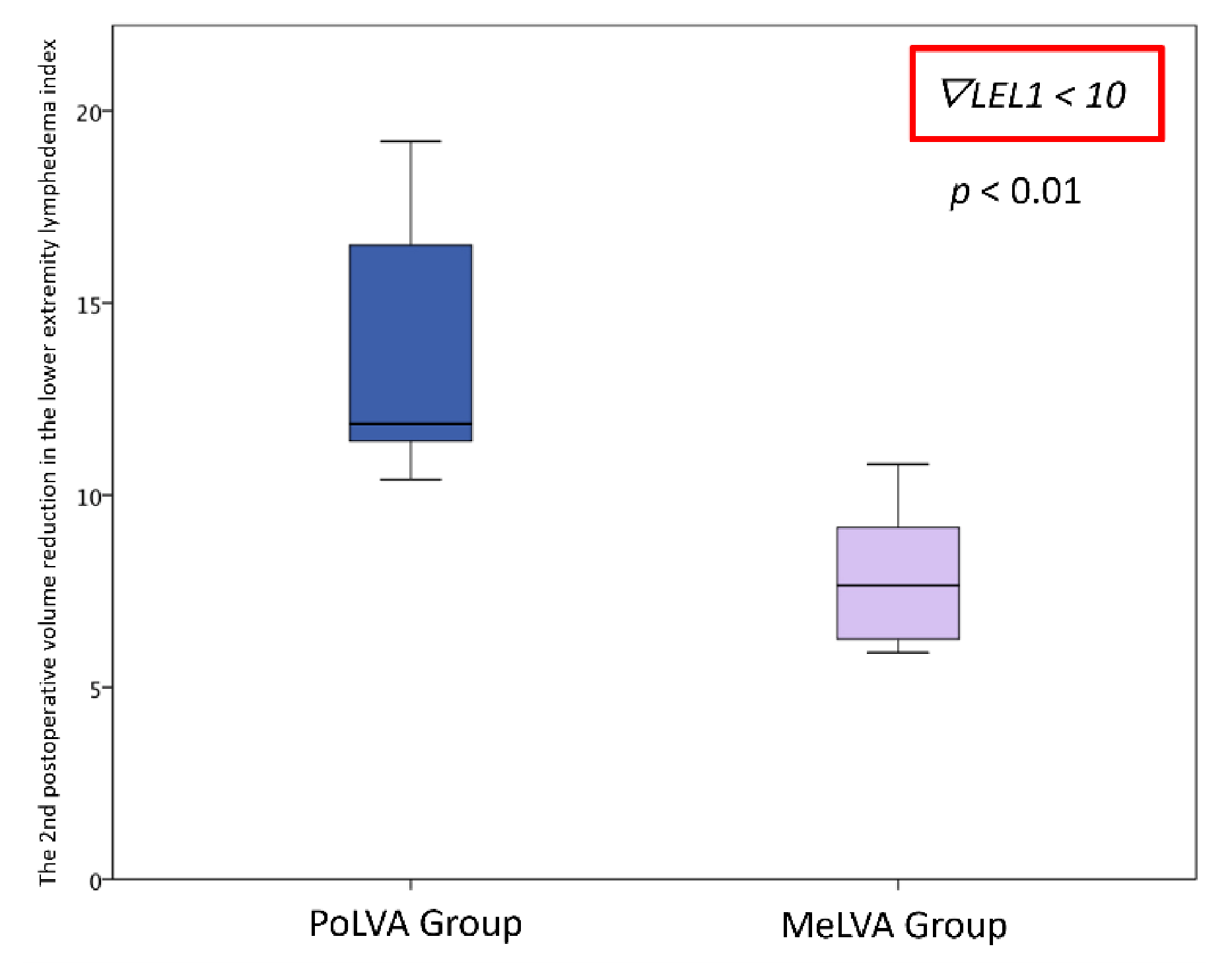
The second postoperative volume reduction in the LEL index was significantly greater in the PoLVA group than in the MeLVA group in all subjects (10.5 ± 4.5 vs. 5.5 ± 3.6; p = 0.008), in the subject with the first postoperative reduced LEL index score of less than 10 (8.0 ± 3.8 vs. 2.5 ± 1.5; p =0.01) and in the subject with the first postoperative reduced LEL index score of more than 10 (13.5 ± 3.5 vs. 7.3 ± 2.8; p < 0.01) (Figure 3, Figure 4 and Figure 5). A significant difference was seen between the PoLVA group and the MeLVA group in the number of anastomoses (3.5 ± 0.6 vs. 4.6 ± 1.0; p = 0.038) and in skin incision length (3.7 ± 1.1 vs. 2.9 ± 0.6 cm; p = 0.042). Incision elongation was necessary in 19 sites out of 46 sites in the PoLVA group, whereas incision elongation was performed in 2 sites out of 59 sites in the MeLVA group. The differences in the diameter of lymphatic vessels and the required time for dissecting lymphatic vessels and veins were not statistically significant between the PoLVA group and the MeLVA group (0.48 ± 0.16 vs. 0.51 ± 0.19 mm; p = 0.629, 15.8 ± 2.9 vs. 12.7 ± 2.4 cm; p = 0.093) (Table 5).
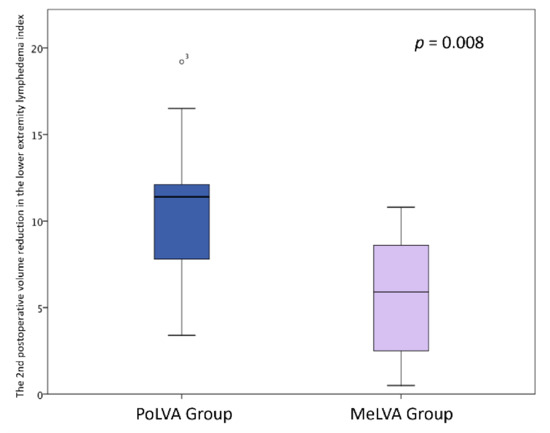
Figure 3.
The second postoperative volume reduction in the lower extremity lymphedema index was significantly greater in the PoLVA group than the MeLVA group in all subjects (10.5 ± 4.5 vs. 5.5 ± 3.6; p = 0.008). Error bars represent standard error.
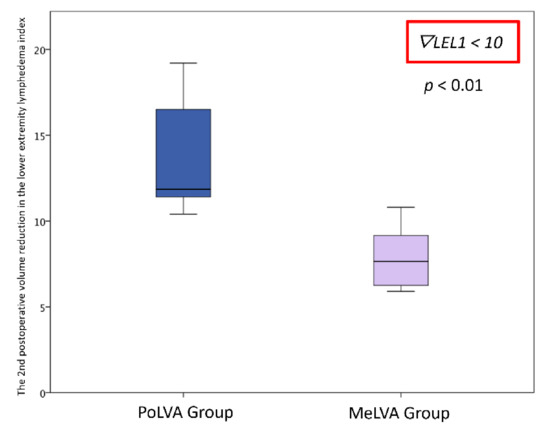
Figure 4.
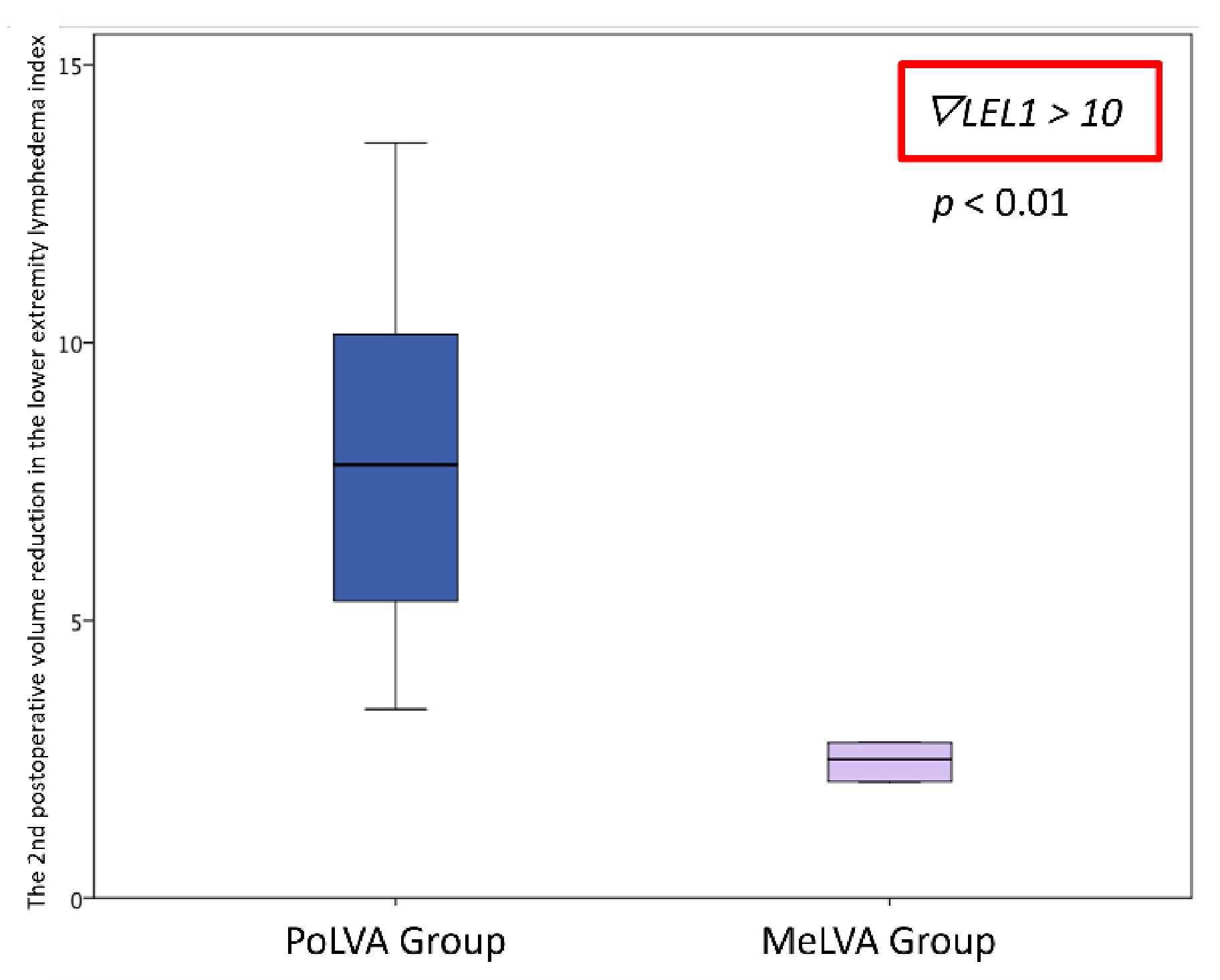
Volume reduction in the lower extremity lymphedema index was significantly greater in the PoLVA group than in the MeLVA group in subject with the first postoperative reduced LEL index score of less than 10 (8.0 ± 3.8 vs. 2.5 ± 1.5; p = 0.01). Error bars represent standard error.
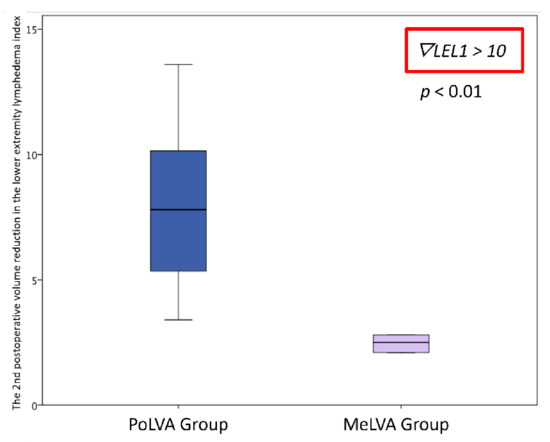
Figure 5.
Volume reduction in the lower extremity lymphedema index was significantly greater in the PoLVA group than in the MeLVA group in subject with the first postoperative reduced LEL index score of more than 10 (13.5 ± 3.5 vs. 7.3 ± 2.8; p < 0.01). Error bars represent standard error.

Table 5.
Comparison between cases of PoLVA and MeLVA groups.
4. Discussion
The present study was conducted to investigate the efficacy of LVA on the posterior side for the treatment of primary lymphedema. The results of this retrospective study demonstrated a statistically significant decrease in postoperative volume in the LEL index and fewer numbers of LVA in the group of those who underwent LVA on the posterior side in the second operation. Thus, LVA on the posterior side is recommended for the second LVA regardless of the results from the first LVA performed on the medial and anterior sides.
Several reports have been published on the efficacy of LVA. O’Brien et al. reported that LVA for primary lymphedema was difficult because the lymphatic vessels were hypoplastic [17]. In contrast, Demirtas et al. stated that LVA was similarly efficacious for both primary and secondary lymphedema [18]. Hara et al. reported that LVA was effective in patients developing primary lymphedema after the age of 11 years [19]. Thus, in this study, we excluded patients whose onset of lymphedema was before the age of 11 years. We often performed LVA more than two times, including not only on the medial and anterior sides but also on the posterior side because all lymphatic drainage routes are likely to be damaged in primary lymphedema. Moreover, we occasionally performed a combined surgical procedure including LVA, vascularized lymph node transfer and a reductive procedure. Even though we excluded patients who underwent a combined procedure in this study, a combination of physiologic and excisional methods was reported to be effective for the treatment in the advanced stages of lymphedema [25,26]. The present retrospective study revealed that the extent of improvement was greater when the second LVA was performed on the posterior side than on the medial and anterior sides.
It has been reported that on the posterior side of the lower extremity, only one or two lymph collecting vessels arise from the lateral side of the Achilles tendon, which travel along the small saphenous vein. Pan et al. demonstrated these vessels drain into the popliteal and the femoral lymph nodes before finally entering the deep inguinal, the external iliac or the inferior gluteal lymph node groups, while most lymphatic vessels in the anterior side drain into the superficial inguinal lymph nodes [23]. Their study revealed that the lymphatic drainage route in the posterior side is completely different from that of the anterior side. This may explain why the decrease in lower extremity lymphedema index was significantly larger in the PoLVA group, making bypasses in completely different lymphatic routes in the second LVA.
In our study, incision elongation was required more often in the PoLVA group, presumably due to the fact that there are fewer lymphatic vessels on the posterior side. For LVA on the posterior side, in addition to an accurate anatomical understanding of the lymphatic routes, a preoperative detection technique of the lymphatic vessels and the branches of the small saphenous vein is also important. ICG lymphography is a minimally invasive imaging modality that can not only evaluate the severity of lymphedema but also determine the location of the lymphatic vessels by visualizing superficial lymph flows [16,21,27,28]. If a linear ICG lymphography pattern is observed, skin incisions can be made on the linear pattern. However, ICG lymphography cannot visualize lymphatic flow in the deep layer of subcutaneous tissue, or one that is masked beneath dermal backflow patterns, particularly in stardust and diffuse patterns [29]. Ultrasonography can detect the lymphatic vessels even in regions with dermal backflow patterns [30,31,32,33]. Ultrasonography provides a roadmap for surgeons when performing LVA. Surgeons may also select the vein with an appropriate size for LVA from among subcutaneous veins and branches of the small saphenous vein on the posterior side by using preoperative ultrasonography on the posterior side. These techniques may aid LVA on the posterior side, prevent intraoperative incision elongation and decrease the operative time.
This study has several limitations. First, we did not statistically examine the relationship between the group that underwent LVA only on the posterior side and the group with LVA only on the medial or anterior side. We usually first perform LVA on the medial and anterior sides for the following reasons: (1) we are familiar with an anatomical understanding of the lymphatic pathways on the medial and anterior sides, and (2) there are more lymphatic vessels on the medial and anterior sides than on the posterior side. Second, the study is limited by its retrospective nature. The effects of LVA include not only volume reduction, but also the reduction in the frequency of cellulitis or softening of the edematous limbs. A prospective study with a large number of patients is needed to take these accounts into consideration.
5. Conclusions
To our knowledge, this is the first attempt to investigate the efficacy of LVA on the posterior side of the lower limb for the treatment of primary lymphedema. Although fewer anastomoses were established, LVA on the posterior side subsequent to LVA on the medial and anterior sides resulted in further improvement of primary lower extremity lymphedema with fewer numbers of anastomoses.
Author Contributions
Conceptualization, A.H., G.V. and J.C.-S.Y.; methodology, A.H.; software, A.H.; validation, N.H. and H.Y.; formal analysis, G.V.; investigation, A.H., G.V. and J.C.-S.Y.; resources, N.H.; data curation, G.V.; writing—original draft preparation, A.H., G.V. and H.Y.; writing—review and editing, A.H., N.H. and H.Y.; visualization, N.H.; supervision, J.C.-S.Y. and H.Y.; project administration, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kameda General Hospital (Approval number: 18–055).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to “publish this paper” if applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koshima, I.; Inagawa, K.; Urushibara, K.; Moriguchi, T. Supermicrosurgical LVA for the treatment of lymphedema in the upper extremities. J. Reconstr. Microsurg. 2000, 16, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kerchner, K.; Fleischer, A.; Yosipovitch, G. Lower extremity lymphedema update: Pathophysiology, diagnosis, and treatment guidelines. J. Am. Acad. Dermatol. 2008, 59, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Beesley, V.; Janda, M.; Eakin, E.; Obermair, A.; Battistutta, D. Lymphedema after gynecological cancer treatment: Prevalence, correlates, and supportive care needs. Cancer 2007, 109, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Villa, G.; Bogliolo, S.; Ferrero, S.; Murelli, F.; Campisi, C. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann. Surg. Oncol. 2009, 16, 703–708. [Google Scholar] [CrossRef]
- Moseley, A.L.; Carati, C.J.; Piller, N.B. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann. Oncol. 2007, 18, 639–646. [Google Scholar] [CrossRef]
- Deltombe, T.; Jamart, J.; Recloux, S.; Legrand, C.; Vandenbroeck, N.; Theys, S.; Hanson, P. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology 2007, 40, 26–34. [Google Scholar]
- Szolnoky, G.; Lakatos, B.; Keskeny, T.; Varga, E.; Varga, M.; Dobozy, A.; Kemény, L. Intermittent pneumatic compression acts synergistically with manual lymphatic drainage in complex decongestive physiotherapy for breast cancer treatment-related lymphedema. Lymphology 2009, 42, 188–194. [Google Scholar]
- Kwan, M.L.; Darbinian, J.; Schmitz, K.H.; Citron, R.; Partee, P.; Kutner, S.E.; Kushi, L.H. Risk factors for lymphedema in a prospective breast cancer survivorship study: The Pathways Study. Arch. Surg. 2010, 145, 1055–1063. [Google Scholar] [CrossRef]
- Lee, B.B.; Andrade, M.; Antignani, P.L.; Boccardo, F.; Bunke, N.; Campisi, C.; Damstra, R.; Flour, M.; Forner-Cordero, I.; Gloviczki, P.; et al. Diagnosis and treatment of primary lymphedema: Consensus document of the International Union of Phlebology (IUP)-2013. Int. Angiol. 2013, 32, 541–574. [Google Scholar]
- Blatt, J.; Powell, C.M.; Burkhart, C.N.; Stavas, J.; Aylsworth, A.S. Genetics of hemangiomas, vascular malformations, and primary lymphedema. J. Pediatr. Hematol. Oncol. 2014, 36, 587–593. [Google Scholar] [CrossRef]
- Rustgi, A.K.; Rotolo, F.S.; Peete, W.P.; Vollmer, R.T.; Meyers, W.C. Successful management of late-onset primary lymphatic hypoplasia. Surgery 1985, 97, 714–720. [Google Scholar]
- Sapountzis, S.; Ciudad, P.; Lim, S.Y.; Chilgar, R.M.; Kiranantawat, K.; Nicoli, F.; Constantinides, J.; Wei, M.Y.; Sönmez, T.T.; Singhal, D.; et al. Modified Charles procedure and lymph node flap transfer for advanced lower extremity lymphedema. Microsurgery 2014, 34, 439–447. [Google Scholar] [CrossRef]
- Zeltzer, A.A.; Hamdi, M. Operative treatment of peripheral lymphedema: A systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplant. Plast. Reconstr. Surg. 2014, 134, 491e–492e. [Google Scholar] [CrossRef]
- Koshima, I.; Nanba, Y.; Tsutsui, T.; Takahashi, Y.; Itoh, S. Long-term followup after LVA for lymphedema in the leg. J. Reconstr. Microsurg. 2003, 19, 209–215. [Google Scholar]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Yoshimatsu, H.; Seki, Y.; Yamamoto, N.; Oka, A.; Hara, H.; Koshima, I. Minimally invasive lymphatic supermicrosurgery (MILS): ICG lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann. Plast. Surg. 2014, 72, 67–70. [Google Scholar] [CrossRef]
- O’Brien, B.M.; Mellow, C.G.; Khazanchi, R.K.; Dvir, E.; Kumar, V.; Pederson, W.C. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast. Reconstr. Surg. 1990, 85, 562–572. [Google Scholar] [CrossRef]
- Demirtas, Y.; Ozturk, N.; Yapici, O.; Topalan, M. Comparison of primary and secondary lower-extremity lymphedema treated with supermicrosurgical lymphaticovenous anastomosis and lymphaticovenous implantation. J. Reconstr. Microsurg. 2010, 26, 137–143. [Google Scholar] [CrossRef]
- Hara, H.; Mihara, M.; Ohtsu, H.; Narushima, M.; Iida, T.; Koshima, I. Indication of Lymphaticovenous Anastomosis for Lower Limb Primary Lymphedema. Plast. Reconstr. Surg. 2015, 136, 883–893. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimatsu, H.; Narusima, M.; Yamamoto, N.; Hayashi, A.; Koshima, I. Indocyanine Green Lymphography Findings in Primary Leg Lymphedema. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 95–102. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsuda, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: The modified dermal backflow stage and concept of subclinical lymphedema. Plast. Reconstr. Surg. 2011, 128, 314e–321e. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yoshimatsu, H.; Yamamoto, N.; Narushima, M.; Iida, T.; Koshima, I. Side-to-end Lymphaticovenular anastomosis through temporary lymphatic expansion. PLoS ONE 2013, 8, e59523. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.R.; Wang, D.G.; Levy, S.M.; Sci, B.M.; Chen, Y. Superficial lymphatic drainage of the lower extremity: Anatomical study and clinical implications. Plast. Reconstr. Surg. 2013, 132, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Matsuda, N.; Todokoro, T.; Yoshimatsu, H.; Narushima, M.; Mihara, M.; Uchida, G.; Koshima, I. Lower extremity lymphedema index: A simple method for severity evaluation of lower extremity lymphedema. Ann. Plast. Surg. 2011, 67, 637–640. [Google Scholar] [CrossRef]
- Campisi, C.C.; Ryan, M.; Boccardo, F.; Campisi, C. Fibro-Lipo-Lymph-Aspiration with a Lymph Vessel Sparing Procedure to Treat Advanced Lymphedema After Multiple Lymphatic-Venous Anastomoses: The Complete Treatment Protocol. Ann. Plast. Surg. 2017, 78, 184–190. [Google Scholar] [CrossRef]
- Bolletta, A.; Taranto, G.; Losco, L.; Elia, R.; Sert, G.; Ribuffo, D.; Cigna, E.; Chen, H.-C. Combined lymph node transfer and suction-assisted lipectomy in lymphedema treatment: A prospective study. Microsurgery 2022. (ahead of print). [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: The generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Doi, K.; Oshima, A.; Yoshimatsu, H.; Todokoro, T.; Ogata, F.; Mihara, M.; Narushima, M.; Iida, T.; et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: A novel severity staging system using dermal backflow patterns. Plast. Reconstr. Surg. 2011, 128, 941–947. [Google Scholar] [CrossRef]
- Ogata, F.; Narushima, M.; Mihara, M.; Azuma, R.; Morimoto, Y.; Koshima, I. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann. Plast. Surg. 2007, 59, 180–184. [Google Scholar] [CrossRef]
- Hayashi, A.; Hayashi, N.; Yoshimatsu, H.; Yamamoto, T. Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J. Surg. Oncol. 2018, 117, 290–298. [Google Scholar] [CrossRef]
- Hayashi, A.; Yamamoto, T.; Yoshimatsu, H.; Hayashi, N.; Furuya, M.; Harima, M.; Narushima, M.; Koshima, I. Ultrasound visualization of the lymphatic vessels in the lower leg. Microsurgery 2016, 36, 397–401. [Google Scholar] [CrossRef]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef]
- Visconti, G.; Bianchi, A.; Hayashi, A.; Salgarello, M. Ultra-high frequency ultrasound preoperative planning of the rerouting method for lymphaticovenular anastomosis in incisions devoid of vein. Microsurgery 2020, 40, 717–718. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).