Benefits of Telemonitoring of Pulmonary Function—3-Month Follow-Up of Home Electronic Spirometry in Patients with Duchenne Muscular Dystrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Respiratory System Assessment
2.3.1. Spirometry (Telemonitoring and Hospital Spirometry Control)
2.3.2. Respiratory Muscle Assessment
2.3.3. Functional Status and Hand Grip Strength
2.4. Telemonitoring Benefits Survey
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Pulmonary Function Test
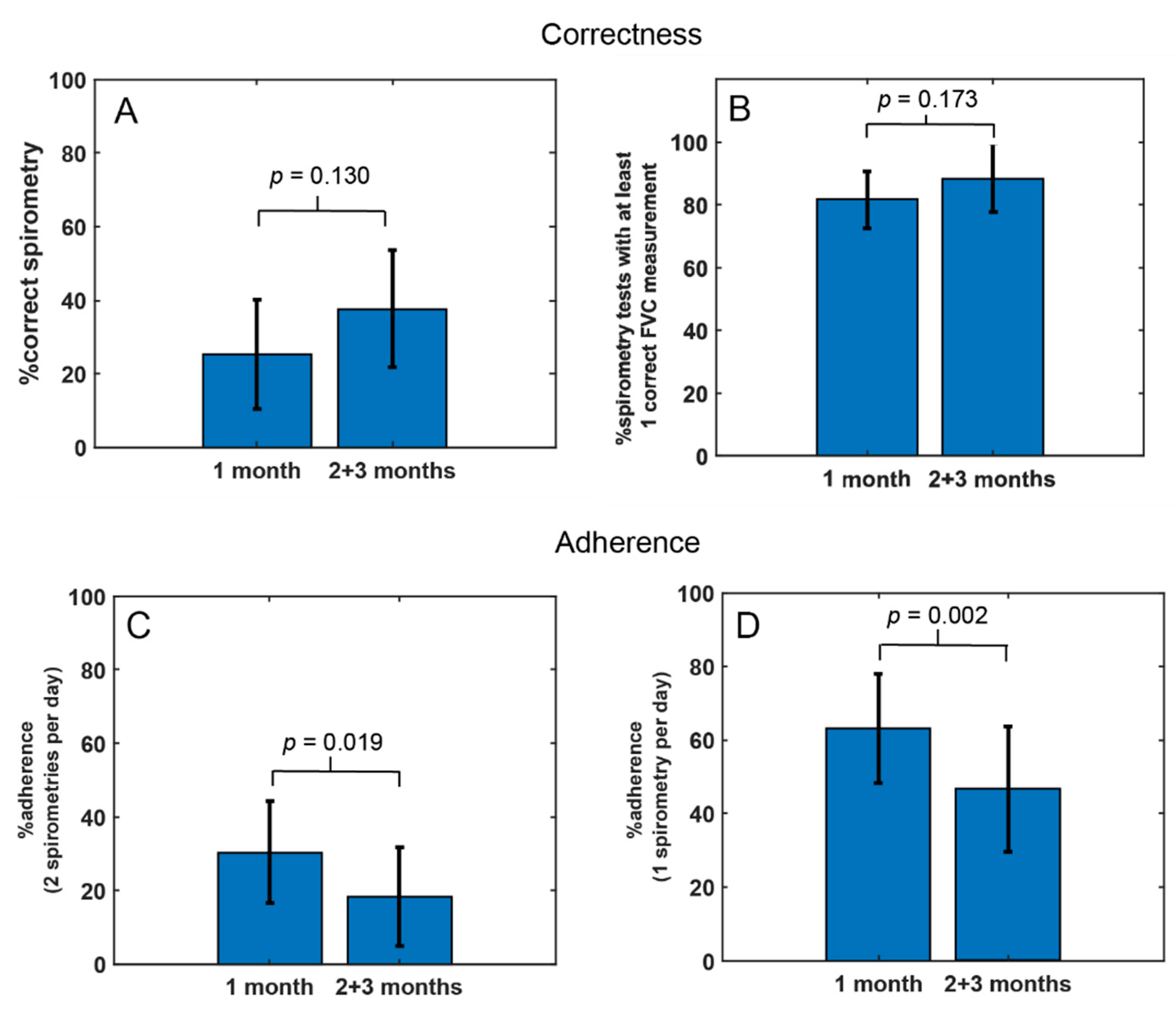
3.2.1. Telemonitoring Compliance
3.2.2. Study Group Spirometry
3.2.3. Individual Spirometry
3.2.4. Respiratory Muscle Assessment
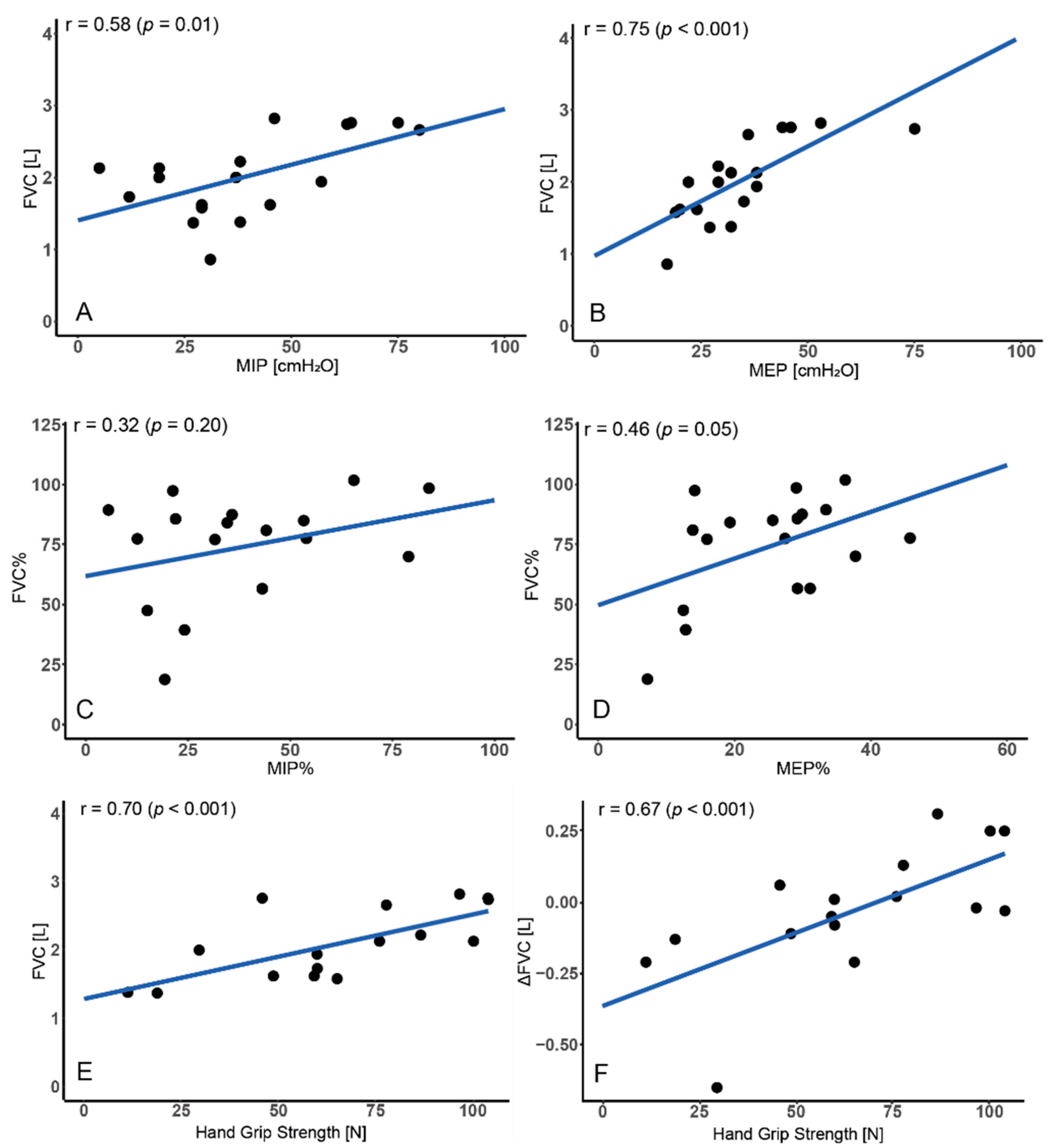
3.3. Relationship between FVC and Respiratory Muscles
3.4. Relationship between FVC and Motor Function
3.5. Regression Analysis
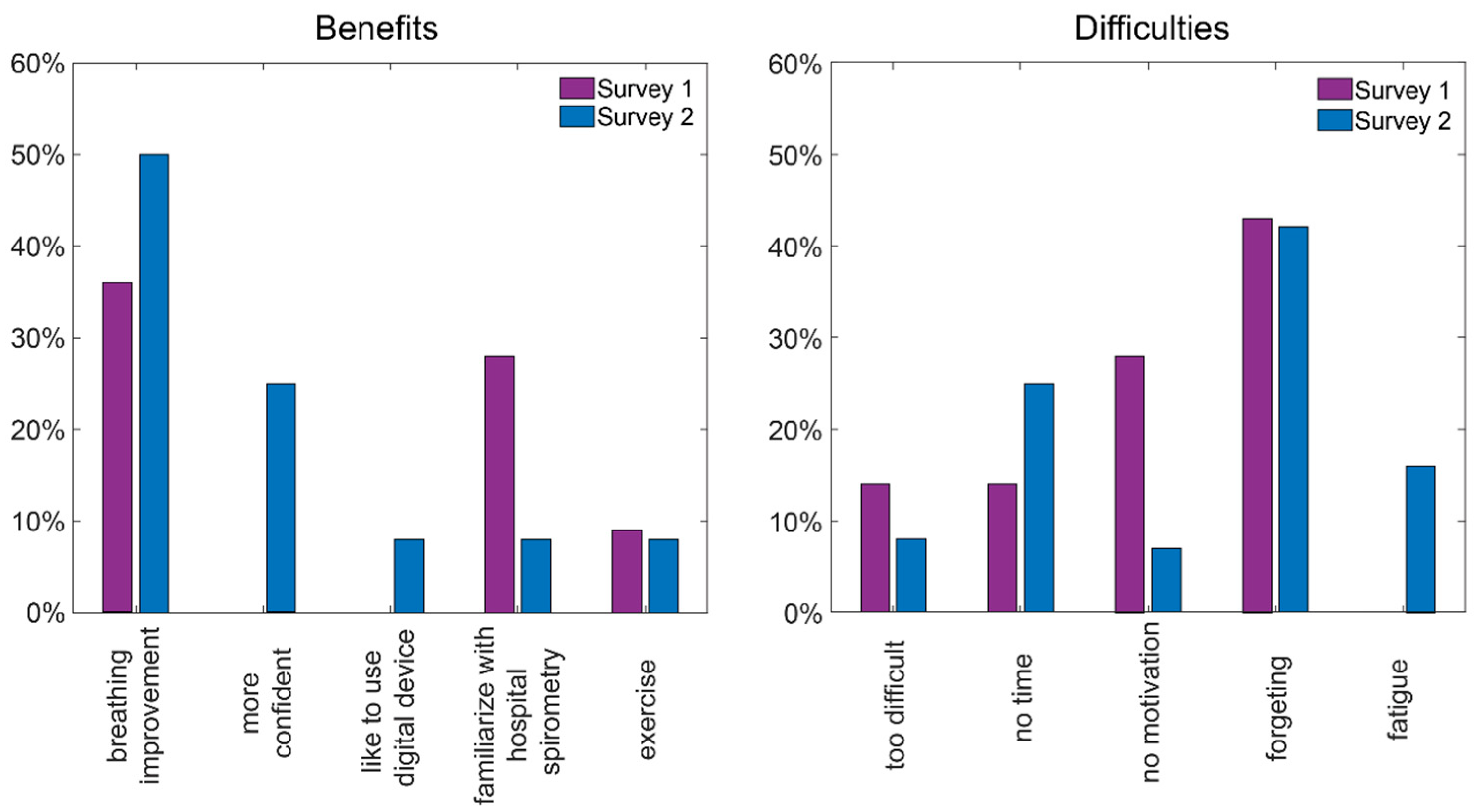
3.6. Telemonitoring Benefits Survey
4. Discussion
5. Summary
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef]
- Gayraud, J.; Ramonatxo, M.; Rivier, F.; Humberclaude, V.; Petrof, B.; Matecki, S. Ventilatory parameters and maximal respiratory pressure changes with age in Duchenne muscular dystrophy patients. Pediatr. Pulmonol. 2010, 45, 552–559. [Google Scholar] [CrossRef]
- Balaban, B.; Matthews, D.J.; Clayton, G.H.; Carry, T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: Long-term effect. Am. J. Phys. Med. Rehabil. 2005, 84, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Biggar, W.D.; Harris, V.A.; Eliasoph, L.; Alman, B. Long-Term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul. Disord. 2006, 16, 249–255. [Google Scholar] [CrossRef]
- LoMauro, A.; Romei, M.; Gandossini, S.; Pascuzzo, R.; Vantini, S.; D’Angetlo, M.G.; Aliverti, A. Evolution of respiratory function in Duchenne muscular dystrophy from childhood to adulthood. Eur. Respir. J. 2018, 51, 1701418. [Google Scholar] [CrossRef]
- Wasilewska, E.; Małgorzewicz, S.; Meyer-Szary, J.; Sledzinska, K.; Niedoszytko, M.B.; Jalssem, E.; Wierzba, J. Pulmonary dysfunction in children with Duchenne muscular dystrophy may appear earlier than we thought—Analysis using novel methodology based on z-scores. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Kato, N.P.; Johansson, P.; Okada, I.; De Vries, A.; Kinugawa, K.; Strömberg, A.; Jaarsma, T.; Hanley, J.; Rodina-Theocharaki, A. Heart Failure Telemonitoring in Japan and Sweden: A Cross-Sectional Survey. J. Med. Internet Res. 2015, 17, e258. [Google Scholar] [CrossRef]
- Vuorinen, A.-L.; Leppänen, J.; Kaijanranta, H.; Kulju, M.; Heliö, T.; Van Gils, M.; Lähteenmäki, J. Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: Randomized controlled trial. J. Med. Internet Res. 2014, 16, e282. [Google Scholar] [CrossRef]
- Aamodt, I.T.; Lycholip, E.; Celutkiene, J.; Strömberg, A.; Atar, D.; Falk, R.S.; Von Lueder, T.; Hellesø, R.; Jaarsma, T.; Lie, I. Health Care Professionals’ Perceptions of Home Telemonitoring in Heart Failure Care: Cross-Sectional Survey. J. Med. Internet Res. 2019, 21, e10362. [Google Scholar] [CrossRef]
- Seto, E.; Leonard, K.J.; Cafazzo, J.A.; Barnsley, J.; Masino, C.; Ross, H.J. Mobile phone-based telemonitoring for heart failure management: A randomized controlled trial. J. Med. Internet Res. 2012, 14, e31. [Google Scholar] [CrossRef]
- Andrès, E.; Meyer, L.; Zulfiqar, A.-A.; Hajjam, M.; Talha, S.; Bahougne, T.; Ervé, S.; Hajjam, J.; Doucet, J.; Jeandidier, N.; et al. Telemonitoring in diabetes: Evolution of concepts and technologies, with a focus on results of the more recent studies. J. Med. Life. 2019, 12, 203–214. [Google Scholar] [CrossRef]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in patients with heart failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The telemedical interventional monitoring in heart failure study. Circulation. 2011, 123, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.H.; Hanley, J.; Lewis, S.C.; McKnight, J.A.; McCloughan, L.B.; Padfield, P.L.; Parker, R.A.; Paterson, M.; Pinnock, H.; Sheikh, A.; et al. Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial. PLoS Med. 2016, 13, e1002098. [Google Scholar] [CrossRef]
- Nicolucci, A.; Cercone, S.; Chiriatti, A.; Muscas, F.; Gensini, G. A Randomized Trial on Home Telemonitoring for the Management of Metabolic and Cardiovascular Risk in Patients with Type 2 Diabetes. Diabetes Technol. Ther. 2015, 17, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.; O’Connor, G.; Friedmann, R.H. Development and implementation of the home asthma telemonitoring (HAT) system to facilitate asthma self-care. Stud. Health Technol. Inform. 2001, 84, 810–814. [Google Scholar]
- Shakkottai, A.; Kaciroti, N.; Kasmikha, L.; Nasr, S.Z. Impact of home spirometry on medication adherence among adolescents with cystic fibrosis. Pediatr. Pulmonol. 2018, 53, 431–436. [Google Scholar] [CrossRef]
- Crimi, C.; Impellizzeri, P.; Campisi, R.; Nolasco, S.; Spanevello, A.; Crimi, N. Practical considerations for spirometry during the COVID-19 outbreak: Literature review and insights. Pulmonology 2021, 27, 438–447. [Google Scholar] [CrossRef]
- Crimi, C.; Impellizzeri, P.; Campisi, R.; Spicuzza, L.; Vancheri, C.; Crimi, N. Resumption of respiratory outpatient services in the COVID-19 era: Experience from Southern Italy. Am. J. Infect. Control. 2020, 48, 1087–1089. [Google Scholar] [CrossRef]
- Trucco, F.; Domingos, J.P.; Tay, C.G.; Ridout, D.; Maresh, K.; Munot, P.; Sarkozy, A.; Robb, S.; Quinlivan, R.; Riley, M.; et al. Cardiorespiratory Progression Over 5 Years and Role of Corticosteroids in Duchenne Muscular Dystrophy: A Single-Site Retrospective Longitudinal Study. Chest 2020, 158, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Buyse, G.M.; Voit, T.; Schara, U.; Straathof, C.S.M.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.-M.; Finkel, R.S.; Goemans, N.; McDonald, C.M.; et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomised placebo-controlled phase 3 trial. Lancet 2015, 385, 1748–1757. [Google Scholar] [CrossRef]
- Buyse, G.M.; Voit, T.; Schara, U.; Straathof, C.S.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.-M.; Finkel, R.S.; Goemans, N.; Rummey, C.; et al. Treatment effect of idebenone on inspiratory function in patients with Duchenne muscular dystrophy. Pediatr. Pulmonol. 2017, 52, 508–515. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Meier, T.; Voit, T.; Schara, U.; Straathof, C.S.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.-M.; Finkel, R.S.; Goemans, N.; et al. Idebenone reduces respiratory complications in patients with Duchenne muscular dystrophy. Neuromuscul. Disord. 2016, 26, 473–480. [Google Scholar] [CrossRef]
- Finder, J.D.; Birnkrant, D.; Carl, J.; Farber, H.J.; Gozal, D.; Iannaccone, S.T.; Kovesi, T.; Kravitz, R.M.; Panitch, H.; Schramm, C.; et al. Respiratory care of the patient with duchenne muscular dystrophy: ATS consensus statement. Am. J. Respir. Crit. Care Med. 2004, 170, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Topin, N.; Matecki, S.; Le Bris, S.; Rivier, F.; Echenne, B.; Prefaut, C.; Ramonatxo, M. Dose-Dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul. Disord. 2002, 12, 576–583. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Carvalho, C.R.F.; Santaella, D.F.; Lorenzi-Filho, G.; Marie, S.K.N. Effects of yoga breathing exercises on pulmonary function in patients with Duchenne muscular dystrophy: An exploratory analysis. J. Bras. Pneumol. Publicacao Soc. Bras. Pneumol. Tisilogia 2014, 40, 128–133. [Google Scholar] [CrossRef]
- Williamson, E.; Pederson, N.; Rawson, H.; Daniel, T. The Effect of Inspiratory Muscle Training on Duchenne Muscular Dystrophy: A Meta-analysis. Pediatr. Phys. Ther. Off. Publ. Sect. Pediatr. Am. Phys. Ther. Assoc. 2019, 31, 323–330. [Google Scholar] [CrossRef]
- Sobierajska-Rek, A.; Mański, Ł.; Jabłońska-Brudło, J.; Śledzińska, K.; Wasilewska, E.; Szalewska, D. Respiratory Telerehabilitation of Boys and Young Men with Duchenne Muscular Dystrophy in the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 6179. [Google Scholar] [CrossRef]
- Wasilewska, E.; Sobierajska-Rek, A.; Małgorzewicz, S.; Soliński, M.; Szalewska, D.; Jassem, E. Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?—A One Center Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 8967. [Google Scholar] [CrossRef]
- Aiocare. Available online: https://healthcloud.aiocare.com (accessed on 1 December 2021).
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.; Spruit, M.A.; Masefield, S.C.; Casaburi, R.; Clini, E.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O.H.; Aliverti, A.; Meier, T. Breathe Duchenne: What natural history studies tell us about the progression of pulmonary morbidity in Duchenne muscular dystrophy. Neuromuscul. Disord. 2018, 28, 910–913. [Google Scholar] [CrossRef]
- Vignos, P.J.J.; Spencer, G.E.J.; Archibald, K.C. Management of progressive muscular dystrophy in childhood. JAMA 1963, 184, 89–96. [Google Scholar] [CrossRef]
- Brooke, M.H.; Griggs, R.C.; Mendell, J.R.; Fenichel, G.M.; Shumate, J.B.; Pellegrino, R.J. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve 1981, 4, 186–197. [Google Scholar] [CrossRef]
- Mayhew, A.G.; Coratti, G.; Mazzone, E.S.; Klingels, K.; James, M.; Pane, M.; Straub, V.; Goemans, N.; Mercuri, E.; Ricotti, V.; et al. Performance of Upper Limb module for Duchenne muscular dystrophy. Dev. Med. Child. Neurol. 2020, 62, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Eaton, T.; Withy, S.; Garrett, J.E.; Mercer, J.; Whitlock, R.M.; Rea, H.H. Spirometry in primary care practice: The importance of quality assurance and the impact of spirometry workshops. Chest 1999, 116, 416–423. [Google Scholar] [CrossRef]
- Kupczyk, M.; Hofman, A.; Kołtowski, Ł.; Kuna, P.; Łukaszyk, M.; Buczyłko, K.; Bodzenta-Łukaszyk, A.; Nastałek, P.; Soliński, M.; Dąbrowiecki, P. Home self-monitoring in patients with asthma using a mobile spirometry system. J. Asthma 2021, 58, 505–511. [Google Scholar] [CrossRef]
- Abboud, S.; Bruderman, I. Assessment of a new transtelephonic portable spirometer. Thorax 1996, 51, 407–410. [Google Scholar] [CrossRef][Green Version]
- Bruderman, I.; Abboud, S. Telespirometry: Novel system for home monitoring of asthmatic patients. Telemed. J. 1997, 3, 127–133. [Google Scholar] [CrossRef]
- Mortimer, K.M.; Fallot, A.; Balmes, J.R.; Tager, I.B. Evaluating the use of a portable spirometer in a study of pediatric asthma. Chest 2003, 123, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Logie, K.; Welsh, L.; Ranganathan, S.C. Telehealth spirometry for children with cystic fibrosis. Arch. Dis. Child. 2020, 105, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.M.; Jurdi, R.; Roberts, C.A.; Hernandez, M.; Horne, R.; Chan, A. A Review of Portable Electronic Spirometers: Implications for Asthma Self-Management. Curr. Allergy Asthma Rep. 2018, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Bashi, N.; Karunanithi, M.; Fatehi, F.; Ding, H.; Walters, D. Remote Monitoring of Patients with Heart Failure: An Overview of Systematic Reviews. J. Med. Internet Res. 2017, 19, e18. [Google Scholar] [CrossRef]
- Richardson, C.H.; Orr, N.J.; Ollosson, S.L.; Irving, S.J.; Balfour-Lynn, I.M.; Carr, S.B. Initiating home spirometry for children during the COVID-19 pandemic—A practical guide. Paediatr. Respir. Rev. 2021. [Google Scholar] [CrossRef]
- Kirkby, J.; Bountziouka, V.; Lum, S.; Wade, A.; Stocks, J. Natural variability of lung function in young healthy school children. Eur. Respir. J. 2016, 48, 411–419. [Google Scholar] [CrossRef]

| DMD Participants n = 21 | |
|---|---|
| Age years | 12.8 (4.4) |
| Weight kg | 51.3 (18.2) |
| Height cm | 148 (16) |
| BMI kg/m2 | 22.7 (5.3) |
| Brooke scale | 2 (4) Median (Q3–Q1) |
| Vignos scale | 8 (7.5) Median (Q3–Q1) |
| Hand grip strength | 66.3 (31.12) |
| Ambulatory yes/no | 10/11 |
| Mutation | Deletions 68.4% Point mutations 15.8% Duplications 10.5% Lack of mutation 5.3% |
| Participants ID | Number of Examinations | Correctness | Adherence | ||
|---|---|---|---|---|---|
| Correct Examinations (ATS/ERS 2019) nb (%) | Examinations with 1 Correct Maneuver nb (%) | Days with 2 Examinations nb (%) | Days with 1 Examination nb (%) | ||
| 1 | 60 | 50 (83.3%) | 58 (96.7%) | 3 (3.3%) | 57 (63.3%) |
| 2 | 176 | 0 (0%) | 47 (26.7%) | 85 (94.4%) | 89 (98.9%) |
| 3 | 3 | 0 (0%) | 1 (0%) | 0 (0%) | 2 (2.22%) |
| 4 | 133 | 61 (45.9%) | 114 (85.7%) | 44 (48.9%) | 82 (91.1%) |
| 5 | 86 | 10 (11.6%) | 65 (75.6%) | 21 (23.3%) | 64 (71.1%) |
| 6 | 170 | 0 (0%) | 165 (97.1%) | 76 (84.4%) | 90 (100%) |
| 7 | 68 | 0 (0%) | 39 (57.4%) | 12 (13.3%) | 55 (61.1%) |
| 8 | 88 | 81 (92%) | 88 (100%) | 1 (1.1%) | 87 (96.7%) |
| 9 | 18 | 5 (27.8%) | 18 (100%) | 5 (5.6%) | 13 (14.4%) |
| 10 | 14 | 3 (21.4%) | 11 (78.6%) | 3 (3.3%) | 10 (11.1%) |
| 11 | 12 | 0 (0%) | 11 (91.7%) | 3 (3.3%) | 9 (10%) |
| 12 | 22 | 5 (22.7%) | 20 (90.9%) | 1 (1.1%) | 21 (23.3%) |
| 13 | 65 | 6 (9.2%) | 57 (87.7%) | 12 (13.3%) | 50 (55.6%) |
| 14 | 42 | 10 (23.8%) | 27 (64.3%) | 8 (8.9%) | 34 (37.8%) |
| 15 | 1 | 0 (0%) | 1 (100%) | 0 (0%) | 1 (1.1%) |
| 16 | 90 | 37 (41.1%) | 89 (98.9%) | 24 (26.7%) | 66 (73.3%) |
| 17 | 106 | 96 (90.6%) | 105 (99.1%) | 34 (37.8%) | 69 (76.7%) |
| 18 | 103 | 49 (47.6%) | 84 (80.6%) | 31 (34.4%) | 71 (78.9%) |
| 19 | 26 | 16 (61.5%) | 26 (100%) | 1 (1.6%) | 25 (41%) |
| 20 | 23 | 0 (0%) | 21 (91.3%) | 7 (21.2%) | 13 (39.4%) |
| 21 | 97 | 21 (21.6%) | 78 (80.4%) | 39 (45.9%) | 46 (54.1%) |
| Total nb | 1403 | 450 | 1125 | 410 | 954 |
| Mean ± SD | 66.8 (52.4) | 21.4 ± 29.3 (28.6% ± 31.1%) | 53.6 ± 43.1 (81.1% ± 25.8%) | 19.5 ± 24.5 (22.5% ± 27.2%) | 45.4 ± 30.7 (52.4% ± 32.9%) |
| DMD Participants n = 19 | ||||||
|---|---|---|---|---|---|---|
| Hospital | p-Value | Home Mean | p-Value | |||
| Visit 1 | Visit 3 | V1 vs. Home | V3 vs. Home | |||
| FVC (L) | 2.02 (0.57) | 2.24(0.55) | 0.211 | 2.08 (0.66) | 0.505 | 0.332 |
| FVC (%pv) | 74.0 (22.2) | 80.1 (18.7) | 0.307 | 74.2 (26.0) | 0.918 | 0.905 |
| FEV1/FVC | 87.8 (7.6) | 88.5 (6.2) | 0.690 | 83.0 (18.3) | 0.224 | 0.435 |
| FEV1 (L) | 1.82 (0.56) | 2.00(0.48) | 0.765 | 1.72 (0.71) | 0.339 | 0.488 |
| FEV1 (%pv) | 77.6 (24.4) | 85.8 (20.0) | 0.141 | 71.3 (33.0) | 0.170 | 0.230 |
| PEF (L/min) | 209 (56) | 228 (55) | 0.653 | 193 (80) | 0.236 | 0.323 |
| PEF (%pv) | 69.0 (23.0) | 74.2 (19.6) | 0.606 | 64.26 (27.88) | 0.322 | 0.371 |
| Participants ID | Spirometry | |||
|---|---|---|---|---|
| Hospital | Home | |||
| ΔFVC (Liter) | ΔFVC (%pv) | ΔFVC (Liter) | ΔFVC (%pv) | |
| 1 | 0.12 | 5.4 | −0.08 * | −3.09 * |
| 2 | −0.14 | −13.8 | −0.51 * | −30.36 * |
| 3 ex | - | - | - | - |
| 4 | −0.04 | 4.3 | 0.31 | 10.36 |
| 5 | 0.24 | 7.7 | −0.02 | −0.55 |
| 6 | −0.01 | −0.8 | 0.25 * | 8.82 * |
| 7 | 0.4 | 16.2 | 0.25 * | 9.82 * |
| 8 | 0.13 | 5.2 | 0.02 | 0.71 |
| 9 | −0.0 | 0.0- | −0.21 | −10.43 |
| 10 | −0.12 | −2.9 | −0.65 | −15.28 |
| 11 | −0.14 | −5.5 | −0.21 | −9.3 |
| 12 | −0.02 | −1.1 | 0.01 | 0.4 |
| 13 | −0.18 | −4.4 | 0.06 | 2.78 |
| 14 | 0.07 | 2 | −0.03 | −0.91 |
| 15 ex | - | - | - | - |
| 16 | 0.25 | −2.7 | 0.13 * | 4.98 * |
| 17 | −0.01 | −0.5 | −0.05 | −2.55 |
| 18 | 0.12 | 0.0 | −0.11 * | −3.37 * |
| 19 | −0.0 | 0.0- | −0.13 | −8.47 |
| 20 | −0.0 | 0.0- | 0.14 | 2.95 |
| 21 | −0.0 | 0.0- | −0.2 | −3.74 |
| Mean (STD) | 0.04 (0.17) | 0.61 (6.83) | −0.05 (0.24) | −2.36 (9.39) |
| DMD Participants | p-Value V1 vs. V3 | ||
|---|---|---|---|
| Visit 1 | Visit 3 | ||
| MIPcmH2O | 39.7 (21.3) | 49.4 (20.0) | 0.169 |
| MIP %pv | 38.3 (22.3) | 46.8 (24.2) | 0.380 |
| MEPcmH2O | 34.2 (14.1) | 40.4 (20.8) | 0.533 |
| MEP %pv | 25.0 (10.5) | 29.1 (17.6) | 0.616 |
| Model 1 (ΔFVC%, e-Spirometry) | ||
| Variables | Coefficient (95%CI) | p-value |
| Intercept | −8.599 (−9.075~−8.409) | 0.090 |
| Age | −0.542 (−5.596~−0.539) | 0.196 |
| Hand Grip Strength | 0.150 (0.149~0.154) | 0.016 |
| Number of measurements | 0.062 (0.063~0.071) | 0.078 |
| Model 2 (ΔFVC (L), hospital spirometry) | ||
| Variables | Coefficient (95%CI) | p-value |
| Intercept | 0.092 (−0.117~0.485) | 0.499 |
| Hand Grip Strength | 0.007 (0.011~0.018) | 0.002 |
| MEP | −0.005 (−0.017~−0.003) | 0.117 |
| BMI | −0.008 (−0.028~−0.003) | 0.197 |
| Number of measurements | −0.002 (−0.006~−0.002) | 0.107 |
| Ambulatory | −0.164 (−0.467~−0.190) | 0.026 |
| Model 3 (ΔFVC%, hospital spirometry) | ||
| Intercept | 4.632 (4.482~5.239) | 0.237 |
| Age | −0.403 (−0.450~−0.339) | 0.131 |
| Hand Grip Strength | −0.255 (−0.259~−0.268) | <0.001 |
| MEP | −0.152 (−0.167~−0.151) | 0.095 |
| MIP | −0.117 (−0.121~−0.114) | 0.010 |
| Number of measurements | −0.065 (−0.070~−0.065) | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, E.; Sobierajska-Rek, A.; Małgorzewicz, S.; Soliński, M.; Jassem, E. Benefits of Telemonitoring of Pulmonary Function—3-Month Follow-Up of Home Electronic Spirometry in Patients with Duchenne Muscular Dystrophy. J. Clin. Med. 2022, 11, 856. https://doi.org/10.3390/jcm11030856
Wasilewska E, Sobierajska-Rek A, Małgorzewicz S, Soliński M, Jassem E. Benefits of Telemonitoring of Pulmonary Function—3-Month Follow-Up of Home Electronic Spirometry in Patients with Duchenne Muscular Dystrophy. Journal of Clinical Medicine. 2022; 11(3):856. https://doi.org/10.3390/jcm11030856
Chicago/Turabian StyleWasilewska, Eliza, Agnieszka Sobierajska-Rek, Sylwia Małgorzewicz, Mateusz Soliński, and Ewa Jassem. 2022. "Benefits of Telemonitoring of Pulmonary Function—3-Month Follow-Up of Home Electronic Spirometry in Patients with Duchenne Muscular Dystrophy" Journal of Clinical Medicine 11, no. 3: 856. https://doi.org/10.3390/jcm11030856
APA StyleWasilewska, E., Sobierajska-Rek, A., Małgorzewicz, S., Soliński, M., & Jassem, E. (2022). Benefits of Telemonitoring of Pulmonary Function—3-Month Follow-Up of Home Electronic Spirometry in Patients with Duchenne Muscular Dystrophy. Journal of Clinical Medicine, 11(3), 856. https://doi.org/10.3390/jcm11030856






