Assessment of the Effectiveness of Obstructive Sleep Apnea Treatment Using Optical Coherence Tomography to Evaluate Retinal Findings
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Kerner, N.A.; Roose, S.P. Obstructive Sleep Apnea is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am. J. Geriatr. Psychiatry 2016, 24, 496–508. [Google Scholar] [CrossRef]
- Vicente, E.; Marin, J.M.; Carrizo, S.J.; Osuna, C.S.; González, R.; Marin-Oto, M.; Forner, M.; Vicente, P.; Cubero, P.; Gil, A.V.; et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur. Respir. J. 2016, 48, 1108–1117. [Google Scholar] [CrossRef]
- May, A.M.; Van Wagoner, D.R.; Mehra, R. OSA and Cardiac Arrhythmogenesis: Mechanistic Insights. Chest 2017, 151, 225–241. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative Stress and Inflammation Biomarker Expression in Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef]
- Lloberes, P.; Durán-Cantolla, J.; Martínez-García, M.Á.; Marín, J.M.; Ferrer, A.; Corral, J.; Masa, J.F.; Parra, O.; Alonso-Álvarez, M.L.; Terán-Santos, J. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch. Broconeumol. 2011, 47, 143–156. [Google Scholar] [CrossRef]
- Iannella, G.; Lechien, J.R.; Perrone, T.; Meccariello, G.; Cammaroto, G.; Cannavicci, A.; Burgio, L.; Maniaci, A.; Cocuzza, S.; Di Luca, M.; et al. Barbed reposition pharyngoplasty (BRP) in obstructive sleep apnea treatment: State of the art. Am. J. Otolaryngol. 2022, 43, 103197. [Google Scholar] [CrossRef]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International Consensus Document on Obstructive Sleep Apnea. Arch. Broconeumol. 2021, 58, T52–T68. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Pace, A.; Iannella, G.; Rossetti, V.; Visconti, I.C.; Gulotta, G.; Cavaliere, C.; De Vito, A.; Maniaci, A.; Cocuzza, S.; Magliulo, G.; et al. Diagnosis of Obstructive Sleep Apnea in Patients with Allergic and Non-Allergic Rhinitis. Medicina 2020, 56, 454. [Google Scholar] [CrossRef]
- Macey, P.M.; Kumar, R.; Woo, M.A.; Valladares, E.M.; Yan-Go, F.L.; Harper, R.M. Brain structural changes in obstructive sleep apnea. Sleep 2008, 31, 967–977. [Google Scholar]
- Purvin, V.A.; Kawasaki, A.; Yee, R.D. Papilledema and obstructive sleep apnea syndrome. Arch. Ophthalmol. 2000, 118, 1626–1630. [Google Scholar] [CrossRef]
- Canessa, N.; Castronovo, V.; Cappa, S.F.; Aloia, M.S.; Marelli, S.; Falini, A.; Alemanno, F.; Ferini-Strambi, L. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am. J. Respir. Crit. Care Med. 2011, 183, 1419–1426. [Google Scholar] [CrossRef]
- Yeung, A. Morphometric and functional connectivity changes in the brain of patients with obstructive sleep apnea: A meta-analysis. J. Sleep Res. 2019, 28, e12857. [Google Scholar] [CrossRef]
- Satue, M.; Obis, J.; Rodrigo, M.J.; Otin, S.; Fuertes, M.I.; Vilades, E.; Gracia, H.; Ara, J.R.; Alarcia, R.; Polo, V.; et al. Optical Coherence Tomography as a Biomarker for Diagnosis, Progression, and Prognosis of Neurodegenerative Diseases. J. Ophthalmol. 2016, 2016, 8503859. [Google Scholar] [CrossRef]
- Kargi, S.H.; Altin, R.; Koksal, M.; Kart, L.; Cinar, F.; Ugurbas, S.H.; Ayoglu, F. Retinal nerve fibre layer measurements are reduced in patients with obstructive sleep apnoea syndrome. Eye 2005, 19, 575–579. [Google Scholar] [CrossRef]
- Lin, P.W.; Friedman, M.; Lin, H.C.; Chang, H.W.; Pulver, T.M.; Chin, C.H. Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea/hypopnea syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 585–593. [Google Scholar] [CrossRef]
- Casas, P.; Ascaso, F.J.; Vicente, E.; Tejero-Garcés, G.; Adiego, M.I.; Cristóbal, J.A. Retinal and optic nerve evaluation by optical coherence tomography in adults with obstructive sleep apnea-hypopnea syndrome (OSAHS). Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1625–1634. [Google Scholar] [CrossRef]
- Adam, M.; Okka, M.; Yosunkaya, S.; Bozkurt, B.; Kerimoğlu, H.; Turan, M. The evaluation of retinal nerve fiber layer thickness in patients with obstructive sleep apnea syndrome. J. Ophthalmol. 2013, 2013, 292158. [Google Scholar] [CrossRef]
- Ferrandez, B.; Ferreras, A.; Calvo, P.; Abadia, B.; Fogagnolo, P.; Wang, Y.; Marin, J.M.; Iester, M. Retinal sensitivity is reduced in patients with obstructive sleep apnea. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7119–7125. [Google Scholar] [CrossRef][Green Version]
- Hwang, Y.H.; Song, M.; Kim, Y.Y.; Yeom, D.J.; Lee, J.H. Interocular symmetry of retinal nerve fibre layer thickness in healthy eyes: A spectral-domain optical coherence tomographic study. Clin. Exp. Optom. 2014, 97, 550–554. [Google Scholar] [CrossRef]
- Sagiv, O.; Fishelson-Arev, T.; Buckman, G.; Mathalone, N.; Wolfson, J.; Segev, E.; Peled, R.; Lavi, I.; Geyer, O. Retinal nerve fibre layer thickness measurements by optical coherence tomography in patients with sleep apnoea syndrome. Clin. Exp. Ophthalmol. 2014, 42, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Wang, J.; Zhang, W.; Wang, L.; Peng, X. Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS). Eye 2014, 28, 415–421. [Google Scholar] [CrossRef]
- Bayhan, H.A.; Aslan Bayhan, S.; İntepe, Y.S.; Muhafiz, E.; Gürdal, C. Evaluation of the macular choroidal thickness using spectral optical coherence tomography in patients with obstructive sleep apnoea syndrome. Clin. Exp. Ophthalmol. 2015, 43, 139–144. [Google Scholar] [CrossRef]
- Yu, J.G.; Mei, Z.M.; Ye, T.; Feng, Y.F.; Zhao, F.; Jia, J.; Fu, X.A.; Xiang, Y. Changes in Retinal Nerve Fiber Layer Thickness in Obstructive Sleep Apnea/Hypopnea Syndrome: A Meta-Analysis. Ophthalmic Res. 2016, 56, 57–67. [Google Scholar] [CrossRef]
- Wang, W.; He, M.; Huang, W. Changes of Retinal Nerve Fiber Layer Thickness in Obstructive Sleep Apnea Syndrome: A Systematic Review and Meta-analysis. Curr. Eye Res. 2017, 42, 796–802. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, K.; Huang, J.; Sun, X.; Jiang, C. Reduced Retinal Vessel Density in Obstructive Sleep Apnea Syndrome Patients: An Optical Coherence Tomography Angiography Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3506–3512. [Google Scholar] [CrossRef]
- Mentek, M.; Aptel, F.; Godin-Ribuot, D.; Tamisier, R.; Pepin, J.L.; Chiquet, C. Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep Med. Rev. 2018, 38, 113–130. [Google Scholar] [CrossRef]
- Uslu, H.; Kanra, A.Y.; Sarac, S. Structural assessment of the optic nerve in patients with obstructive sleep apnea syndrome: Case-control study. Eur. J. Ophthalmol. 2021, 31, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Moyal, L.; Blumen-Ohana, E.; Blumen, M.; Blatrix, C.; Chabolle, F.; Nordmann, J.P. Parafoveal and optic disc vessel density in patients with obstructive sleep apnea syndrome: An optical coherence tomography angiography study. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Zengin, M.O.; Tuncer, I.; Karahan, E. Retinal nerve fiber layer thickness changes in obstructive sleep apnea syndrome: One year follow-up results. Int. J. Ophthalmol. 2014, 7, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; Lin, H.C.; Friedman, M.; Chang, H.W.; Salapatas, A.M.; Lin, M.C.; Chen, Y.C. Effects of CPAP for patients with OSA on visual sensitivity and retinal thickness. Sleep Med. 2020, 67, 156–163. [Google Scholar] [CrossRef]
- Lin, P.W.; Lin, H.C.; Friedman, M.; Chang, H.W.; Salapatas, A.M.; Lin, M.C.; Chin, C.H. Effects of OSA Surgery on Ophthalmological Microstructures. Ann. Otol. Rhinol. Laryngol. 2019, 128, 938–948. [Google Scholar] [CrossRef]
- Kaya, H.; Pekel, G.; Kaya, D.; Kara, C.O.; Hıraali, M.C. The Effects of Surgical Treatment on Retina-Choroidal Findings in Patients With Obstructive Sleep Apnea Syndrome. Ophthalmic Surg. Lasers Imaging Retin. 2019, 51, 35–42. [Google Scholar] [CrossRef]
- Shrier, E.M.; Adam, C.R.; Spund, B.; Glazman, S.; Bodis-Wollner, I. Interocular asymmetry of foveal thickness in Parkinson disease. J. Ophthalmol. 2012, 2012, 728457. [Google Scholar] [CrossRef]
- Mohammadieh, A.; Sutherland, K.; Cistulli, P.A. Sleep disordered breathing: Management update. Intern. Med. J. 2017, 47, 1241–1247. [Google Scholar] [CrossRef]
- De Vito, A.; Woodson, B.T.; Koka, V.; Cammaroto, G.; Iannella, G.; Bosi, M.; Pelucchi, S.; Filograna-Pignatelli, G.R.; El Chater, P.; Vicini, C. OSA Upper Airways Surgery: A Targeted Approach. Medicina 2021, 57, 690. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Ascaso, F.J.; Mateo, J.; Cabezón, L.; Casas, P.; Grzybowski, A. Other Neurological Disorders: Migraine, Neurosarcoidosis, Schizophrenia, Obstructive Sleep Apnea-Hypopnea Syndrome and Bipolar Disorder. In OCT and Imaging in Central Nervous System Diseases; Grzybowski, A., Barboni, P., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 317–342. [Google Scholar] [CrossRef]
- Küçük, B.; Sırakaya, E.; Delibaş, Ş. Posterior segment assessment in patients with obstructive sleep apnea syndrome. Sleep Breath. 2019, 23, 997–1005. [Google Scholar] [CrossRef]
- Ngoo, Q.Z.; Nazihatul Fikriah, A.; Baharudin, A.; Wh, W.H. Evaluation of Retinal Nerve Fiber Layer Thickness and Optic Nerve Head Parameters in Obstructive Sleep Apnoea Patients. Korean J. Ophthalmol. 2021, 35, 223–230. [Google Scholar] [CrossRef]
- Guven, S.; Kilic, D.; Bolatturk, O.F. Thinning of the inner and outer retinal layers, including the ganglion cell layer and photoreceptor layers, in obstructive sleep apnea and hypopnea syndrome unrelated to the disease severity. Int. Ophthalmol. 2021, 41, 3559–3569. [Google Scholar] [CrossRef]
- Naranjo-Bonilla, P.; Muñoz-Villanueva, M.C.; Giménez-Gómez, R.; Jurado-Gámez, B. Retinal and choroidal thickness measurements in obstructive sleep apnea: Impacts of continuous positive airway pressure treatment. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3381–3393. [Google Scholar] [CrossRef]
- Jayakumar, K.; Bansal, S.; Markan, A.; Agarwal, A.; Bansal, R.; Mahajan, S.; Agrawal, R.; Gupta, V. Reversibility of retinochoroidal vascular alteration in patients with obstructive sleep apnea after continuous positive air pressure and surgical intervention. Indian J. Ophthalmol. 2021, 69, 1850–1855. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Gualtierotti, R.; Pierro, L.; Del Turco, C.; Miserocchi, E.; Schioppo, T.; Meroni, P.L.; ACUTE Study Group. Choroidal impairment and macular thinning in patients with systemic sclerosis: The acute study. Microvasc. Res. 2015, 97, 31–36. [Google Scholar] [CrossRef]
- D’Souza, H.; Kapoor, K.G. Retinal vascular manifestations of obstructive sleep apnea. Curr. Opin. Ophthalmol. 2020, 31, 508–513. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Cirrus OCT Normative Database Study Group. Interocular symmetry in peripapillary retinal nerve fiber layer thickness measured with the Cirrus HD-OCT in healthy eyes. Am. J. Ophthalmol. 2011, 151, 514–521.e1. [Google Scholar] [CrossRef]
- Ray, W.A.; O’Day, D.M. Statistical analysis of multi-eye data in ophthalmic research. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1186–1188. [Google Scholar]
- Rosner, B. Statistical methods in ophthalmology: An adjustment for the intraclass correlation between eyes. Biometrics 1982, 38, 105–114. [Google Scholar] [CrossRef]
- Ray, W.A.; O’Day, D.M.; Head, W.S.; Robinson, R. Statistical analysis for experimental models of ocular disease: Continuous response measures. Curr. Eye Res. 1985, 4, 585–597. [Google Scholar] [CrossRef]
- Liu, P.K.; Chiu, T.Y.; Wang, N.K.; Levi, S.R.; Tsai, M.J. Ocular Complications of Obstructive Sleep Apnea. J. Clin. Med. 2021, 10, 3422. [Google Scholar] [CrossRef]
- Nakayama, L.F.; Tempaku, P.F.; Bergamo, V.C.; Polizelli, M.U.; Santos da Cruz, N.F.; Bittencourt, L.; Regatieri, C. Obstructive sleep apnea and the retina: A review. J. Clin. Sleep Med. 2021, 17, 1947–1952. [Google Scholar] [CrossRef]
- Monteiro-Henriques, I.; Rocha-Sousa, A.; Barbosa-Breda, J. Optical coherence tomography angiography changes in cardiovascular systemic diseases and risk factors: A Review. Acta Ophthalmol. 2021, 100, e1–e15. [Google Scholar] [CrossRef]



| Variable (n) | Mean ± SD | ||
|---|---|---|---|
| Pre-Treatment | Post-Treatment | Treatment Effect | |
| (Comparison pre/post) | |||
| F(d.f.); p-Value; (eta2) | |||
| AHI mild–moderate OSA | F(1.15) = 1.902; p = 0.188 | ||
| Surgery (10) | 18.2 ± 7.3 | 11.6 ± 15.9 | (0.113) |
| CPAP + surgery (7) | 24.8 ± 4.8 | 21.4 ± 13.4 | |
| Total (17) | 20.9 ± 7.1 | 15.6 ± 15.3 | |
| AHI severe OSA | F(1.16) = 31.162; p < 0.001 | ||
| Surgery (5) | 43.2 ± 14.6 | 11.2 ± 8.0 | (−0.661) |
| CPAP + surgery (13) | 69.7 ± 25.6 | 38.2 ± 23.4 | |
| Total (18) | 62.4 ± 25.7 | 30.7 ± 23.6 | |
| Variable | Mean ± SD | p-Value | |
|---|---|---|---|
| Mild–Moderate OSA (n = 41) | Severe OSA (n = 55) | ||
| Foveal thickness (µm) | 214.6 ± 22.28 | 206.7 ± 27.38 | 0.138 |
| Inner ring macular thickness (µm) | 281.4 ± 13.4 | 271.9 ± 15.85 | 0.003 |
| Outer ring macular thickness (µm) | 241.3 ± 1.17 | 236.4 ± 13.55 | 0.054 |
| Macular volume (mm3) | 7 ± 0.33 | 6.9 ± 0.39 | 0.041 |
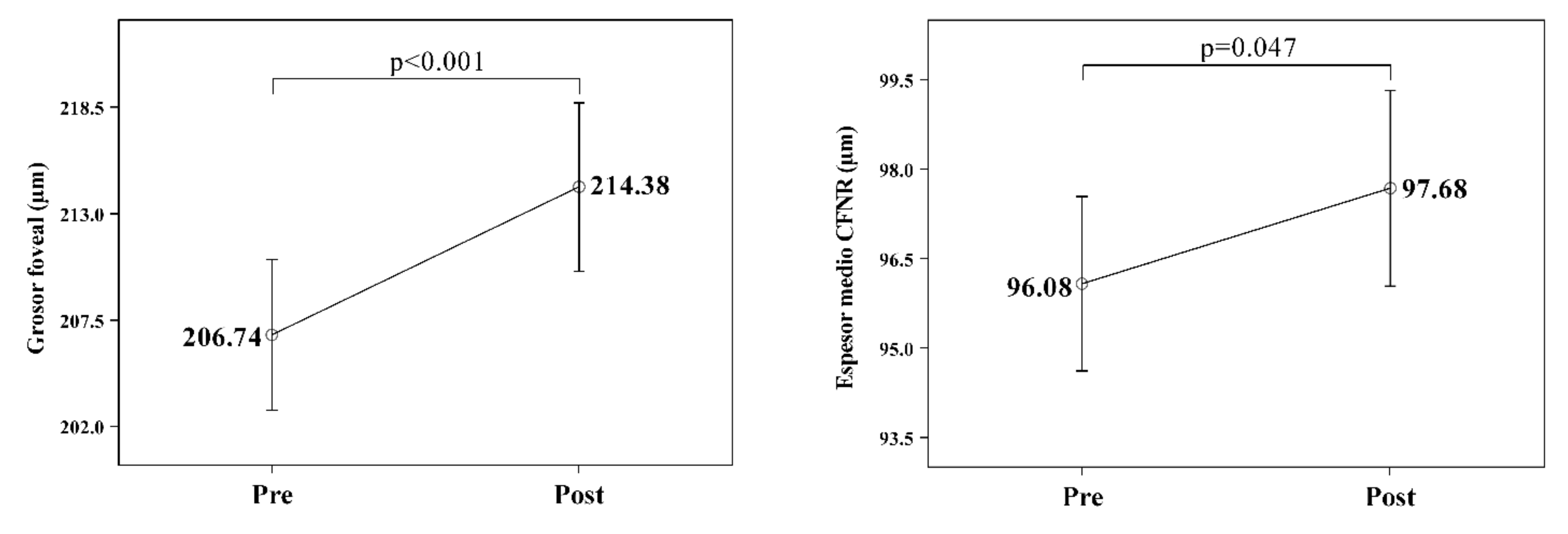
| RNFL average thickness (µm) | 102.3 ± 9.06 | 96.1 ± 1.27 | 0.003 |
| RNFL superior quadrant thickness (µm) | 129.1 ± 12.56 | 122.3 ± 18.21 | 0.044 |
| RNFL nasal quadrant thickness (µm) | 75.7 ± 16.27 | 73.3 ± 15.02 | 0.458 |
| RNFL inferior quadrant thickness (µm) | 128.6 ± 16.99 | 120.1 ± 16.08 | 0.014 |
| RNFL temporal quadrant thickness(µm) | 75.6 ± 11.3 | 68.7 ± 13.04 | 0.008 |
| VIRA (mm3) | 0.8 ± 0.51 | 0.6 ± 0.31 | 0.102 |
| HIRW (mm2) | 2 ± 0.3 | 1.8 ± 0.28 | 0.01 |
| Disc area (mm2) | 2.8 ± 0.69 | 2.6 ± 0.56 | 0.115 |
| Cup area (mm2) | 0.5 ± 0.52 | 0.9 ± 1.06 | 0.028 |
| Rim area (mm2) | 2.1 ± 0.7 | 1.9 ± 0.81 | 0.279 |
| Cup/disc area ratio | 0.2 ± 0.18 | 0.3 ± 0.27 | 0.078 |
| Cup/disc horizontal ratio | 0.4 ± 0.19 | 0.5 ± 0.24 | 0.123 |
| Cup/disc vertical ratio | 0.4 ± 0.17 | 0.5 ± 0.24 | 0.108 |
| Variable (n) | Mean ± SD | ||
|---|---|---|---|
| Pre-Treatment | Post-Treatment | Treatment Effect (Comparison pre/post) | |
| F(d.f.); p-Value; (eta2) | |||
| Foveal thickness (µm) | F(1.38) = 5.391; p = 0.026 | ||
| CPAP (10) | 215.1± 20.3 | 209.2 ± 18.1 | (0.124) |
| Surgery (19) | 215.9 ± 23.9 | 215.6 ± 20.7 | |
| CPAP + surgery (12) | 212.1 ± 22.9 | 205.2 ± 28.0 | |
| Total | 214.6 ± 22.3 | 211 ± 22.4 | |
| Macular inner ring thickness (µm) | F(1.38) = 8.074; p = 0.007 | ||
| CPAP (10) | 284.7 ± 9.7 | 281.7 ± 12.9 | (0.175) |
| Surgery (19) | 281.5 ± 16.0 | 279.5 ± 16.5 | |
| CPAP + Surgery (12) | 278.5 ± 11.7 | 269.2 ± 16.7 | |
| Total | 281.4 ± 13.4 | 277 ± 16.3 | |
| Macular outer ring thickness (µm) | F(1.38) = 6.485; p = 0.015 | ||
| CPAP (10) | 243.8 ± 8.5 | 240.2 ± 14.6 | (0.146) |
| Surgery (19) | 242.2 ± 10.7 | 241.9 ± 12.5 | |
| CPAP + Surgery (12) | 237.8 ± 10.4 | 231.3 ± 12.7 | |
| Total | 241.3 ± 10.2 | 238.4 ± 13.6 | |
| Macular volume (mm3) | F(1.38) = 6.515; p = 0.015 | ||
| CPAP (10) | 7.1 ± 0.3 | 7 ± 0.4 | (0.146) |
| Surgery (19) | 7.1 ± 0.4 | 7.1 ± 0.4 | |
| CPAP + Surgery (12) | 6.9 ± 0.3 | 6.7 ± 0.4 | |
| Total | 7 ± 0.3 | 6.9 ± 0.4 | |
| Variable (n) | Mean ± SD | ||
|---|---|---|---|
| Pre-treatment | Post-treatment | Treatment Effect (comparison pre/post) | |
| F(f.g.); p-Value (eta2) | |||
| Foveal thickness (µm) | F(1.50) = 16.780; p < 0.001 | ||
| CPAP (22) | 215.2 ± 35.7 | 225.5 ± 38.1 | (0.251) |
| Surgery (10) | 192.8 ± 15.6 | 204.3 ± 24.6 | |
| CPAP + surgery (23) | 204.5 ± 17.9 | 207.6 ± 20.2 | |
| Total | 206.7 ± 27.4 | 214.4 ± 30.6 | |
| Macular inner ring thickness (µm) | F(1.50) = 0.267; p = 0.608 | ||
| CPAP (22) | 275.2 ± 18.2 | 275.6 ± 19.2 | (0.005) |
| Surgery (10) | 260.4 ± 12.5 | 261.8 ± 13.9 | |
| CPAP + surgery (23) | 273.8 ± 12.3 | 274.5 ± 13.3 | |
| Total | 271.9 ± 15.8 | 272.6 ± 16.7 | |
| Macular outer ring thickness (µm) | F(1.50) = 1.438; p = 0.236 | ||
| CPAP (22) | 237.5 ± 16.3 | 237.7 ± 17.9 | (0.028) |
| Surgery (10) | 230.7 ± 8 | 235.2 ± 11.5 | |
| CPAP + surgery (23) | 237.8 ± 12.2 | 238.9 ± 10.1 | |
| Total | 236.3 ± 13.6 | 237.7 ± 13.9 | |
| Macular volume (mm3) | F(1.50) = 0.834; p = 0.365 | ||
| CPAP (22) | 6.9 ± 0.5 | 6.8 ± 0.5 | (0.016) |
| Surgery (10) | 6.6 ± 0.2 | 6.8 ± 0.3 | |
| CPAP + surgery (23) | 6.9 ± 0.3 | 6.9 ± 0.3 | |
| Total | 6.9 ± 0.4 | 6.9 ± 0.4 | |
| n | OCT RETINAL Relevant Findings | |
| Lin et al. [19] | 210 | Peripapillary all quadr. RNFL lower in OSA vs. healthy subjects |
| Sagiv et al. [24] | 108 | Peripapillary all quadr. RNFL lower in OSA vs. healthy subjects |
| Casas et al. [20] | 96 | Only peripapillary nasal RNFL lower in OSA vs. healthy subjects |
| Adam et al. [21] | 43 | No differences between OSA and healthy subjects |
| Kücük et al. [42] | 45 | No differences between OSA and healthy subjects |
| Yu et al. [29] | 69 | Average RNFL thickness lower in severe OSA |
| Ngoo et al. [43] | 44 | Peripapillary all quadr. RNFL lower in OSA vs. healthy subjects |
| Guven et al. [44] | 31 | Average nasal RNFL thickness lower in severe OSA |
| Tejero-Garcés et al. | 98 | Average RNFL thickness lower in severe OSA |
| n | OCT OPTIC NERVE Relevant Findings | |
| Lin et al. [19] | 210 | Peripapillary all quadr. RNFL decreased in OSA vs. healthy subjects |
| Casas et al. [20] | 96 | Only peripapillary nasal RNFL decreased in OSA vs. healthy subjects |
| Kücük et al. [42] | 45 | No differences between OSA and healthy subjects |
| Tejero-Garcés et al. | 98 | Peripapillary atrophy in severe OSA |
| n | OCT as Monitor of OSA after Treatment | |
| Zengin et al. [33] | 44 | After CPAP, all quadr. RNFL decreased compared to controls |
| Lin et al. [34] | 32 | After CPAP, inferior and nasal-inf. quadr. RNFL improved compared to controls |
| Lin et al. [35] | 108 | After UAS, macular thickness improved compared to controls |
| Kaya et al. [36] | 34 | After pharyngoplasty, no significant changes |
| Naranjo-Bonilla et al. [45] | 40 | After CPAP, normalization of choroidal thickness |
| Jayakumar et al. [46] | 36 | After UAS and CPAP, choroidal thickness and vascularity improved |
| Tejero-Garcés et al. | 98 | After UAS and CPAP, foveal thickness and retinal nerve fibers improved in severe OSA compared to controls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejero-Garcés, G.; Ascaso, F.J.; Casas, P.; Adiego, M.I.; Baptista, P.; O’Connor-Reina, C.; Vicente, E.; Plaza, G. Assessment of the Effectiveness of Obstructive Sleep Apnea Treatment Using Optical Coherence Tomography to Evaluate Retinal Findings. J. Clin. Med. 2022, 11, 815. https://doi.org/10.3390/jcm11030815
Tejero-Garcés G, Ascaso FJ, Casas P, Adiego MI, Baptista P, O’Connor-Reina C, Vicente E, Plaza G. Assessment of the Effectiveness of Obstructive Sleep Apnea Treatment Using Optical Coherence Tomography to Evaluate Retinal Findings. Journal of Clinical Medicine. 2022; 11(3):815. https://doi.org/10.3390/jcm11030815
Chicago/Turabian StyleTejero-Garcés, Gloria, Francisco J. Ascaso, Paula Casas, Maria I. Adiego, Peter Baptista, Carlos O’Connor-Reina, Eugenio Vicente, and Guillermo Plaza. 2022. "Assessment of the Effectiveness of Obstructive Sleep Apnea Treatment Using Optical Coherence Tomography to Evaluate Retinal Findings" Journal of Clinical Medicine 11, no. 3: 815. https://doi.org/10.3390/jcm11030815
APA StyleTejero-Garcés, G., Ascaso, F. J., Casas, P., Adiego, M. I., Baptista, P., O’Connor-Reina, C., Vicente, E., & Plaza, G. (2022). Assessment of the Effectiveness of Obstructive Sleep Apnea Treatment Using Optical Coherence Tomography to Evaluate Retinal Findings. Journal of Clinical Medicine, 11(3), 815. https://doi.org/10.3390/jcm11030815








