The Role of Sclerostin in Bone Diseases
Abstract
1. Introduction
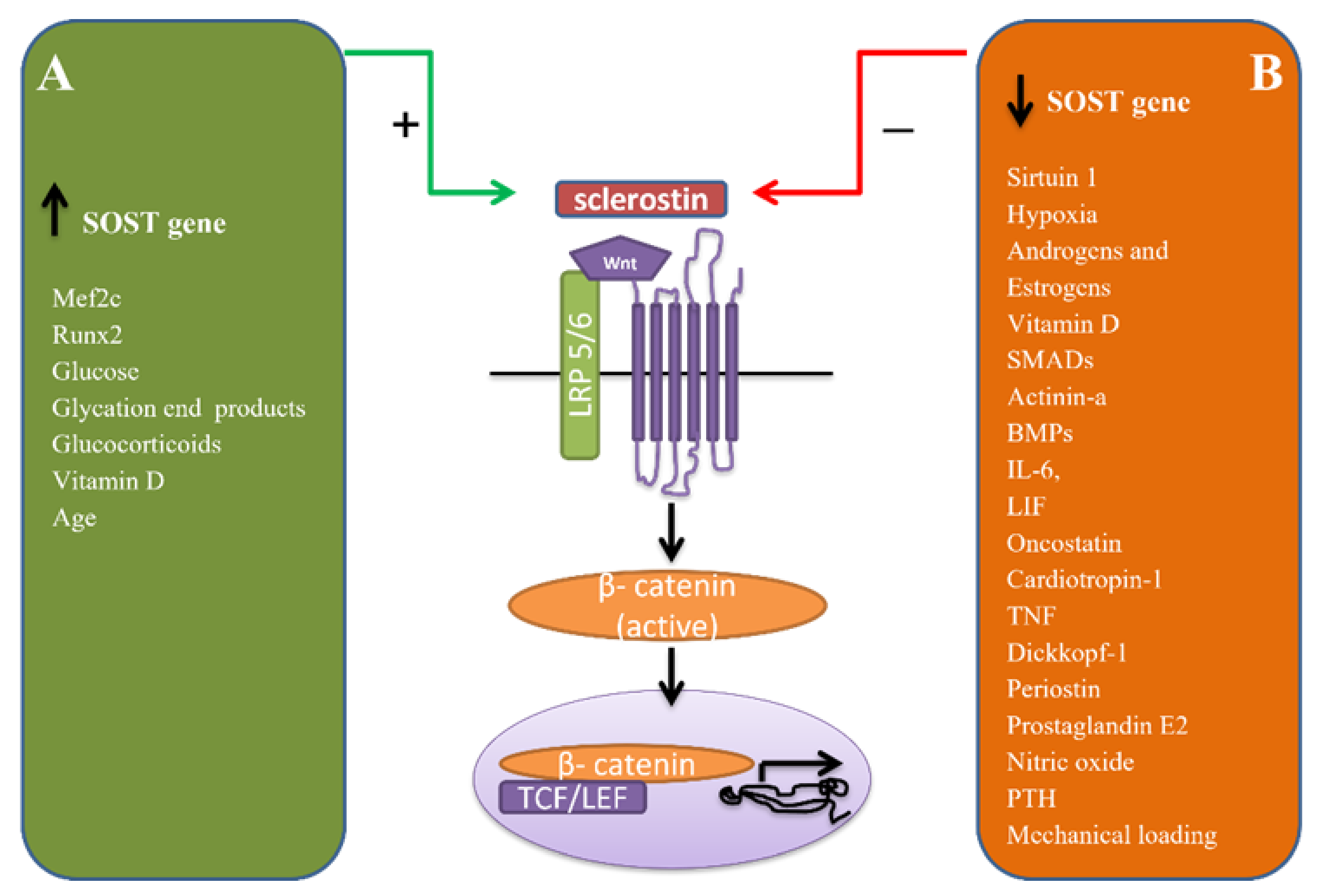
2. Regulation of Sclerostin
3. Role of Sclerostin in Skeletal Diseases
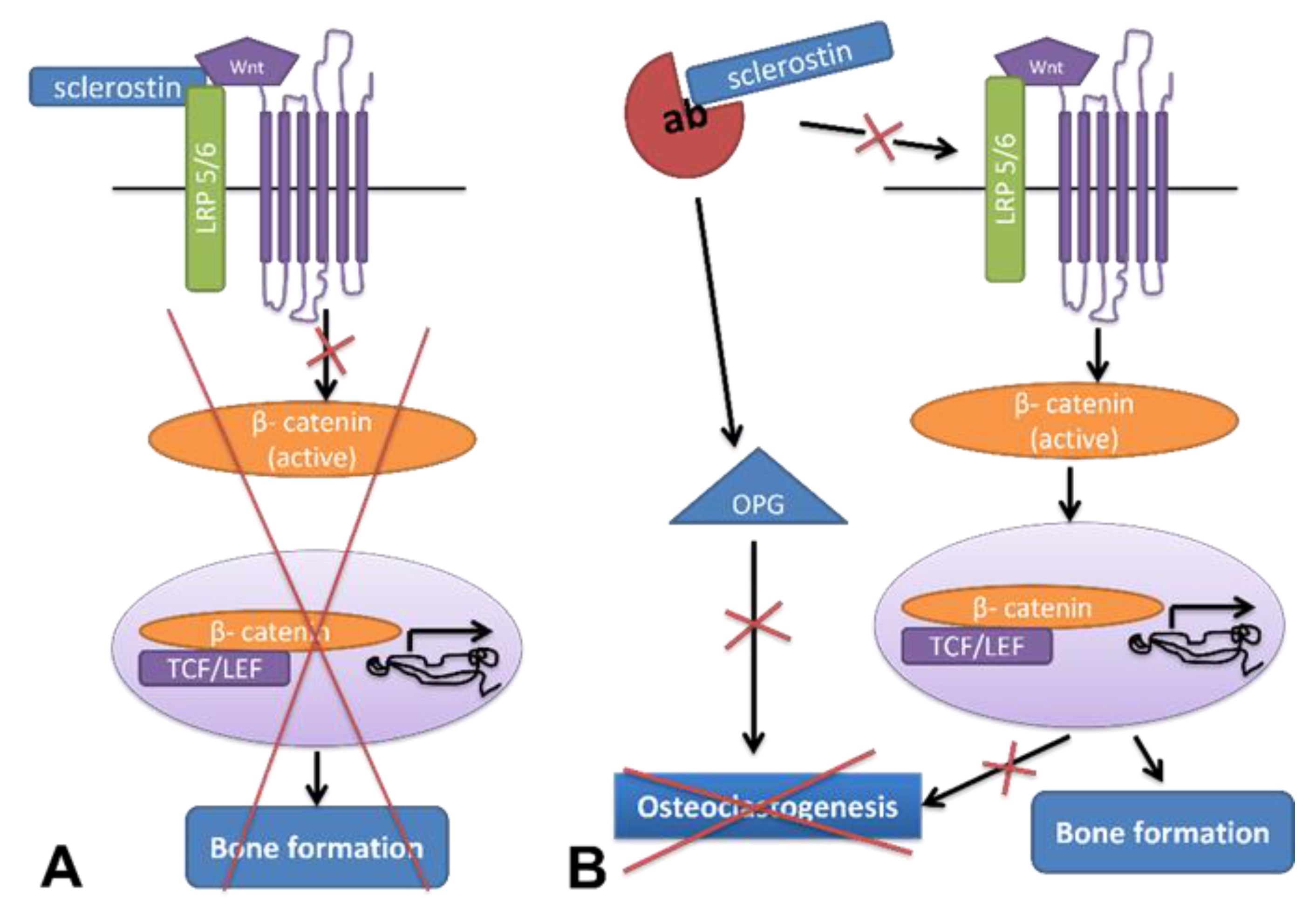
3.1. Osteoporosis
3.2. Glucocortosteroid Induced Bone Loss
3.3. Osteonecrosis
3.4. Osteoarthritis
3.5. Rheumatoid Arthritis
3.6. Ankylosing Spondylitis
3.7. Bone Tumors
3.7.1. Primary Bone Tumors
3.7.2. Bone Metastasis
3.7.3. Multiple Myeloma
3.8. Osteogenesis Imperfecta
3.9. Hypophosphatasia
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hamersma, H.; Gardner, J.; Beighton, P. The natural history of sclerosteosis. Clin. Genet. 2003, 63, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Van Buchem, F.; Hadders, H.; Hansen, J.; Woldring, M. Hyperostosis corticalis generalisata: Report of seven cases. Am. J. Med. 1962, 33, 387–397. [Google Scholar] [CrossRef]
- Van Bezooijen, R.L.; Roelen, B.A.; Visser, A.; Van Der Wee-Pals, L.; De Wilt, E.; Karperien, M.; Hamersma, H.; Papapoulos, S.E.; Dijke, P.T.; Löwik, C.W. Sclerostin Is an Osteocyte-expressed Negative Regulator of Bone Formation, But Not a Classical BMP Antagonist. J. Exp. Med. 2004, 199, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Evenity MG HCP English—Amgen. Available online: https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/evenity/evenity_mg_hcp_english.ashx. (accessed on 28 November 2021).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evenity (accessed on 28 November 2021).
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef]
- Kusu, N.; Laurikkala, J.; Imanishi, M.; Usui, H.; Konishi, M.; Miyake, A.; Thesleff, I.; Itoh, N. Sclerostin Is a Novel Secreted Osteoclast-derived Bone Morphogenetic Protein Antagonist with Unique Ligand Specificity. J. Biol. Chem. 2003, 278, 24113–24117. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Youssef, S.J.; Oursler, M.J. Sclerostin expression and functions beyond the osteocyte. Bone 2016, 96, 45–50. [Google Scholar] [CrossRef]
- Collette, N.; Yee, C.S.; Murugesh, D.; Sebastian, A.; Taher, L.; Gale, N.W.; Economides, A.; Harland, R.M.; Loots, G.G. SOST and its paralog SOSTdc1 coordinate digit number in a Gli3-dependent manner. Dev. Biol. 2013, 383, 90–105. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Kramann, R.; Koos, R.; Krüger, T.; Schurgers, L.; Mühlenbruch, G.; Hübner, S.; Gladziwa, U.; Drechsler, C.; Ketteler, M. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2013, 14, 219. [Google Scholar] [CrossRef]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt Signaling in Differentiated Osteoblasts Controls Osteoclast Differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Stolina, M.; Dwyer, D.; Niu, Q.-T.; Villasenor, K.S.; Kurimoto, P.; Grisanti, M.; Han, C.-Y.; Liu, M.; Li, X.; Ominsky, M.S.; et al. Temporal changes in systemic and local expression of bone turnover markers during six months of sclerostin antibody administration to ovariectomized rats. Bone 2014, 67, 305–313. [Google Scholar] [CrossRef]
- Tu, X.; Delgado-Calle, J.; Condon, K.W.; Maycas, M.; Zhang, H.; Carlesso, N.; Taketo, M.M.; Burr, D.B.; Plotkin, L.I.; Bellido, T. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc. Natl. Acad. Sci. USA 2015, 112, E478–E486. [Google Scholar] [CrossRef]
- Albers, J.; Keller, J.; Baranowsky, A.; Beil, F.T.; Catala-Lehnen, P.; Schulze, J.; Amling, M.; Schinke, T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J. Cell Biol. 2013, 200, 537–549. [Google Scholar] [CrossRef]
- Koide, M.; Kobayashi, Y. Regulatory mechanisms of sclerostin expression during bone remodeling. J. Bone Miner. Metab. 2018, 37, 9–17. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by RunxInt. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sañudo, C.; Bolado, A.; Fernández, A.F.; Arozamena, J.; Pascual-Carra, M.A.; Rodriguez-Rey, J.C.; Fraga, M.F.; Bonewald, L.; A Riancho, J. DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J. Bone Miner. Res. 2011, 27, 926–937. [Google Scholar] [CrossRef]
- Cohen-Kfir, E.; Artsi, H.; Levin, A.; Abramowitz, E.; Bajayo, A.; Gurt, I.; Zhong, L.; D’Urso, A.; Toiber, D.; Mostoslavsky, R.; et al. Sirt1 Is a Regulator of Bone Mass and a Repressor of SOST Encoding for Sclerostin, a Bone Formation Inhibitor. Endocrinology 2011, 152, 4514–4524. [Google Scholar] [CrossRef]
- Genetos, D.C.; Toupadakis, C.A.; Raheja, L.F.; Wong, A.; Papanicolaou, S.E.; Fyhrie, D.P.; Loots, G.; Yellowley, C.E. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J. Cell. Biochem. 2010, 110, 457–467. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Yamaguchi, T.; Kanazawa, I.; Sugimoto, T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem. Biophys. Res. Commun. 2015, 461, 193–199. [Google Scholar] [CrossRef]
- Jensen, P.R.; Andersen, T.L.; Chavassieux, P.; Roux, J.-P.; Delaissé, J.-M. 2015 Annual Meeting of the American Society for Bone and Mineral Research Seattle, WA October 9-12, J. Bone Miner. Res. 2015, 30, S1. [Google Scholar] [CrossRef]
- Sato, A.Y.; Cregor, M.; Delgado-Calle, J.; Condon, K.W.; Allen, M.R.; Peacock, M.; Plotkin, L.I.; Bellido, T. Protection From Glucocorticoid-Induced Osteoporosis by Anti-Catabolic Signaling in the Absence of SOST/Sclerostin. J. Bone Miner. Res. 2016, 31, 1791–1802. [Google Scholar] [CrossRef]
- Kim, R.Y.; Yang, H.J.; Song, Y.M.; Kim, I.S.; Hwang, S.J. Estrogen Modulates Bone Morphogenetic Protein-Induced Sclerostin Expression Through the Wnt Signaling Pathway. Tissue Eng. Part A 2015, 21, 2076–2088. [Google Scholar] [CrossRef]
- Di Nisio, A.; De Toni, L.; Speltra, E.; Rocca, M.S.; Taglialavoro, G.; Ferlin, A.; Foresta, C. Regulation of Sclerostin Production in Human Male Osteocytes by Androgens: Experimental and Clinical Evidence. Endocrinology 2015, 156, 4534–4544. [Google Scholar] [CrossRef][Green Version]
- Marenzana, M.; Greenslade, K.; Eddleston, A.; Okoye, R.; Marshall, D.; Moore, A.; Robinson, M.K. Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Care Res. 2011, 63, 2385–2395. [Google Scholar] [CrossRef]
- Wijenayaka, A.R.; Prideaux, M.; Yang, D.; Morris, H.A.; Findlay, D.M.; Anderson, P.H.; Atkins, G.J. Early response of the human SOST gene to stimulation by 1α,25-dihydroxyvitamin DJ. Steroid Biochem. Mol. Biol. 2016, 164, 369–373. [Google Scholar] [CrossRef]
- John, H.C.S.; Hansen, S.J.; Pike, J.W. Analysis of SOST expression using large minigenes reveals the MEF2C binding site in the evolutionarily conserved region (ECR5) enhancer mediates forskolin, but not 1,25-dihydroxyvitamin D3 or TGFβ1 responsiveness. J. Steroid Biochem. Mol. Biol. 2015, 164, 277–280. [Google Scholar] [CrossRef]
- Loots, G.G.; Keller, H.; Leupin, O.; Murugesh, D.; Collette, N.M.; Genetos, D.C. TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone 2012, 50, 663–669. [Google Scholar] [CrossRef]
- Roforth, M.M.; Fujita, K.; McGregor, U.I.; Kirmani, S.; McCready, L.K.; Peterson, J.M.; Drake, M.T.; Monroe, D.G.; Khosla, S. Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone 2013, 59, 1–6. [Google Scholar] [CrossRef]
- Bellido, T.; Saini, V.; Pajevic, P.D. Effects of PTH on osteocyte function. Bone 2012, 54, 250–257. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Plotkin, L.I.; Galli, C.; Goellner, J.J.; Gortazar, A.R.; Allen, M.R.; Robling, A.G.; Bouxsein, M.; Schipani, E.; Turner, C.H.; et al. Control of Bone Mass and Remodeling by PTH Receptor Signaling in Osteocytes. PLoS ONE 2008, 3, e2942. [Google Scholar] [CrossRef]
- Kim, S.W.; Pajevic, P.D.; Selig, M.; Barry, K.J.; Yang, J.-Y.; Shin, C.S.; Baek, W.-Y.; Kim, J.-E.; Kronenberg, H.M. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. Res. 2012, 27, 2075–2084. [Google Scholar] [CrossRef]
- Rhee, Y.; Lee, E.-Y.; Lezcano, V.; Ronda, A.C.; Condon, K.W.; Allen, M.R.; Plotkin, L.I.; Bellido, T. Resorption Controls Bone Anabolism Driven by Parathyroid Hormone (PTH) Receptor Signaling in Osteocytes. J. Biol. Chem. 2013, 288, 29809–29820. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Ali, A.A.; Gubrij, I.; Plotkin, L.I.; Fu, Q.; O’Brien, C.A.; Manolagas, S.C.; Jilka, R.L. Chronic Elevation of Parathyroid Hormone in Mice Reduces Expression of Sclerostin by Osteocytes: A Novel Mechanism for Hormonal Control of Osteoblastogenesis. Endocrinology 2005, 146, 4577–4583. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Kneissel, M. SOST is a target gene for PTH in bone. Bone 2005, 37, 148–158. [Google Scholar] [CrossRef] [PubMed]
- 2013 Annual Meeting of the American Society for Bone and Mineral Research Baltimore, MD October 4–7, 2013. J. Bone Miner. Res. 2013, 28, S1. [CrossRef] [PubMed]
- Robling, A.G.; Kedlaya, R.; Ellis, S.N.; Childress, P.J.; Bidwell, J.P.; Bellido, T.; Turner, C.H. Anabolic and Catabolic Regimens of Human Parathyroid Hormone 1–34 Elicit Bone- and Envelope-Specific Attenuation of Skeletal Effects in SOST-Deficient Mice. Endocrinology 2011, 152, 2963–2975. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Loots, G.; Studer, A.; Keller, H.; Kneissel, M. Parathyroid hormone (PTH)–induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 2009, 25, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L.; Khosla, S. Physiology of Bone Loss. Radiol. Clin. N. Am. 2010, 48, 483–495. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, E.; Zhang, H.; Wang, J.; Wu, N.; Chen, X.; Wang, N.; Wen, S.; Nan, G.; Deng, F.; et al. Crosstalk between Wnt/β-Catenin and Estrogen Receptor Signaling Synergistically Promotes Osteogenic Differentiation of Mesenchymal Progenitor Cells. PLoS ONE 2013, 8, e82436. [Google Scholar] [CrossRef]
- Kondoh, S.; Inoue, K.; Igarashi, K.; Sugizaki, H.; Shirode-Fukuda, Y.; Inoue, E.; Yu, T.; Takeuchi, J.K.; Kanno, J.; Bonewald, L.F.; et al. Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone 2013, 60, 68–77. [Google Scholar] [CrossRef]
- Chung, Y.E.; Lee, S.H.; Kim, S.-Y.; Kim, H.-H.; Mirza, F.S.; Lorenzo, J.A.; Kim, G.S.; Koh, J.-M. Long-term treatment with raloxifene, but not bisphosphonates, reduces circulating sclerostin levels in postmenopausal women. Osteoporos. Int. 2011, 23, 1235–1243. [Google Scholar] [CrossRef]
- Ota, K.; Quint, P.; Ruan, M.; Pederson, L.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. TGF-β Induces Wnt10b in Osteoclasts From Female Mice to Enhance Coupling to Osteoblasts. Endocrinology 2013, 154, 3745–3752. [Google Scholar] [CrossRef]
- Padhi, D.; Allison, M.; Kivitz, A.J.; Gutierrez, M.J.; Stouch, B.; Wang, C.; Jang, G. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: A randomized, double-blind, placebo-controlled study. J. Clin. Pharmacol. 2013, 54, 168–178. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.L.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men with Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K.; et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594. [Google Scholar] [CrossRef]
- Cai, Y.; Cai, T.; Chen, Y. Wnt Pathway in Osteosarcoma, from Oncogenic to Therapeutic. J. Cell. Biochem. 2014, 115, 625–631. [Google Scholar] [CrossRef]
- Recker, R.R.; Benson, C.T.; Matsumoto, T.; Bolognese, M.A.; Robins, D.A.; Alam, J.; Chiang, A.Y.; Hu, L.; Krege, J.H.; Sowa, H.; et al. A Randomized, Double-Blind Phase 2 Clinical Trial of Blosozumab, a Sclerostin Antibody, in Postmenopausal Women with Low Bone Mineral Density. J. Bone Miner. Res. 2015, 30, 216–224. [Google Scholar] [CrossRef]
- Witcher, P.; Miner, S.E.; Horan, D.J.; Bullock, W.; Lim, K.-E.; Kang, K.S.; Adaniya, A.L.; Ross, R.D.; Loots, G.; Robling, A.G. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Florio, M.; Gunasekaran, K.; Stolina, M.; Li, X.; Liu, L.; Tipton, B.; Salimi-Moosavi, H.; Asuncion, F.J.; Li, C.; Sun, B.; et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat. Commun. 2016, 7, 11505. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Jia, D.; Plotkin, L.I.; Bellido, T.; Powers, C.C.; Stewart, S.A.; Manolagas, S.C.; Weinstein, R.S. Glucocorticoids Act Directly on Osteoblasts and Osteocytes to Induce Their Apoptosis and Reduce Bone Formation and Strength. Endocrinology 2004, 145, 1835–1841. [Google Scholar] [CrossRef]
- Kim, H.-J.; Zhao, H.; Kitaura, H.; Bhattacharyya, S.; Brewer, J.A.; Muglia, L.J.; Ross, F.P.; Teitelbaum, S. Glucocorticoids suppress bone formation via the osteoclast. J. Clin. Investig. 2006, 116, 2152–2160. [Google Scholar] [CrossRef]
- Thiele, S.; Hannemann, A.; Winzer, M.; Baschant, U.; Weidner, H.; Nauck, M.; Thakker, R.V.; Bornhäuser, M.; Hofbauer, L.C.; Rauner, M. Regulation of sclerostin in glucocorticoid-induced osteoporosis (GIO) in mice and humans. Endocr. Connect. 2019, 8, 923–934. [Google Scholar] [CrossRef]
- Yao, W.; Dai, W.; Jiang, L.; Lay, E.Y.-A.; Zhong, Z.; Ritchie, R.O.; Li, X.; Ke, H.; Lane, N.E. Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporos. Int. 2015, 27, 283–294. [Google Scholar] [CrossRef]
- Van Lierop, A.H.; Hamdy, N.A.; Papapoulos, S.E. Glucocorticoids are not always deleterious for bone. J. Bone Miner. Res. 2010, 25, 2796–2800. [Google Scholar] [CrossRef]
- Chen, X.; Yang, F.; Chen, Z.-Q.; He, M.-C.; Hong, G.-J.; Huang, J.-Y.; Zhou, Y.-C.; Qin, Y.-X.; Wei, Q.-S.; He, W. Association of reduced sclerostin expression with collapse process in patients with osteonecrosis of the femoral head. Int. Orthop. 2018, 42, 1675–1682. [Google Scholar] [CrossRef]
- Calder, J.D.F.; Buttery, L.; Revell, P.A.; Pearse, M.; Polak, J.M. Apoptosis--a significant cause of bone cell death in osteonecrosis of the femoral head. J. Bone Jt. Surgery. Br. Vol. 2004, 86, 1209–1213. [Google Scholar] [CrossRef]
- Hadaya, D.; Gkouveris, I.; Soundia, A.; Bezouglaia, O.; Boyce, R.W.; Stolina, M.; Dwyer, D.; Dry, S.M.; Pirih, F.Q.; Aghaloo, T.L.; et al. Clinically Relevant Doses of Sclerostin Antibody Do Not Induce Osteonecrosis of the Jaw (ONJ) in Rats with Experimental Periodontitis. J. Bone Miner. Res. 2018, 34, 171–181. [Google Scholar] [CrossRef]
- Van Bezooijen, R.; Bronckers, A.; Gortzak, R.; Hogendoorn, P.; Van Der Wee-Pals, L.; Balemans, W.; Oostenbroek, H.; Van Hul, W.; Hamersma, H.; Dikkers, F.; et al. Sclerostin in Mineralized Matrices and van Buchem Disease. J. Dent. Res. 2009, 88, 569–574. [Google Scholar] [CrossRef]
- Chan, B.; Fuller, E.; Russell, A.; Smith, S.; Jackson, M.; Cake, M.; Read, R.; Bateman, J.; Sambrook, P.; Little, C. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthr. Cartil. 2011, 19, 874–885. [Google Scholar] [CrossRef]
- Staines, K.; Madi, K.; Mirczuk, S.M.; Parker, S.; Burleigh, A.; Poulet, B.; Hopkinson, M.; Bodey, A.J.; Fowkes, R.; Farquharson, C.; et al. Endochondral Growth Defect and Deployment of Transient Chondrocyte Behaviors Underlie Osteoarthritis Onset in a Natural Murine Model. Arthritis Rheumatol. 2015, 68, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Roudier, M.; Li, X.; Niu, Q.-T.; Pacheco, E.; Pretorius, J.K.; Graham, K.; Yoon, B.-R.P.; Gong, J.; Warmington, K.; Ke, H.Z.; et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Care Res. 2012, 65, 721–731. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, D.; Wu, Q.; Hao, S.; Chen, M.; Xie, C.; Rosier, R.N.; O’Keefe, R.J.; Zuscik, M.; Chen, D. Activation of β-Catenin Signaling in Articular Chondrocytes Leads to Osteoarthritis-Like Phenotype in Adult β-Catenin Conditional Activation Mice. J. Bone Miner. Res. 2009, 24, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, W.; Funck-Brentano, T.; Lin, H.; Marty, C.; Ea, H.-K.; Hay, E.; Cohen-Solal, M. Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent Wnt pathways. Arthritis Res. Ther. 2015, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, I.; Kostopoulou, F.; Malizos, K.N.; Tsezou, A. DNA methylation regulates sclerostin (SOST) expression in osteoarthritic chondrocytes by bone morphogenetic protein 2 (BMP-2) induced changes in Smads binding affinity to the CpG region of SOST promoter. Arthritis Res. Ther. 2015, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Mabey, T.; Honsawek, S.; Tanavalee, A.; Wilairatana, V.; Yuktanandana, P.; Saetan, N.; Zhan, D. Plasma and synovial fluid sclerostin are inversely associated with radiographic severity of knee osteoarthritis. Clin. Biochem. 2014, 47, 547–551. [Google Scholar] [CrossRef]
- Dequeker, J.; Johnell, O.; Dilsen, G.; Gennari, C.; Vaz, A.; Lyritis, G.; Mazzuoli, G.; Miravet, L.; Passeri, M.; Pérez-Cano, R.; et al. Osteoarthritis protects against femoral neck fracture: The MEDOS study experience. Bone 1993, 14, 51–56. [Google Scholar] [CrossRef]
- Deshmukh, V.; O’Green, A.; Bossard, C.; Seo, T.; Lamangan, L.; Ibanez, M.; Ghias, A.; Lai, C.; Do, L.; Cho, S.; et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease-modifying approach for knee osteoarthritis treatment. Osteoarthr. Cartil. 2019, 27, 1347–1360. [Google Scholar] [CrossRef]
- Lietman, C.; Wu, B.; Lechner, S.; Shinar, A.; Sehgal, M.; Rossomacha, E.; Datta, P.; Sharma, A.; Gandhi, R.; Kapoor, M.; et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Brabnikova-Maresova, K.; Jarosova, K.; Pavelka, K.; Stepan, J.J. Serum sclerostin in high-activity adult patients with juvenile idiopathic arthritis. Arthritis Res. Ther. 2014, 16, 460. [Google Scholar] [CrossRef]
- Wehmeyer, C.; Frank, S.; Beckmann, D.; Böttcher, M.; Cromme, C.; König, U.; Fennen, M.; Held, A.; Paruzel, P.; Hartmann, C.; et al. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci. Transl. Med. 2016, 8, 330ra35. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar] [CrossRef]
- Sen, M.; Carson, D.A. Wnt signaling in rheumatoid synoviocyte activation. Mod. Rheumatol. 2002, 12, 5–9. [Google Scholar] [CrossRef]
- Lories, R.J.; Corr, M.; Lane, N.E. To Wnt or not to Wnt: The bone and joint health dilemma. Nat. Rev. Rheumatol. 2013, 9, 328–339. [Google Scholar] [CrossRef]
- Rauner, M.; Stein, N.; Winzer, M.; Goettsch, C.; Zwerina, J.; Schett, G.; Distler, J.H.; Albers, J.; Schulze, J.; Schinke, T.; et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J. Bone Miner. Res. 2011, 27, 575–585. [Google Scholar] [CrossRef]
- Enomoto, M.; Hayakawa, S.; Itsukushima, S.; Ren, D.Y.; Matsuo, M.; Tamada, K.; Oneyama, C.; Okada, M.; Takumi, T.; Nishita, M.; et al. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene 2009, 28, 3197–3208. [Google Scholar] [CrossRef]
- Vincent, C.; Findlay, D.M.; Welldon, K.J.; Wijenayaka, A.R.; Zheng, T.S.; Haynes, D.R.; Fazzalari, N.L.; Evdokiou, A.; Atkins, G.J. Pro-Inflammatory Cytokines TNF-Related Weak Inducer of Apoptosis (TWEAK) and TNFα Induce the Mitogen-Activated Protein Kinase (MAPK)-Dependent Expression of Sclerostin in Human Osteoblasts*. J. Bone Miner. Res. 2009, 24, 1434–1449. [Google Scholar] [CrossRef]
- Marenzana, M.; Vugler, A.; Moore, A.; Robinson, M. Effect of sclerostin-neutralising antibody on periarticular and systemic bone in a murine model of rheumatoid arthritis: A microCT study. Arthritis Res. Ther. 2013, 15, R125. [Google Scholar] [CrossRef]
- Chen, X.-X.; Baum, W.; Dwyer, D.; Stock, M.; Schwabe, K.; Ke, H.-Z.; Stolina, M.; Schett, G.; Bozec, A. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann. Rheum. Dis. 2013, 72, 1732–1736. [Google Scholar] [CrossRef]
- Appel, H.; Ruiz-Heiland, G.; Listing, J.; Zwerina, J.; Herrmann, M.; Mueller, R.; Haibel, H.; Baraliakos, X.; Hempfing, A.; Rudwaleit, M.; et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Care Res. 2009, 60, 3257–3262. [Google Scholar] [CrossRef]
- Tsui, F.W.L.; Tsui, H.W.; Heras, F.L.; Pritzker, K.P.H.; Inman, R.D. Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Ann. Rheum. Dis. 2013, 73, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; Appel, H.; Braun, J.; Rudwaleit, M. Critical appraisal of assessment of structural damage in ankylosing spondylitis: Implications for treatment outcomes. Arthritis Care Res. 2008, 58, 649–656. [Google Scholar] [CrossRef]
- Sakellariou, G.T.; Anastasilakis, A.D.; Bisbinas, I.; Oikonomou, D.; Gerou, S.; Polyzos, S.A.; Sayegh, F.E. Circulating periostin levels in patients with AS: Association with clinical and radiographic variables, inflammatory markers and molecules involved in bone formation. Rheumatology 2014, 54, 908–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inagaki, Y.; Hookway, E.S.; Kashima, T.G.; Munemoto, M.; Tanaka, Y.; Hassan, A.B.; Oppermann, U.; A Athanasou, N. Sclerostin expression in bone tumours and tumour-like lesions. Histopathology 2016, 69, 470–478. [Google Scholar] [CrossRef]
- Sevetson, B.; Taylor, S.; Pan, Y. Cbfa1/RUNX2 Directs Specific Expression of the Sclerosteosis Gene (SOST). J. Biol. Chem. 2004, 279, 13849–13858. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Villanueva, D.; Zeef, L.; Shore, P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFβ-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011, 13, R106. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, H.; Li, X.; Li, X.; Cong, M.; Peng, F.; Yu, J.; Zhang, X.; Yang, Q.; Hu, G. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKKNat. Cell Biol. 2017, 19, 1274–1285. [Google Scholar] [CrossRef]
- Yuen, H.-F.; Chan, Y.-P.; Cheung, W.-L.; Wong, Y.-C.; Wang, X.; Chan, K.-W. The prognostic significance of BMP-6 signaling in prostate cancer. Mod. Pathol. 2008, 21, 1436–1443. [Google Scholar] [CrossRef]
- Hudson, B.D.; Hum, N.; Thomas, C.B.; Kohlgruber, A.; Sebastian, A.; Collette, N.; Coleman, M.A.; Christiansen, B.; Loots, G.G. SOST Inhibits Prostate Cancer Invasion. PLoS ONE 2015, 10, e0142058. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; van Lierop, A.H.; Hamdy, N.A.; Rizzoli, R.; Papapoulos, S.E. Serum sclerostin levels in Paget’s disease and prostate cancer with bone metastases with a wide range of bone turnover. Bone 2012, 51, 153–157. [Google Scholar] [CrossRef][Green Version]
- García-Fontana, B.; Morales-Santana, S.; Varsavsky, M.; García-Martín, A.; García-Salcedo, J.A.; Reyes-García, R.; Muñoz-Torres, M. Sclerostin serum levels in prostate cancer patients and their relationship with sex steroids. Osteoporos. Int. 2013, 25, 645–651. [Google Scholar] [CrossRef]
- Roodman, G.D. Pathogenesis of myeloma bone disease. Leukemia 2008, 23, 435–441. [Google Scholar] [CrossRef]
- Eisenberger, S.; Ackermann, K.; Voggenreiter, G.; Sültmann, H.; Kasperk, C.; Pyerin, W. Metastases and multiple myeloma generate distinct transcriptional footprints in osteocytes in vivo. J. Pathol. 2008, 214, 617–626. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Anderson, J.; Cregor, M.D.; Hiasa, M.; Chirgwin, J.M.; Carlesso, N.; Yoneda, T.; Mohammad, K.S.; Plotkin, L.I.; Roodman, G.D.; et al. Bidirectional Notch Signaling and Osteocyte-Derived Factors in the Bone Marrow Microenvironment Promote Tumor Cell Proliferation and Bone Destruction in Multiple Myeloma. Cancer Res. 2016, 76, 1089–1100. [Google Scholar] [CrossRef]
- Wang, X.-T.; He, Y.-C.; Zhou, S.-Y.; Jiang, J.-Z.; Huang, Y.-M.; Liang, Y.-Z.; Lai, Y.-R. Bone marrow plasma macrophage inflammatory protein protein-1 alpha(MIP-1 alpha) and sclerostin in multiple myeloma: Relationship with bone disease and clinical characteristics. Leuk. Res. 2014, 38, 525–531. [Google Scholar] [CrossRef]
- Loredana, S.; Santo, L.; Wein, M.N.; Hu, D.Z.; Cirstea, D.D.; Nemani, N.; Tai, Y.-T.; Raines, S.E.; Kuhstoss, S.A.; Munshi, N.C.; et al. Regulation of Sclerostin Expression in Multiple Myeloma by Dkk-1: A Potential Therapeutic Strategy for Myeloma Bone Disease. J. Bone Miner. Res. 2016, 31, 1225–1234. [Google Scholar] [CrossRef]
- Terpos, E.; Christoulas, D.; Katodritou, E.; Bratengeier, C.; Gkotzamanidou, M.; Michalis, E.; Delimpasi, S.; Pouli, A.; Meletis, J.; Kastritis, E.; et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: Reduction post-bortezomib monotherapy. Int. J. Cancer 2011, 131, 1466–1471. [Google Scholar] [CrossRef]
- Brunetti, G.; Oranger, A.; Mori, G.; Specchia, G.; Rinaldi, E.; Curci, P.; Zallone, A.; Rizzi, R.; Grano, M.; Colucci, S. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann. N. Y. Acad. Sci. 2011, 1237, 19–23. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Anderson, J.; Cregor, M.D.; Condon, K.W.; Kuhstoss, S.A.; Plotkin, L.I.; Bellido, T.; Roodman, G.D. Genetic deletion of SOST or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia 2017, 31, 2686–2694. [Google Scholar] [CrossRef]
- McDonald, M.M.; Reagan, M.R.; Youlten, S.E.; Mohanty, S.T.; Seckinger, A.; Terry, R.L.; Pettitt, J.A.; Simic, M.K.; Cheng, T.L.; Morse, A.; et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 2017, 129, 3452–3464. [Google Scholar] [CrossRef]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2015, 387, 1657–1671. [Google Scholar] [CrossRef]
- Fahiminiya, S.; Majewski, J.; Mort, J.; Moffatt, P.; Glorieux, F.H.; Rauch, F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 2013, 50, 345–348. [Google Scholar] [CrossRef]
- Roschger, A.; Roschger, P.; Keplingter, P.; Klaushofer, K.; Abdullah, S.; Kneissel, M.; Rauch, F. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone 2014, 66, 182–188. [Google Scholar] [CrossRef]
- Cardinal, M.; Tys, J.; Roels, T.; Lafont, S.; Ominsky, M.S.; Devogelaer, J.-P.; Chappard, D.; Mabilleau, G.; Ammann, P.; Nyssen-Behets, C.; et al. Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 2019, 124, 137–147. [Google Scholar] [CrossRef]
- Glorieux, F.H.; Devogelaer, J.-P.; Durigova, M.; Goemaere, S.; Hemsley, S.; Jakob, F.; Junker, U.; Ruckle, J.; Seefried, L.; Winkle, P.J. BPS804 Anti-Sclerostin Antibody in Adults with Moderate Osteogenesis Imperfecta: Results of a Randomized Phase 2a Trial. J. Bone Miner. Res. 2017, 32, 1496–1504. [Google Scholar] [CrossRef]
- Millán, J.L.; Whyte, M.P. Alkaline Phosphatase and Hypophosphatasia. Calcif. Tissue Res. 2015, 98, 398–416. [Google Scholar] [CrossRef]
- McKiernan, F.E.; Berg, R.L.; Fuehrer, J. Clinical and Radiographic Findings in Adults with Persistent Hypophosphatasemia. J. Bone Miner. Res. 2014, 29, 1651–1660. [Google Scholar] [CrossRef]
- Seefried, L.; Baumann, J.; Hemsley, S.; Hofmann, C.; Kunstmann, E.; Kiese, B.; Huang, Y.; Chivers, S.; Valentin, M.-A.; Borah, B.; et al. Efficacy of anti-sclerostin monoclonal antibody BPS804 in adult patients with hypophosphatasia. J. Clin. Investig. 2017, 127, 2148–2158. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliadis, E.S.; Evangelopoulos, D.-S.; Kaspiris, A.; Benetos, I.S.; Vlachos, C.; Pneumaticos, S.G. The Role of Sclerostin in Bone Diseases. J. Clin. Med. 2022, 11, 806. https://doi.org/10.3390/jcm11030806
Vasiliadis ES, Evangelopoulos D-S, Kaspiris A, Benetos IS, Vlachos C, Pneumaticos SG. The Role of Sclerostin in Bone Diseases. Journal of Clinical Medicine. 2022; 11(3):806. https://doi.org/10.3390/jcm11030806
Chicago/Turabian StyleVasiliadis, Elias S., Dimitrios-Stergios Evangelopoulos, Angelos Kaspiris, Ioannis S. Benetos, Christos Vlachos, and Spyros G. Pneumaticos. 2022. "The Role of Sclerostin in Bone Diseases" Journal of Clinical Medicine 11, no. 3: 806. https://doi.org/10.3390/jcm11030806
APA StyleVasiliadis, E. S., Evangelopoulos, D.-S., Kaspiris, A., Benetos, I. S., Vlachos, C., & Pneumaticos, S. G. (2022). The Role of Sclerostin in Bone Diseases. Journal of Clinical Medicine, 11(3), 806. https://doi.org/10.3390/jcm11030806






