Pain Burden in Post-COVID-19 Syndrome following Mild COVID-19 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaires
2.3. Calculation of Generalised Pain and Fibromyalgia Diagnosis According to the 2016 Criteria
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics of Participants
3.2. Comorbidities and Medication
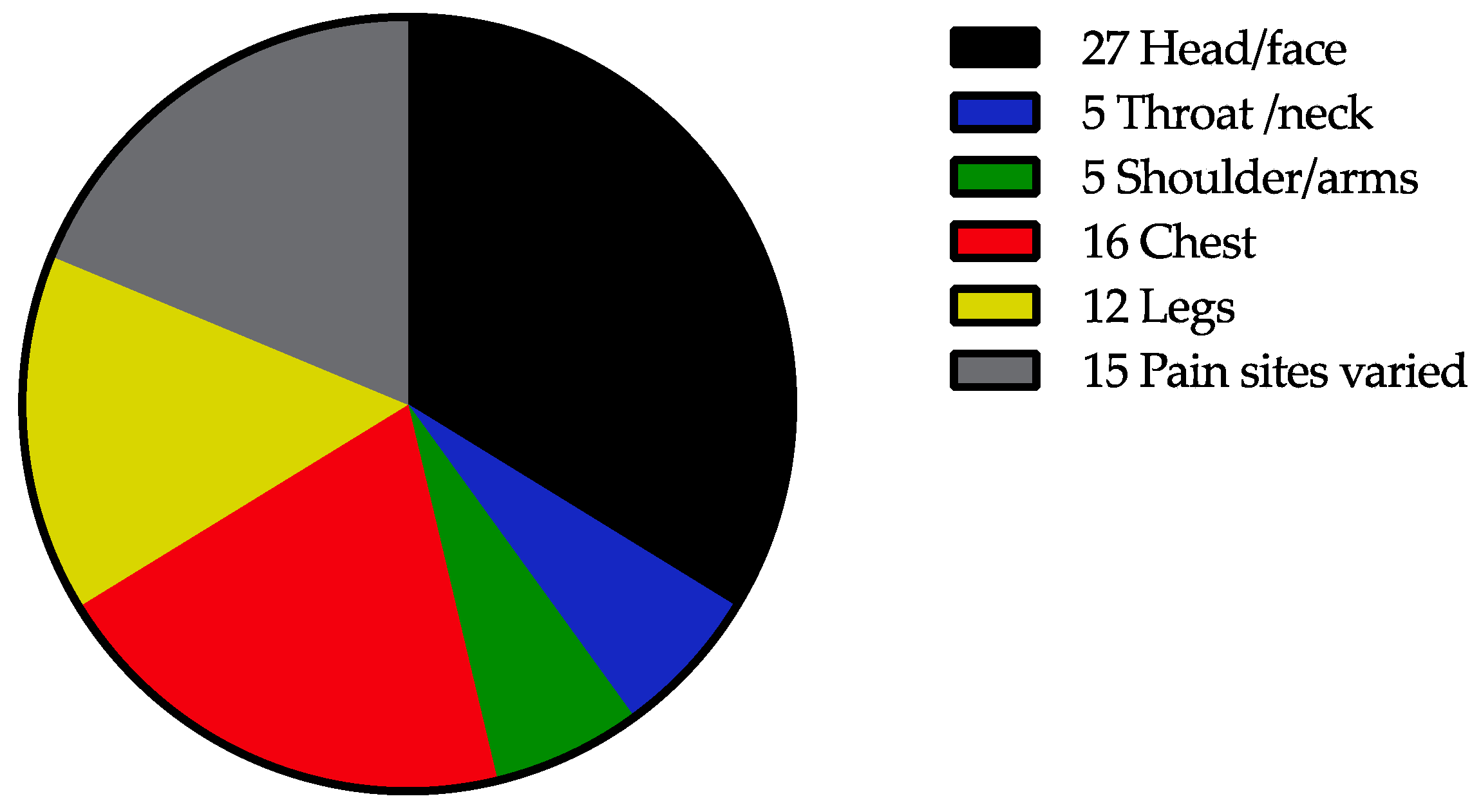
3.3. Pain Characteristics in Participants
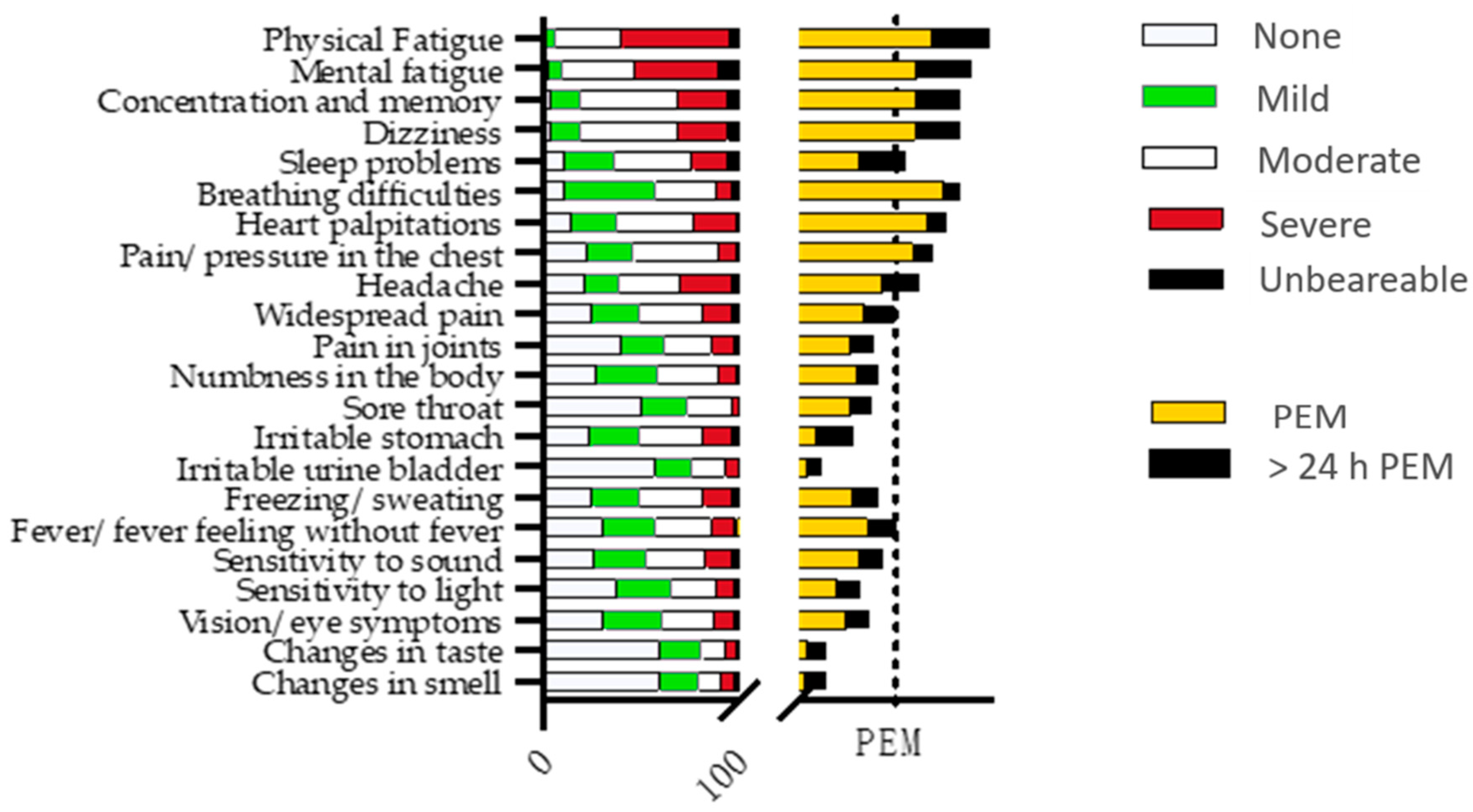
3.4. The Symptom Questionnaire
3.5. EQ5D, MFI-20, HADS, PHQ-9, GAD-7, and ISI
3.6. Widespread Pain and Estimation of Fibromyalgia Diagnosis According to the 2016 Criteria and Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yachou, Y.; El Idrissi, A.; Belapasov, V.; Ait Benali, S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: Understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020, 41, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Masi, P.; Hekimian, G.; Lejeune, M.; Chommeloux, J.; Desnos, C.; Pineton De Chambrun, M.; Martin-Toutain, I.; Nieszkowska, A.; Lebreton, G.; Brechot, N.; et al. Systemic Inflammatory Response Syndrome Is a Major Contributor to COVID-19-Associated Coagulopathy: Insights From a Prospective, Single-Center Cohort Study. Circulation 2020, 142, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Group, C.-I. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Lambert, N. Covid-19 “Long Hauler” Symptom Survey Report. 2020. Available online: https://dig.abclocal.go.com/wls/documents/2020/072720-wls-covid-symptom-study-doc.pdf (accessed on 30 January 2022).
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 30 January 2022).
- Carfi, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Liang, K.; Li, G.; Min, L.; Xing, Z.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Weng, L.M.; Su, X.; Wang, X.Q. Pain Symptoms in Patients with Coronavirus Disease (COVID-19): A Literature Review. J. Pain Res. 2021, 14, 147–159. [Google Scholar] [CrossRef]
- Fernandez-de-Las-Penas, C.; Navarro-Santana, M.; Gomez-Mayordomo, V.; Cuadrado, M.L.; Garcia-Azorin, D.; Arendt-Nielsen, L.; Plaza-Manzano, G. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: A meta-analysis of the current literature. Eur. J. Neurol. 2021, 28, 3820–3825. [Google Scholar] [CrossRef]
- Karaarslan, F.; Demircioglu Guneri, F.; Kardes, S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: Prospective follow-up by phone interviews. Rheumatol. Int. 2021, 41, 1263–1271. [Google Scholar] [CrossRef]
- Norrefalk, J.R.; Borg, K.; Bileviciute-Ljungar, I. Self-scored impairments in functioning and disability in post-COVID syndrome following mild COVID-19 infection. J. Rehabil. Med. 2021, 53, jrm00239. [Google Scholar] [CrossRef] [PubMed]
- Szende, A.; Janssen, B.; Cabases, J. Self-Reported Population Helath: An International Perspective Based on EQ-5D; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Sullivan, M.; Karlsson, J. The Swedish SF-36 Health Survey III. Evaluation of criterion-based validity: Results from normative population. J. Clin. Epidemiol. 1998, 51, 1105–1113. [Google Scholar] [CrossRef]
- Sullivan, M.; Karlsson, J.; Ware, J.E., Jr. The Swedish SF-36 Health Survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc. Sci. Med. 1995, 41, 1349–1358. [Google Scholar] [CrossRef]
- Ericsson, A.; Bremell, T.; Mannerkorpi, K. Usefulness of multiple dimensions of fatigue in fibromyalgia. J. Rehabil. Med. 2013, 45, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lungh Hagelin, C.; Wengström, Y.; Runesdotter, S.; Furst, C.-J. The psychometric properties of the Swedish Multidimentional Ftigue Inventory MFI-20 in four different populations. Acta Oncol. 2007, 46, 97–104. [Google Scholar] [CrossRef]
- Zigmont, A.S.; Snaith, R.P. The Hospital and Anxiety Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Löwe, B. The Patient Health Questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. Gen. Hosp. Psychiatry 2010, 32, 345–359. [Google Scholar] [CrossRef]
- Levis, B.; Benedetti, A.; Thombs, B.D. Accuracy of patient health questionnaire-9 (PHQ-9) for screening to detect major depression: Individual participant data meta-analysis. BMJ 2019, 365, 1476. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-de-Las-Penas, C.; Palacios-Cena, D.; Gomez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Tiosano, S.; Amital, H. The complexities of fibromyalgia and its comorbidities. Curr. Opin. Rheumatol. 2018, 30, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kleykamp, B.A.; Ferguson, M.C.; McNicol, E.; Bixho, I.; Arnold, L.M.; Edwards, R.R.; Fillingim, R.; Grol-Prokopczyk, H.; Turk, D.C.; Dworkin, R.H. The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: An ACTTION systematic review. Semin. Arthritis Rheum. 2021, 51, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016, 157, 55–64. [Google Scholar] [CrossRef]
- Andrews, P.; Steultjens, M.; Riskowski, J. Chronic widespread pain prevalence in the general population: A systematic review. Eur. J. Pain. 2018, 22, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Vanichkachorn, G.; Newcomb, R.; Cowl, C.T.; Murad, M.H.; Breeher, L.; Miller, S.; Trenary, M.; Neveau, D.; Higgins, S. Post-COVID-19 Syndrome (Long Haul Syndrome): Description of a Multidisciplinary Clinic at Mayo Clinic and Characteristics of the Initial Patient Cohort. Mayo Clin. Proc. 2021, 96, 1782–1791. [Google Scholar] [CrossRef]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef]
| Disorders | Disorders before COVID-19 | Taking Medication after COVID-19 | Taking Medication after COVID-19, Healthy before, n = 68 | Taking Medication after COVID-19, Unhealthy before, n = 32 | p-Value |
|---|---|---|---|---|---|
| Total number of persons with disorders before infection | 32 | ||||
| Cardiovascular disorders | 7 | 26 | 15 | 11 | p = 0.1 |
| Metabolic diseases: | 6 | ||||
| 4 1 1 | 7 1 | 2 0 | 5 1 | p = 0.03 p = 0.3 |
| Lung disorders: | 11 | ||||
| 10 1 | 29 | 12 | 17 | p < 0.001 |
| Allergies | 2 | 19 | 11 | 8 | p = 0.2 |
| Psychiatric disorders: | 12 | ||||
| 1 6 2 2 1 | 23 | 10 | 13 | p = 0.005 |
| Inflammatory disorders: | 9 | NSAID, biological drugs | 9 | 3 | p = 0.4 |
| 2 2 2 3 | tricyclic/tetracyclic antidepressants opioids antiepileptics paracetamol | 9 1 4 1 | 4 0 2 2 | p = 0.6 p = 0.7 p = 0.6 p = 0.2 |
| Vitamin deficiency | 2 | ||||
| Sleep disorders: | |||||
| 1 | 17 | 9 | 8 | p = 0.1 |
| Herpes virus | 1 | ||||
| Anaemia | 1 | ||||
| ME/CFS | 1 | ||||
| BMI (mean, standard deviation in kg/m2) | 26.5, 5.9 | 25.2, 3.9 | 29.3, 8.2 | p = 0.01 |
| Questionnaires | Mean, SD, and Range | Number of Persons with Abnormal Values |
|---|---|---|
| EQ5D index | 0.51 (0.2) 0.14–1.00 | 99 |
| EQ5D VAS | 42.6 (19.5) 10–83 | 100 |
| Bodily Pain SF-36 | 46 (23) 10–100 | 84 |
| MFI-20 General fatigue | 18.5 (2.2) 8–20 | 98 |
| MFI-20 Physical fatigue | 18.1 (2.4) 7–20 | 99 |
| MFI-20 Reduced activity | 17.4 (3.0) 5–20 | 97 |
| MFI-20 Reduced motivation | 11.7 (3.7) 4–20 | 78 |
| MFI-20 Mental fatigue | 14.7 (3.7) 4–2 | 96 |
| HADS Anxiety | 8.0 (3.2) 2–21 | 14 |
| HADS Depression | 8.7 (4.1) 0–19 | 28 |
| PHQ-9 | 12.7 (6.1) 0–28 | 56 |
| GAD-7 | 5.2 (4.6) 0–19 | 20 |
| Insomnia Severity Index | 12.7 (6.1) 0–28 | 34 |
| Widespread Pain Index and Number of Participants (Max 19 Points) | Symptom Severity Scale (Max 12 Points) | Fibromyalgia Diagnosis, Number of Participants |
|---|---|---|
| 0 (n = 1) | 3 | 0 |
| 3 (n = 1) | 4 | 0 |
| 4 (n = 1 | 11 | 1 |
| 5 (n = 3) | 7–8 | 0 |
| 6 (n = 8) | 5–10 | 3 |
| 7 (n = 8) | 8–11 | 8 |
| 8 (n = 3) | 7–9 | 3 |
| 9 (n = 6) | 8–11 | 6 |
| 10 (n = 5) | 9–12 | 5 |
| 11 (n = 5) | 8–12 | 5 |
| 12 (n = 1) | 11 | 1 |
| 13 (n = 1) | 10 | 1 |
| 14 (n = 1) | 12 | 1 |
| 15 (n = 3) | 7–10 | 3 |
| 16 (n = 2) | 10–12 | 2 |
| 19 (n = 1) | 8 | 1 |
| Pain Drugs After COVID-19, Median and Range | Total Drugs After COVID-19, Median and Range | No Generalised Pain After COVID-19, n = 50 | Generalised Pain After COVID-19, n = 50 | Comparison “No Generalised Pain” vs. “Generalised Pain” | No Fibromyalgia After COVID-19, n = 60 | Fibromyalgia After COVID-19, n = 40 | Comparison “No Fibromyalgia” vs. “Fibromyalgia” | |
|---|---|---|---|---|---|---|---|---|
| n = 100 | ||||||||
| Healthy before COVID-19, n = 68 | 0 (0–6) | 1 (0–9) | 38 | 30 | 45 | 23 | ||
| Unhealthy, n = 32 | 1 (0–5) | 2 (0–7) | 12 | 20 | 15 | 17 | ||
| Comparison “Healthy” vs. “Unhealthy” | p = 0.13 M-W | p < 0.001 M-W | p = 0.13 Chi-square | p = 0.082 Chi-square | ||||
| Total comorbidities before COVID-19, median and range | 0 (0–2) | 0 (0–5) | p = 0.031 M-W | 0 (0–4) | 0 (0–5) | p = 0.027 M-W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bileviciute-Ljungar, I.; Norrefalk, J.-R.; Borg, K. Pain Burden in Post-COVID-19 Syndrome following Mild COVID-19 Infection. J. Clin. Med. 2022, 11, 771. https://doi.org/10.3390/jcm11030771
Bileviciute-Ljungar I, Norrefalk J-R, Borg K. Pain Burden in Post-COVID-19 Syndrome following Mild COVID-19 Infection. Journal of Clinical Medicine. 2022; 11(3):771. https://doi.org/10.3390/jcm11030771
Chicago/Turabian StyleBileviciute-Ljungar, Indre, Jan-Rickard Norrefalk, and Kristian Borg. 2022. "Pain Burden in Post-COVID-19 Syndrome following Mild COVID-19 Infection" Journal of Clinical Medicine 11, no. 3: 771. https://doi.org/10.3390/jcm11030771
APA StyleBileviciute-Ljungar, I., Norrefalk, J.-R., & Borg, K. (2022). Pain Burden in Post-COVID-19 Syndrome following Mild COVID-19 Infection. Journal of Clinical Medicine, 11(3), 771. https://doi.org/10.3390/jcm11030771







